Randomized clinical trial comparing outcomes after fentanyl or ketamine-dexmedetomidine analgesia in thoracolumbar spinal surgery in dogs
Abstract
Background
Opioids are widely used for perioperative pain control in dogs undergoing spinal surgery, but alternatives may be required because data suggest that opioids exacerbate inflammation in the injured spinal cord and veterinary access to opioids may become more restricted in the future.
Objectives
To compare recovery of ambulation and other functions between spinal cord-injured dogs receiving peri-operative fentanyl and those receiving a ketamine-dexmedetomidine combination.
Animals
A total of 102 client-owned dogs undergoing decompressive surgery for thoracolumbar intervertebral disc herniation.
Methods
Randomized clinical trial. Dogs were randomized 1:1 to fentanyl or a ketamine-dexmedetomidine combination for intra and postoperative analgesia. Primary outcome was time to recovery of ambulation; secondary outcomes were the postoperative Colorado Acute Pain Scale, the short-form Glasgow Composite Measure Pain Scale, time to recovery of voluntary urination and time to unassisted eating.
Results
No difference was found in time to recovery of ambulation between groups (adjusted sub-hazard ratio, 0.83; 95% confidence interval [CI], 0.55-1.24; P = .36) or in pain scores (Colorado: χ2 = 14.74; P = .32; Glasgow: χ2 = 6.61; P = .76). Differences in time to recovery of eating and urination were small but favored ketamine-dexmedetomidine (adjusted odds ratios, 3.31; 95% CI, 1.53-7.16; P = .002 and 2.43; 95% CI, 1.00-5.96; P = .05, respectively).
Conclusions and Clinical Importance
There was no evidence that, at the doses used, fentanyl impaired ambulatory outcome after surgery for thoracolumbar intervertebral disc herniation in dogs. Pain control appeared similar between groups. Secondary outcomes suggested minor benefits associated with ketamine-dexmedetomidine. The ketamine-dexmedetomidine combination appears to be a reasonable alternative to peri-operative opioids.
Abbreviations
-
- CI
-
- confidence interval
-
- CMPS-SF
-
- short-form Glasgow Composite Measure Pain Scale
-
- CRI
-
- constant rate infusion
-
- CSU
-
- Colorado State University
-
- CT
-
- computed tomography
-
- IQR
-
- interquartile range
-
- IV
-
- intravenous
-
- MRI
-
- magnetic resonance imaging
-
- NMDA
-
- N-methyl-D-aspartate
-
- OR
-
- odds ratio
-
- SD
-
- standard deviation
-
- SHR
-
- subhazard ratio
-
- TLR
-
- toll-like receptor
1 INTRODUCTION
Although specific postsurgical analgesic protocols vary among veterinary hospitals, opioids are the most effective drug class for managing acute pain,1 and currently form the cornerstone of postoperative pain management.2 Despite these advantages, adverse effects associated with opioid analgesics in dogs include bradycardia,3-5 hypoventilation,3, 5 gastroesophageal reflux,6 and dysphoria,7 as well as a single report of myoclonus and urinary retention following subarachnoid administration.8 Postoperatively, the most prominent adverse effects include dysphoria, nausea, vomiting, ileus and panting.6, 9-11
Opioids have additional possible drawbacks. First, widespread opiate addiction in people in the United States has prompted consideration of further restriction on veterinary access to opioid analgesics,12 potentially limiting their use for postoperative pain control in animals. If this was to occur, readily available opioid-free alternatives to manage pain after surgical procedures will be required, as being developed in human medicine,13 and another option might be ketamine.14, 15 However, a previous study in dogs suggested that the analgesic effect of ketamine in combination with dexmedetomidine with or without lidocaine after ovariohysterectomy was less than that associated with fentanyl alone.16 Nevertheless, ketamine has many other effects that may be beneficial, especially in neurosurgery, such as neuroprotection (by blockade of the N-methyl-D-aspartate [NMDA] receptor),17 antiinflammatory effects,18, 19 decreased requirement for inhalant anesthetic,4 and decrease in postoperative shivering.20 Dexmedetomidine also has been reported to have neuroprotective21 and antiinflammatory22 effects.
