Ondansetron in dogs with nausea associated with vestibular disease: A double-blinded, randomized placebo-controlled crossover study
Lea Henze and Sarah Foth had equal contribution to this study as joined first authors.
Funding information: Deutsche Forschungsgemeinschaft; gkf - Gesellschaft zur Förderung Kynologischer Forschung e.V.; Stiftung Tierärztliche Hochschule Hannover
Abstract
Background
Nausea and emesis can be, among other signs, common manifestations of acute vestibular system dysfunction in dogs. Currently, antiemetic drugs, such as maropitant and metoclopramide, are used commonly, but do not appear to control nausea. A non-placebo-controlled preliminary study suggested good efficacy of 5-HT3-receptor antagonists, such as ondansetron, against nausea in dogs with vestibular syndrome.
Objectives
To assess and confirm the effect of ondansetron on behavior suggestive of nausea in dogs with vestibular syndrome.
Animals
Fourteen dogs with vestibular syndrome and clinical signs of nausea presented to a neurology service.
Methods
Placebo-controlled, double-blinded, crossover study. Behavioral assessment was performed hourly for 4 hours using an established numerical rating scale. The criteria salivation, lip licking, vocalization, restlessness, lethargy, and general nausea were scored. The occurrence of emesis was recorded. After scoring at T0 (pre-dose) and T2 (2 hours post-dose) either ondansetron (0.5 mg/kg) or placebo was injected IV. Two hours post-dose, treatments were switched. Blood samples were collected to measure serum arginine vasopressin (AVP) concentration, which previously has been shown to correlate with clinical signs of nausea.
Results
Clinical resolution of nausea was observed 1 hour after administration of ondansetron, whereas serum AVP concentration decreased 4 hours after ondansetron administration.
Conclusion and Clinical Importance
Administration of ondansetron IV is beneficial for dogs with nausea secondary to acute vestibular syndrome. Ondansetron substantially and rapidly decreased clinical signs of nausea behavior and stopped emesis.
Abbreviations
-
- AVP
-
- arginine-vasopressin
-
- IQR
-
- interquartile range
-
- MRI
-
- magnetic resonance imaging
-
- NRS
-
- numerical rating scale
1 INTRODUCTION
The vestibular system is the primary sensory system that maintains an animal's balance and normal orientation relative to the gravitational field of the earth.1 When disorders of this system occur, typical clinical signs include abnormalities of gait, head position, and body posture, such as ataxia, head tilt, and nystagmus.2, 3 Clinical signs such as nausea and vomiting also can be observed. Evaluating nausea in dogs as compared to humans can be challenging because human beings can communicate their sensations to the clinicians in contrast to dogs. Therefore, it is important to precisely observe signs that indicate nausea in dogs (eg, facial expression, behavior, hypersalivation, lip licking).4 A previous study developed a numerical rating scale (NRS),4 which was further in another study.5
A more objective test for evaluating nausea is the measurement of serum arginine vasopressin (AVP) concentration, which is positively correlated with increasing nausea scores.6-8 Although the precise physiological mechanisms relating to the role of AVP are still unclear, it is thought to induce nausea.9 Various studies showed that IV or intracerebroventricular administration of AVP induces nausea and vomiting in humans and animals.10-14
Regarding treatment of nausea, a previous study15 found that in human patients, most disorders causing nausea require intervention using anti-nausea medications. In veterinary medicine, medications commonly used against vomiting include maropitant and metoclopramide. Several studies have shown that they successfully limit vomiting but have only a limited anti-nausea effect.4, 8, 16-23 Because nausea is rated by people as worse than vomiting itself,24, 25 medications that target nausea should be considered in veterinary medicine.