Also, recent studies using experimental rodents suggested that opioids might worsen functional outcome after spinal cord injury by exacerbating the inflammation and tissue damage that follow contusive lesions.23-25 On the other hand, these effects have not been consistently observed, with some researchers reporting similar outcomes in a sub-acute spinal cord injury model between fentanyl- and ketamine-treated animals.26 In the clinic, long-standing evidence links use of opioid receptor blockers to preservation of spinal cord tissue after traumatic injury,27 culminating in a study that found a lack of benefit associated with naloxone.28 A possible link between opioid use and impaired recovery after spinal cord injury in dogs has been suggested by 2 recent studies in which a longer duration of anesthesia (or surgery) was associated with decreased likelihood of recovery after decompressive surgery for acute thoracolumbar disc herniation.29, 30 Although numerous explanations are possible for this association, prolonged use of opioids during anesthesia is a plausible factor.
We investigated the possibility that fentanyl might be detrimental to recovery after spinal cord injury in dogs. We compared the rapidity of recovery of ambulation and other outcomes between dogs randomized to receive fentanyl or a ketamine-dexmedetomidine combination after decompressive surgery for acute thoracolumbar disc herniation.
2 MATERIALS AND METHODS
2.1 Animals
The study was approved by the Clinical Research Review Committee and the Institutional Animal Care and Use Committee of Texas A&M University and informed owner consent was obtained before enrollment for all dogs.
Client-owned dogs presented to the Texas A&M Small Animal Hospital between September 2019 and February 2022 for evaluation of neurologic dysfunction consistent with thoracolumbar myelopathy were eligible for enrollment unless they met the following exclusion criteria: preexisting conditions that increase the risk of aspiration pneumonia (e.g., megaesophagus), or cardiac disease that potentially could preclude the safe administration of dexmedetomidine and ketamine as a constant rate infusion (CRI). The number of hours that elapsed between when each dog was first observed to be unable to walk and when the dog was presented to the hospital was recorded for all nonambulatory or paraplegic patients. Corticosteroid or nonsteroidal antiinflammatory drugs administered in the 24 hours before anesthesia were noted.
Body weight and signalment data were recorded for each patient. All dogs underwent physical and neurologic examinations and preanesthetic blood testing consisting of venous blood gas analysis and serum electrolyte concentrations, total solids concentration and packed cell volume, or CBC and serum biochemistry profile. Preoperative neurologic status was categorized as: (a) ambulatory, (b) nonambulatory with paraparesis, (c) paraplegic with intact deep pain sensation or (d) paraplegic with absent deep pain sensation. Deep pain sensation was assessed as described previously.31
Dogs were randomly assigned either to Group F (fentanyl) or Group K (ketamine-dexmedetomidine combination). Random allocation was achieved by opening numbered envelopes, each containing treatment assignment written on a slip of paper, after each dog had been accepted into the study and the owner had given consent. Randomization was 1:1 between treatment groups and batches of envelopes of variable number (between 8 and 20) were prepared before and throughout the study to ensure that, if recruitment problems occurred, an approximately equal number of dogs would be allocated to each treatment arm.
Dogs that had undergone computed tomography (CT) and been diagnosed with thoracolumbar intervertebral disc herniation were enrolled and randomized after imaging and before general anesthesia for surgery. Dogs undergoing magnetic resonance imaging (MRI) under general anesthesia to investigate the cause of myelopathy with intent to proceed directly to surgery were enrolled and randomized before imaging. If MRI findings led to a diagnosis of noncompressive intervertebral disc herniation or a different cause of myelopathy (e.g., neoplasia) they were then excluded from the study. The location of disk herniation was recorded based on cross-sectional images. Dogs diagnosed with intervertebral disc herniations caudal to L3/4 were excluded from the study to remove the possible confounding influence of lower motor neuron involvement on neurologic outcome.
2.2 Anesthetic protocols
The trial protocol did not restrict medications used for preoperative pain control or premedication before induction. Therefore, pain management strategies between admission and diagnostic evaluation varied, but the majority of dogs received IV methadone (Mylan Institutional LLC, Rockford, Illinois; 0.2 mg/kg). For induction, all dogs received IV midazolam (Almaject, Morristown, New Jersey; 0.25 mg/kg) and propofol (Zoetis, Kalamazoo, Michigan; up to 4 mg/kg to effect, until the trachea could be intubated). Endotracheal tubes were attached to a rebreathing circuit through which dogs received sevoflurane (Akorn, Lake Forest, Illinois) in 100% oxygen.
Heart rate, respiratory rate, end-tidal CO2, rectal or esophageal temperature, pulse arterial oxygen saturation, and oscillometric blood pressure were recorded every 5 minutes throughout the anesthetic period using a multiparameter anesthesia monitor (Mindray, Mahwah, New Jersey). A volume-controlled mechanical ventilator was used to provide positive pressure ventilation and maintain end-tidal CO2 between 35 and 45 mm Hg.
After induction and placement of monitoring devices, anesthesia was maintained using sevoflurane in dogs undergoing MRI. Once intervertebral disc herniation with an indication for surgery was confirmed, the assigned analgesic treatment was started while the surgical site was prepared. Dogs with a CT diagnosis of intervertebral disc herniation received the assigned drug after induction and placement of monitoring devices, concurrent with surgical preparation. Patients assigned to receive fentanyl (Group F) received a 2 to 5 μg/kg IV bolus of fentanyl (Hospira, Lake Forest, Illinois), followed by a CRI of fentanyl at 3 to 20 μg/kg/h. Infusion rate was adjusted intraoperatively based on fluctuations in physiological variables (±20% change) and anesthetic depth. If fentanyl was deemed insufficient to control intraoperative pain (i.e., sustained tachycardia and mean arterial pressure >100 mm Hg), lidocaine (MWI, Boise, Idaho) was administered IV at a dosage of 2 mg/kg followed by a CRI of 2 to 3 mg/kg/h. Dogs assigned to ketamine-dexmedetomidine (Group K) received dexmedetomidine (Zoetis, Kalamazoo, Michigan; 0.5-1 μg/kg IV over 10 min followed by a CRI of dexmedetomidine at 0.5-1 μg/kg/h). In addition, they received an IV bolus of ketamine (Akorn Inc, Lake Forest, Illinois; 0.5-1 mg/kg) followed by a CRI of 0.3 to 0.6 mg/kg/h. Similar to Group F, physiologic variables and anesthetic depth were used to assess analgesia intraoperatively and, if dogs were determined to be painful, a 0.5 μg/kg bolus of dexmedetomidine was administered. If this dose was insufficient, it was followed by a bolus of 0.5 to 1 mg/kg dose of ketamine and the infusion rates of both drugs were titrated as needed. If the combined analgesic effects of dexmedetomidine and ketamine were deemed insufficient, lidocaine was administered at a dosage of 2 mg/kg IV followed by a CRI of 2 to 3 mg/kg/h.
Routine decompressive surgery was performed under general anesthesia by a board-certified neurologist, a neurology resident, a surgery resident or a combination thereof. Patients in both groups were recovered from anesthesia and extubated. No restrictions were placed on the administration of sedative medications (including dexmedetomidine) upon recovery, if indicated.