Ondansetron, a selective 5-HT3 receptor antagonist, proved to be successful in the treatment of chemotherapy-related nausea in humans and animals.26 In vitro, ondansetron has high affinity and selectivity for 5-HT3 receptors and antagonizes the effects of serotonin.27 In a preliminary study of 16 dogs with vestibular disease, ondansetron significantly decreased the intensity of nausea within 2 hours after administration.28
Our aim was to evaluate the efficacy and safety of ondansetron in dogs with vestibular syndrome using a randomized, placebo-controlled, double-blinded, crossover study. An additional aim was to assess the drug's effect on nausea objectively by measuring serum AVP concentrations pre- and post-administration. Last, the occurrence of emesis was noted with the aim of assessing whether or not nausea occurs in the absence of emesis, thus potentially emphasizing the importance of specific medication for nausea and the need for clinicians to assess nausea in patients not exhibiting vomiting.
2 MATERIAL AND METHODS
2.1 Animals
Eighteen client-owned dogs presented between July 2020 and October 2021 to our neurology service with signs of vestibular syndrome and nausea were examined. If it was known that a dog had received maropitant or metoclopramide, a 15-hour washout period for prior maropitant treatment or a 13-hour washout period for prior metoclopramide treatment was required before the dog's inclusion in the trial.8
Using block randomization, 2 groups with 9 dogs each were formed. One of the groups first received placebo and the other group ondansetron. Treatment was switched for the second drug administration 2 hours later. All dogs were discharged after a few days of hospitalization. Out of the initial 18 analyzed dogs, 4 were excluded because it was later reported that they had received an antiemetic drug shortly before ondansetron, and therefore there were 6 dogs in the placebo-first and 8 dogs in the ondansetron-first group. Serum AVP concentration was measured in 7 dogs treated in 2021, because of the requirement of a short storage time for the samples.
2.2 Study protocol
Ethical approval: Dogs were enrolled in the study under the regulations of the Ethics Committee of Lower Saxony (Niedersächsisches Landesamt für Verbraucherschutz und Lebensmittelsicherheit [LAVES], trial number 33.8-42502-04-20/3340).
Dogs were admitted to the hospital and water as well as soft bedding were provided. Intravenous access was established (Vasofix, B. Braun Melsungen AG, Germany), and IV fluid therapy (Sterofundin, ISO, B. Braun Melsungen AG, Germany) was administered at the rate of 2 mL/kg/hour.
2.3 Study design
An independent clinician opened a randomized envelope indicating which medication should be administered at each time point, T0 and T2 (2 hours after administration of the first medication). The time until crossover was chosen to be 2 hours, although the washout period of ondansetron is longer, to guarantee ethical handling of the dogs that received placebo first. The person rating the severity of nausea was unaware of the treatment given. The randomization procedure was performed before the start of the study, using the website www.random.org. The owners also were blinded to which medication their dogs received.
2.4 Treatments
Treatments were administered at T0, pre-dose, and T2, 2 hours post-dose (Figure 1). Ondansetron (Cellondan, STADAPHARM GmbH, Germany) was prepared in a syringe at a dose of 0.5 mg/kg and diluted 1:1 with 0.9% saline to minimize the risk of phlebitis. In another syringe, the same volume of 0.9% saline (placebo) was prepared. The medication or placebo was administered IV as a bolus.
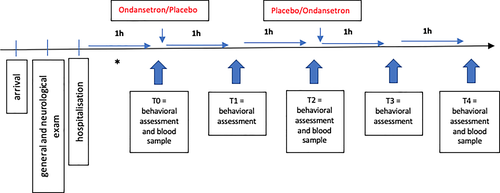
2.5 Assessments
Assessments were performed at 5 different time points (Figure 1); pre-dose (T0) and at 1-hour intervals post-dose (T1 to T4). At T0, the first behavioral assessment (Table 1) was performed, a blood sample was collected, and subsequently the medication (ondansetron or placebo) was administered. At T2, the same procedure was repeated as in T0 with the only difference being that dogs that received ondansetron first now received placebo and those that received placebo first received ondansetron. At T1 and T3 (1 hour after administration each), only behavioral assessments were performed. At T4, a final behavioral assessment was performed, and a final blood sample was collected. The behavioral assessments were conducted using the NRS. The criteria salivation, lip licking, vocalization, restlessness, lethargy, and general nausea were scored on scales from 0 to 5, with a maximum possible score of 30.