Postoperatively, dogs in Group F continued to receive a fentanyl CRI starting at 3 μg/kg/h and the rate was adjusted as needed based on clinical assessment of comfort within a range 1 to 4 μg/kg/h. Dogs in Group K continued to receive a CRI of ketamine combined with dexmedetomidine starting at 0.3 mg/kg/h of ketamine and 0.5 μg/kg/h of dexmedetomidine and the infusion rate was adjusted as needed within a range of 0.15 to 0.6 mg/kg/h ketamine and 0.25 to 1 μg/kg/h dexmedetomidine. Because these drugs were combined in a single syringe postoperatively, any adjustment resulted in proportionally equal changes in ketamine and dexmedetomidine doses. All CRIs were delivered by syringe pump (B Braun Medical, Bethlehem, Pennsylvania). Concurrent administration and doses of nonsteroidal antiinflammatory drugs or other PO analgesics (e.g., gabapentin) was based on patient-specific factors and clinician preference. Dogs in both groups were maintained on their assigned CRI for a period of 24 to 48 h postoperatively, with the dose being gradually decreased before discontinuation of IV analgesia if clinical assessment deemed the dogs to be comfortable.
Postoperatively, dogs were given the opportunity to urinate q6h. All dogs that did not urinate voluntarily underwent bladder palpation and, if necessary, ultrasound examination, to determine whether they had inappropriate urine retention (defined in this group as a bladder diameter of >6 cm). All dogs with urine retention were managed by manual bladder expression until recovery of voluntary control, which was recorded as a single outcome measure (see below).
2.3 Outcome measures
The primary outcome measure was the time elapsed until recovery of independent walking in each dog, which was defined as taking 10 consecutive unassisted steps without falling. In dogs that remained nonambulatory at discharge, the time to recover ambulation was recorded based on in-person reevaluations, telephone, or email follow-up with owners.
To assess pain control between groups, all patients were evaluated and assigned pain scores using the Colorado (CSU) Acute Pain Scale,32 and the short-form Glasgow Composite Measure Pain Scale (CMPS-SF)33 the morning after surgery by an observer blinded to their treatment. Secondary outcome measures included the time (days) elapsed before dogs were willing to eat and before they gained control of urination, which were obtained from hospital records or from follow-up with the owners.
2.4 Statistical analysis
Appropriate sample size for the study was difficult to determine because of a lack of preexisting similar data. The closest parallel was experiments carried out in rats in which behavioral differences were detected using groups of 9 rats,23 corresponding to a large effect size (Cohen's d = 1.2). Because the effects in dogs were likely more difficult to detect as a consequence of multiple clinical factors such as breed, size, and severity of injury that might make measurement of outcome more variable, we opted to collect data on 100 dogs, for which power calculations indicated we would be able to detect a hazard ratio of 1.75 in Cox proportional hazards regression analysis, which corresponds to a Cohen's d of 0.44, usually regarded as small to medium effect size.34
Results initially were assembled in spreadsheets and summary statistics (mean, median, standard deviation, interquartile range [IQR]) were calculated. Because we were attempting to determine whether a different effect existed between the 2 analgesics, the analysis followed a per protocol approach, only including dogs that met the inclusion criteria (and not the exclusion criteria) and that also received treatment as allocated by randomization.
We intended to use Cox proportional hazards regression for examining differences in recovery of ambulation after surgery, but this approach was replaced with competing risks regression35 because some animals died at various stages after surgery and could not be regarded as censored. The χ2 test was used to compare pain scores between groups and ordinal logistic regression was used to analyze recovery of eating and urination (because, post hoc, the data were found to be clustered at specific time points). All analyses were carried out using Stata 17 (StataCorp, College Station, Texas) and P values < .05 were considered significant. Graphs were prepared using GraphPad Prism (Prism 7 for Windows, San Diego, California).
3 RESULTS
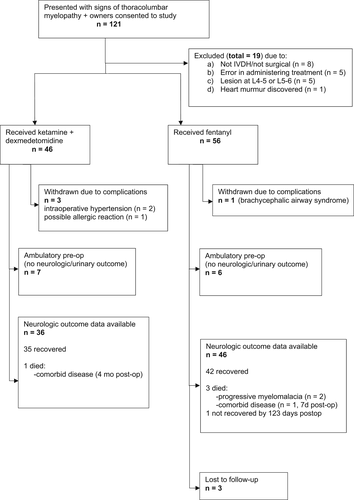
The initial study population consisted of 121 dogs, of which 19 were excluded from further analysis because they did not meet our inclusion and exclusion criteria (Figure 1). A total of 102 dogs that received their allocated intra and postoperative analgesic medication according to the protocol were included in the analysis of outcomes, of which 56 dogs received fentanyl and 46 received the ketamine-dexmedetomidine combination. An imbalance occurred in numbers between the groups because of errors in administering the randomized treatment and restriction of investigation to per protocol analysis. The demographic features were similar between the 2 groups (as expected based on randomization and despite errors in allocation) and these data are summarized in Table 1. Peri-operative variables are summarized in Table 2. It is evident that a minor imbalance occurred between groups in the proportion of Grade 2 cases (nonambulatory paraparesis).
| Variable | Fentanyl | Ketamine | ||
|---|---|---|---|---|
| Number (%) | Median (IQR) | Number (%) | Median (IQR) | |
| Total number | 55 | 43 | ||
| Male | 30 (55%) | 20 (47%) | ||
| Weight (kg) | 7.1 (5.6-10.2) | 7.2 (5.4-10.5) | ||
| Age (years) | 6 (4-9) | 6 (4-8) | ||
| Neutered | 7 (13%) | 5 (12%) | ||
- Abbreviation: IQR, interquartile range.
| Variable | Fentanyl | Ketamine | ||
|---|---|---|---|---|
| Number (%) | Mean (SD) or Median (IQR) | Number (%) | Mean (SD) or Median (IQR) | |
| Neurologic signs | ||||
| Grade 1 | 7 (13%) | 9 (21%) | ||
| Grade 2 | 34 (62%) | 19 (44%) | ||
| Grade 3 | 13 (24%) | 12 (28%) | ||
| Grade 4 | 1 (2%) | 3 (7%) | ||
| Duration of signs (h) | 22 (11-36) | 24 (12-48) | ||
| Preoperative meds | ||||
| NSAID | 13 (24%) | 15 (35%) | ||
| C/S | 15 (27%) | 10 (23%) | ||
| Anesthesia time (min) | 255 (57) | 260 (67) | ||
| Surgery time (min) | 132 (35) | 127 (42) | ||
- Abbreviations: IQR, interquartile range; SD, standard deviation.
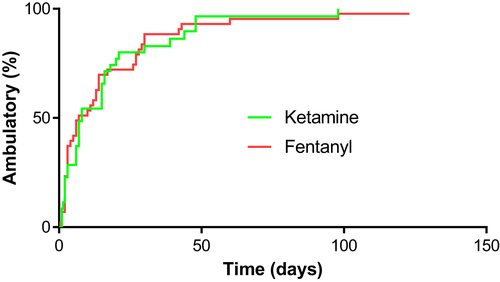
Our primary outcome measure was the time to recover independent ambulation (defined as 10 steps without falling). For the 82 dogs for which we had follow-up for this outcome, 4 dogs were presented deep pain-negative: 3 in Group K (of which 2 recovered and 1 died of concurrent disease [brain glioma] at 131 days) and 1 in Group F (that was lost to follow-up at 11 days). Overall, 4 dogs died (1 in Group K and 3 in Group F), 2 of progressive myelomalacia and 2 of comorbidities (1 multisystem endocrine-related disease and 1 brain tumor), and 1 dog (Group F) had not recovered ambulation by 123 days after surgery. The remaining 77 dogs recovered independent ambulation between 12 h and 98 days after decompressive surgery. Kaplan-Meier analysis (Figure 2) indicated little difference in time to recover ambulation between treatment groups (medians of 7.5 and 7 days for the ketamine and fentanyl groups, respectively). Competing risks regression was used to compare recovery of ambulation between the fentanyl and ketamine groups. Both crude (subhazard ratio [SHR] = 0.95; 95% confidence interval [CI], 0.62-1.46; P = .82) and adjusted (incorporating severity of initial presentation as a covariate: SHR = 0.80; 95% CI, 0.54-1.20; P = .28) analyses did not indicate association of treatment type with recovery.