| Score value | ||||||
|---|---|---|---|---|---|---|
| 0 (None) | 1 (Mild) | 2 (Mild/Moderate) | 3 (Moderate) | 4 (Moderate/Severe) | 5 (Severe) | |
| General nausea | No nausea | Short period of mild nausea | Longer period of mild nausea or short period of moderate nausea | Longer period of severe nausea | Longer period of severe nausea | Constant nausea |
| Salivation | None | Slight dampness around the mouth | Wet around the muzzle | Pools of saliva around the lips | Dripping saliva | Strings of saliva |
| Lip licking | None | Occasional lip licking | Frequent lip licking | Constant lip licking for periods up to a few minutes | Frequent lip licking for periods up to several minutes | Permanent, constant lip licking |
| Vocalization | None | Occasional short whining | Occasional whining | Frequent whining | Constant whining or crying for periods of a few minutes | Constant whining or crying |
| Restlessness | None | Eg, occasional panting/turning/circling/digging | Eg, shows longer panting/turning/circling/digging behavior, but calms down after a short time | Eg, anxious, repeated panting/turning/circling/digging | Eg, restless panting/turning/circling/digging behavior, only very short calm periods between phases | Eg, does not come to rest, constant panting/turning/circling/digging |
| Lethargy | None | Sleeping, responsive to stimuli | Sleeping, responsive to repeated stimuli | Sleeping for long periods, responsive to stimuli | Sleeping for long periods, responsive to repeated stimuli | Sleeping for unusually long periods, unresponsive to stimuli |
The blood samples for AVP measurement, taken at T0, T2 and T4, were collected in EDTA tubes prepared with 0.05 mL Trasylol/ml blood (Trasylol 500 000 KIE/50 mL, Nordic Pharma GmbH, Germany). The samples were centrifuged at 4°C and 2000 rpm for 15 min, serum was harvested and frozen immediately afterwards at −80°C until assay. The essay is only reliable for samples stored <6 months and thus only these samples were used. The blood samples were analyzed using a canine AVP ELISA kit (AVP ELISA Kit MBS1604344, MyBiosource, Inc, USA), according to the manufacturer's recommendations.
2.6 Statistical analysis
Statistical analysis was performed using GraphPad Prism 9 (GraphPad Software, Inc., La Jolla, California, USA). Data were analyzed using a repeated measures 2-way analysis of variance followed by a post-hoc test with correction for multiple comparisons, either a Šidák's test (nausea overall score) or a Dunnett's multiple comparisons test (single factors and serum AVP concentration), where appropriate. A P-value ≤ .05 was considered significant. Serum concentrations of AVP measured at the different time points were assessed for correlation with the nausea scores.
3 RESULTS
Fourteen dogs were included in the study (mixed breeds [n = 6], Border Collie [n = 2], and 1 of the following breeds: Havanese, German Shepherd Dog, Boxer, Jack Russell Terrier, Shiba Inu, Podenco, Beagle, White Shepherd Dog, and French Bulldog). Median age at the day of presentation was 127 months (range, 6-180 months; interquartile range [IQR], 81-168 months). Four more dogs were previously scored, but then excluded because it was determined that they had received antiemetic medication shortly before entry into the study, which violated the protocol.