The effect of both medication types on postoperative pain appeared comparable; 24-h pain scores on both scales generally were similar between treatment groups and χ2 tests gave no indication of differences between treatment groups (Tables 3 and 4). Of the 55 dogs treated with fentanyl and included in the analysis for which we had pain scores, 9 (16%) had scores >1 on the CSU Acute Pain scale and for ketamine the equivalent number was 4 (9%). Similarly, for the CMPS-SF 16 dogs (29%) treated with fentanyl scored >2 and 11 dogs (26%) scored >2 for ketamine.
| CAPS | 0 | 0.0 | 0.25 | 0.5 | 0.6 | 0.7 | 0.75 | 0.8 | 0.9 | 1 | 1.25 | 1.5 | 2 | 3 | TOTAL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ketamine | 8 | 1 | 0 | 11 | 0 | 0 | 6 | 2 | 2 | 9 | 1 | 2 | 1 | 0 | 43 |
| Fentanyl | 5 | 2 | 2 | 7 | 1 | 1 | 16 | 1 | 0 | 11 | 1 | 6 | 1 | 1 | 55 |
| TOTAL | 13 | 3 | 2 | 18 | 1 | 1 | 22 | 3 | 2 | 20 | 2 | 8 | 2 | 1 | 98 |
- Note: Analysis: Χ2 = 14.74; P = .32.
| Glasgow | 0 | 1 | 1.5 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 10 | TOTAL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ketamine | 7 | 14 | 1 | 10 | 5 | 2 | 0 | 1 | 1 | 2 | 0 | 43 |
| Fentanyl | 6 | 16 | 0 | 17 | 7 | 5 | 1 | 1 | 0 | 1 | 1 | 55 |
| TOTAL | 13 | 30 | 1 | 27 | 12 | 7 | 1 | 2 | 1 | 3 | 1 | 98 |
- Note: Analysis: Χ2 = 6.61; P = .76.
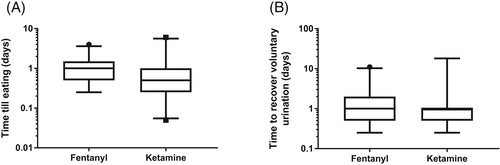
Our secondary outcomes of time to voluntarily eat and time to voluntarily urinate produced data that were clustered into narrow time periods (because of the way these functions are monitored after surgery), but little clinically meaningful difference in the time to recover eating was observed (Figure 3). However, because of the clustering, the data were re-classified into 3 time periods (<6 hours, 6-24 hours, and >24 hours) to correspond to the time intervals when food was made available to the dogs after surgery. In this analysis, using ordinal logistic regression and adjusting effect of treatment allocation for severity of neurologic presentation, dogs that received fentanyl were more likely to first eat in later time periods than those treated with ketamine (odds ratio [OR] = 3.31; 95% CI, 1.53-7.16; P = .002) but recovery of voluntary eating did not seem to be influenced by severity of presentation (OR = 1.46; 95% CI, 0.89-2.40; P = .13). Similar analysis of recovery of voluntary urination confirmed that dogs treated with fentanyl were more likely to urinate later after surgery (OR = 2.44; 95% CI, 1.00-5.96; P = .05) and more severe initial presentation had a similar effect in delaying recovery of urinary control (OR = 4.97; 95% CI, 2.10-11.73; P < .001).
3.1 Intraoperative observations
Surgeons were not blinded to the intraoperative study drug and their subjective impression was that intraoperative hemorrhage was more severe in dogs that received ketamine-dexmedetomidine, but this impression was not reflected in overall surgery time (Group F: 132 ± 35 min vs Group K: 127 ± 42 min). Problems with prolonged and excessive hypertension led to withdrawal of 2 dogs from the ketamine group at the discretion of the anesthesiologist. These 2 dogs were not included in the outcome analysis.