Half of the recruited dogs [n = 7] were diagnosed with central vestibular syndrome, and half were diagnosed with peripheral vestibular syndrome. All central vestibular syndrome cases had magnetic resonance imaging (MRI) assessment of the brain. Two of the 7 dogs with peripheral vestibular syndrome had MRI of their brain, whereas the other 5 were diagnosed based on clinical history, clinical progression, and results from an otoscopic examination alone. Two were diagnosed with hypothyroidism, 1 with otitis media and the other 4 with idiopathic peripheral vestibular syndrome.29, 30
After randomization, 6 dogs received placebo first and 8 dogs received ondansetron. The difference in the number of dogs in each group occurred because the exclusion of 4 dogs. At the behavioral assessment, both groups had similar pre-treatment nausea scores at T0 (13.17 [IQR, 11-15; range, 11-16], placebo; 13.63 [IQR, 12-15.5; range, 11-16], ondansetron). Dogs that received ondansetron showed a rapid decrease in nausea behaviors, with lower nausea scores at T1 and T2, compared to those that received placebo first (Figure 2). In the group that received ondansetron as first treatment at T0, the score significantly decreased post-treatment (3.88 [IQR, 3-4.5] at T1 [P ≤ .001]; 1.68 [IQR, 1-2.5] at T2 [P ≤ .0001]; 0.75 [IQR, 0-1.5] at T3 [P ≤ .001]; 1.65 [IQR, 0-2.5] at T4 [P ≤ .001]). The placebo group had similar scores at T1 and T2 compared to baseline. After administration of ondansetron at T2 in this group, the score significantly decreased to 3.34 (IQR, 2-3, P ≤ .001) at T3 and 2.08 (IQR, 1-3) at T4 (P ≤ .001). For each criterion that was scored, apart from vocalization, a significant decrease of the score was noted after administration of ondansetron (see Figure 3A-E). No statistical analysis was performed for vocalization because only 4 dogs showed vocalization, with a score of 2 out of 5 being the maximal score.
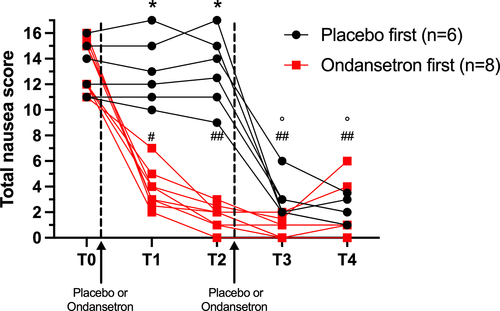
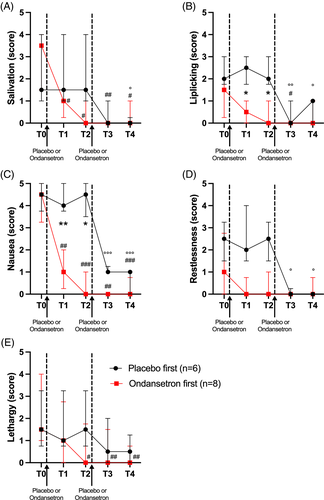
Only 4 dogs (28.6%) vomited in addition to clinical signs of nausea at T0. No dog experienced emesis after ondansetron treatment. Plasma samples were analyzed for AVP in a single batch after finishing the clinical portion of the study to avoid inter-assay variability. Serum AVP concentration decreased significantly after administration of ondansetron when concentrations were compared at T0 (6.72 ng/L ± 1.37 [mean + SD]; range, 4.8-8.9 ng/L; median, 6.7) to the level at T4 (5.0 ng/L ± 1.5; range, 2.9-7.2; median, 6.1 P: .04).
4 DISCUSSION
Our objective was to further evaluate the efficacy of ondansetron in a randomized, placebo-controlled, double-blinded, cross-over study, after promising results in a previous preliminary study.28 Our study confirmed the results of the first open-labeled study. Clinical signs suggestive of nausea were significantly decreased in the behavioral assessment after administration of ondansetron. In addition, serum AVP concentration was significantly decreased after ondansetron administration, supporting the anti-nausea effect of ondansetron. Of the 14 dogs, only 4 vomited in addition to signs of nausea before ondansetron treatment. After ondansetron treatment, none of the dogs vomited. Our study provides evidence for the benefit of ondansetron in the treatment of dogs with vestibular syndrome-induced nausea. It also indicates the importance of differentiating nausea from vomiting clinically and, hence, the value of treating each sign independently, as necessary.