4 DISCUSSION
Our study sought to determine if peri-operative use of fentanyl has a detrimental effect on outcome after decompressive spinal surgery (as compared with a ketamine-dexmedetomidine combination). No difference was found in time to recover independent ambulation between groups. However, some evidence supported a minor effect of fentanyl on recovery of eating and urination. The lack of evidence of an effect on recovery of ambulation suggests that it is unlikely that prolonged use of fentanyl during surgery can account for the previously noted association between duration of anesthesia and surgery with decreased likelihood of recovery after decompressive thoracolumbar spinal surgery.29, 30
The finding of an association of fentanyl use with delayed recovery of eating and urination was not unexpected, because both of these effects previously have been associated with opioid administration,36-39 although not consistently,40 and fentanyl appears to have a lesser effect on urinary control than some other opioids.36 In addition, the clinical implications of these findings are likely fairly limited, because 93/98 (95%) of dogs resumed eating within 48 h after surgery. Nevertheless, evidence suggests that early recovery of eating after surgery decreases the risks of postoperative complications,41 and a limited period of postoperative anorexia may provide some patient benefit. In view of the findings that fentanyl decreases,42 and dexmedetomidine increases,43 urine production, it might be expected that loss of urinary control would be more apparent in dogs in Group K. We found the opposite, and perhaps the relative benefit of ketamine and dexmedetomidine on voluntary urination in our study was stronger than first appreciated. Nevertheless, any such effects are unlikely to be clinically relevant because 68/78 (87%) dogs recovered voluntary urination within 48 hours of surgery and it is well-recognized (as found in our study) that severity of spinal cord injury plays the dominant role in determining when urinary control is regained and any detrimental effect of fentanyl is comparatively minor.
On the other hand, our data show that there is no reason to suppose that the analgesia provided by the ketamine-dexmedetomidine combination was inferior to that provided by fentanyl (Tables 3 and 4), implying that ketamine likely could be substituted for fentanyl without risking loss of pain control. Indeed, our data provide support for substitution of fentanyl by ketamine in clinics or geographical regions where veterinary access to opioids may be difficult. The pain score data were congregated at high frequency at specific values and little overall variability was found both within and between treatment groups. This finding might indicate that postoperative pain is generally well-controlled in most dogs undergoing decompressive spinal surgery but, on the other hand, it might indicate that the validated pain scoring schemes that we used are not appropriate for evaluating this type of surgical patient and do not readily allow distinction among different levels of pain. Measuring pain in animals is difficult because of their inability to self-report44 and, especially for clinical cases, an additional problem is knowing how best to interpret what appears as restlessness or anxiety. Pain scoring in animals relies entirely upon interpretation of behavioral signs and these are susceptible to misinterpretation. For example, reluctance to interact with veterinarians and technicians might be a sign of pain, but also may be normal behavior for an individual dog treated in a veterinary hospital. As written, the pain scoring schemes we used interpret signs consistent with anxiety or restlessness, which were displayed by some of the dogs in our study, as compatible with pain. However, in our study such signs may not always have been deemed indicative of pain by the observers carrying out the scoring if specific individual animals were known to behave that way before surgery.
An imbalance occurred between the numbers of dogs allocated to Group F and Group K. This imbalance arose because fentanyl CRI is the routine analgesic protocol for spinal surgery at our hospital, and so dogs excluded for errors in administration all were in the ketamine-dexmedetomidine group. These dogs were inadvertently given the standard analgesic (fentanyl) before the anesthetic team was aware that they should have been randomized. Because we wished to rely upon per protocol analysis these cases had to be excluded.
When a study finds no difference between compared groups, the question of study power is critical. Before carrying out the study, it was difficult to determine what sample size was appropriate because preexisting data were limited. However, in rat studies that had detected differences in ambulatory outcomes, sample sizes of 9 were used and a difference in outcome was apparent within a week.23 Power calculations on our much larger group sizes (34 vs 42 dogs with recorded ambulation events) suggested reliable detection of a much smaller effect size in dogs; post hoc calculation implies detection of hazard ratio of 1.9 (corresponding to a small to medium effect size) with a power of 0.8. Therefore, our conclusion that there is no meaningful clinical detrimental effect of fentanyl, within our dose range, on recovery of locomotion in dogs is likely to be reliable. However, another possible explanation is that ketamine is equally detrimental to ambulatory outcomes, but there is little reason to suppose that conclusion is correct. In fact, substantial evidence suggests that ketamine is neuroprotective after central nervous system (CNS) injury.17, 45-47 A further limitation is that, as permitted by our protocol, the majority of dogs were given methadone as a premedication, and it must be considered whether this single dose could have been enough to create a sufficiently detrimental environment in the spinal cord that different effects of our 2 intra and postoperative analgesic protocols could not be distinguished. Before beginning the study, we believed this possibility to be unlikely, because those detrimental effects are associated with prolonged and high dose opioid administration (see below), but the possibility cannot be entirely discounted.
The detrimental activity of opioids in CNS injury is thought to be mediated largely by pro-inflammatory and excitotoxic effects, although several mechanisms exist.23, 48 In many spinal cord injury experiments, the focus has been on morphine (rather than fentanyl) which acts on mu, kappa and delta opiate receptors.24 After experimental spinal cord injury, morphine given intrathecally or IV increases inflammation and expression of pro-inflammatory cytokines,24, 49, 50 by binding to receptors on microglia, including the nonopioid receptor toll-like receptor (TLR) 4.51 Such mediators promote additional cycles of inflammatory cell invasion, loss of blood-brain barrier integrity and circulatory impairment.52 Also, dynorphin, which is increased in the damaged spinal cord by sustained opioid administration, is associated with decreased functional recovery after spinal cord injury.53 The mechanism is thought to be through increases in concentrations of excitatory amino acids in the spinal cord,54 which have toxic effects (through an increase in intracellular calcium concentration) on both neurons and oligodendrocytes by activation of the NMDA receptor (i.e., excitotoxicity), thereby degrading neural networks through loss of both neurons and myelin55 and causing delayed and decreased recovery of locomotion.25, 50 Current explanations for the detrimental effects of morphine after spinal cord injury focus on activation of the kappa receptor,24 but, opioids also can produce pro-inflammatory effects through nonclassical opioid receptors.56 Although we chose to investigate fentanyl because of its veterinary relevance compared to the more widespread use of morphine in human medicine,57 there also is evidence that fentanyl enhances production of pro-inflammatory cytokines in the undamaged spinal cord58 and brain.19 The detrimental effects of fentanyl in the brain have been associated with cell loss and impaired functional outcome.59, 60 There are several possible reasons for our inability to detect similar effects in dogs, including different dosages or species differences in distribution and response to mu receptor activation. In addition, the wide range of severity of cord injury in clinical dogs may complicate the analysis.
In conclusion, we found no evidence to suggest that peri-operative use of opioids is likely to impair or delay recovery of ambulation after decompressive thoracolumbar surgery for disc herniation in dogs. Some evidence suggested that fentanyl may have minor effects of delaying return of appetite and urinary control after this surgery, but these findings are likely to be of limited clinical relevance. The apparent similarity of the 2 analgesic regimens in control of pain and the lack of substantial differences in functional outcome measures suggest that ketamine-dexmedetomidine is an appropriate alternative to fentanyl for postoperative pain control in dogs undergoing thoracolumbar spinal surgery, but formal equivalence studies would be needed to conclusively confirm their interchangeability.
ACKNOWLEDGMENT
No funding was received for this study.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest. This was a blinded randomized clinical trial. Randomization was by means of opening opaque envelopes containing treatment allocation after enrolment into study and owner consent. Blinded observers obtained follow-up information.
OFF-LABEL ANTIMICROBIAL DECLARATION
Authors declare no off-label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the Clinical Research Review Committee and the IACUC of Texas A&M University. Animal Use Protocol IACUC 2021-0220 CA.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.