The pathophysiological mechanisms of nausea are complex and still not completely understood, which makes appropriate pharmacological management challenging. The vestibular nuclei play a central role in the relay of vestibular information and are involved in the pathway for the induction of nausea in the tractus solitarius. By activation of projections to the dorsal vagal complex and ascending projections to higher brain areas, such as the thalamus, lateral postcentral gyrus, insular cortex and temporoparietal cortex, the induction of nausea can be modulated.25 A previous study identified 2 networks of neurons located in different vestibular nuclei the activity of which correlated with the severity of motion sickness symptoms.31 At the receptor level, various receptors can be found in the vestibular nuclei. Attention has been focused on the 5-HT3 receptors. They can be found in the forebrain, brainstem, and spinal cord.32 Their distribution suggests that 5-HT3 receptors may mediate the known serotonergic inhibition of pyramidal cell populations via excitation of inhibitory interneurons.33 The vestibular system seems to be at least 1 possible origin for nauseogenic stimuli based on the high numbers of 5-HT3 receptors and the anti-nausea effect of receptor antagonists.
Ondansetron already is used in veterinary medicine for the treatment of nausea after chemotherapy.8, 34 Studies showed a decrease of the nausea score, as well as in the frequency of vomiting. These results have been observed in other experimental studies, such as in dogs with renal disease or when used against preoperative nausea.19, 22, 35 After promising results when using ondansetron against vestibular nausea,28 our current study emphasizes further the potential for the use of ondansetron to treat nausea resulting from vestibular syndrome in dogs.
Our study had some limitations, including the behavioral assessment and relatively small sample size. The observed patients included a variety of breeds and different pre-existing medical histories. However, because the clinical effect was so evident, including a higher number of patients could be perceived as unethical. Dogs were exposed to a stressful environment by hospitalizing them, although efforts were made to minimize the stress by giving the dogs at least 1 hour of acclimatization. Nevertheless, stress certainly could have influenced the dogs' behavior and therefore the score achieved in the behavioral assessment. This factor was mitigated by the inclusion of a placebo group, because the ondansetron-first and the placebo-first groups were exposed to the same stressors.
As mentioned above, the time until crossover was chosen to be a period of only 2 hours, although the washout period of ondansetron is longer. This timepoint was recommended by the ethical review board to limit the time dogs had to experience nausea before receiving treatment.
Last, nausea can be an important, but subjective, experience and can be difficult to assess in humans, and even more so in animals. Therefore, it was important to observe the dogs closely and to use a standardized NRS adapted to the situation. For our study, the NRS developed previously4 and modified later5 was used. Although the score is easily applied, every dog was assessed by 1 of the 2 observers with specialized training to minimize inconsistencies in interpretation. To further optimize the study, it could have been useful to have scores from an additional observer to compare inter-observer accuracy.
5 CONCLUSION
Nausea was detectable in all 14 dogs included in our study whereas only 4 (29%) dogs also exhibited vomiting. Administration of ondansetron IV was beneficial for dogs with nausea secondary to acute vestibular syndrome. Ondansetron substantially and rapidly decreased clinical signs of nausea behavior and stopped emesis.
ACKNOWLEDGMENT
Funding provided by German Research Foundation within the program Development of an Objective Means of Assessing Nausea in Dogs “Open Access Publication Costs,” GKF—Society for the Promotion of Cynological Research e.V., and Foundation of the University of Veterinary Medicine Hanover (Deutsche Forschungsgemeinschaft, GKF—Gesellschaft zur Förderung Kynologischer Forschung e.V., and Stiftung Tierärztliche Hochschule Hannover). Preliminary data was presented at the annual meeting of the German Veterinary Association 2022 (Innere Medizin und klinische Labordiagnostik [InnLab] 2022). The authors express gratitude to the GKF - Gesellschaft zur Förderung Kynologischer Forschung e.V. for the financial support, the team at the neurology service at the Department of Small Animal Medicine & Surgery, University of Veterinary Medicine Hannover (TiHo) for their help and assistance during the data collection and to Steven R. Talbot for his help with statistical questions.
CONFLICT OF INTEREST DECLARATION
The funders played no role in the design, analysis, and reporting of the study. Authors declare no conflict of interest.
OFF-LABEL ANTIMICROBIAL DECLARATION
Authors declare no off-label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Dogs were enrolled in this study under the regulations of the Ethics Committee of Lower Saxony (Niedersächsisches Landesamt für Verbraucherschutz und Lebensmittelsicherheit [LAVES], trial number 33.8-42502-04-20/3340).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.




