Effect of calcifediol supplementation on renin-angiotensin-aldosterone system mediators in dogs with chronic kidney disease
Funding information: VCA Animal Hospitals
Abstract
Background
Chronic kidney disease (CKD) leads to low serum concentrations of vitamin D metabolites. Thus, hypovitaminosis D associated with CKD might contribute to disease progression via increased concentration of renin angiotensin aldosterone system (RAAS) mediators.
Objectives
To evaluate whether supplementation with calcifediol affects equilibrium concentrations of selected mediators of the RAAS. We hypothesized that vitamin D supplementation will decrease concentration of circulating RAAS mediators in dogs with CKD.
Animals
Six client-owned adult dogs with IRIS Stage 2 and 3 CKD.
Methods
Prospective study. Serum 25-hydroxyvitamin D (25[OH]D), 1,25-dihydroxyvitamin D (1,25[OH]2D), 24,25-dihydroxyvitamin D (24,25[OH]2D), RAAS mediators (angiotensin I/II/III/IV/1-5/1-7, and aldosterone), and surrogate angiotensin converting enzyme (ACE) activity (calculated by the ratio of angiotensin II to angiotensin I) were evaluated at baseline, after 3 months of calcifediol supplementation, and 2 months after discontinuing administration of supplement.
Results
All serum vitamin D metabolite concentrations increased significantly by month 3 (P < .001): 25(OH)D (median 250 ng/mL; range, 204-310), compared to baseline (median 43.2 ng/mL; range, 33.8-58.3 ng/mL); 1,25(OH)2D (median 66.1 pg/mL; range, 57.3-88.1 pg/mL) compared to baseline (median 35.2 pg/mL; range, 29.3-56.7 pg/mL); 24,25(OH)2D (median 68.4 ng/mL; range, 22.1-142.0 ng/mL) compared to baseline (median 14.4 ng/mL; range, 9.0-21.3 ng/mL). Calculated ACE activity was significantly lower at month 3 (median 0.5; range, 0.4-1.0) compared to baseline (median 0.7; range, 0.6-1.3; P = .01). There were no significant differences in any of the evaluated RAAS variables at any other time-point.
Conclusions and Clinical Importance
Short-term calcifediol supplementation in this small group of CKD dogs appeared to decrease ACE activity.
Abbreviations
-
- ACE
-
- angiotensin-converting enzyme
-
- ALT
-
- alternative RAAS
-
- BCS
-
- body condition score
-
- CKD
-
- chronic kidney disease
-
- MCS
-
- muscle condition score
-
- PRA
-
- plasma renin activity
-
- RAAS
-
- renin angiotensin aldosterone system
-
- UPC
-
- urine protein:creatinine
-
- USG
-
- urine specific gravity
1 INTRODUCTION
The renin-angiotensin-aldosterone system (RAAS) plays a key role in maintaining fluid and electrolyte balance and in the regulation of blood pressure. However, up-regulation of RAAS mediators (eg, angiotensin II [AngII] and aldosterone) can result in negative sequelae.1 RAAS upregulation occurs in chronic kidney disease (CKD) in multiple species and is thought to contribute to the development of hypertension and proteinuria as well as exacerbation of tubulointerstitial injury and glomerular damage because of pro-inflammatory and pro-fibrotic effects.2-6 In turn, hypertension at the time of CKD diagnosis in dogs has been linked to decreased survival,7 and proteinuria has been shown to be associated with faster progression of CKD and decreased survival in dogs.8
Dogs with CKD have lower vitamin D metabolite concentrations than healthy dogs9 and hypovitaminosis D has been associated with progression of kidney disease and decreased survival in people.10, 11 Vitamin D receptor (VDR) knockout mice have increased plasma AngII concentrations and increased renal renin gene expression.12, 13 In remnant kidney model rats, supplementation with paricalcitol, a VDR agonist similar to calcitriol, decreases renal renin gene expression, and leads to lower serum creatinine concentration, hypertension, proteinuria, and glomerular and tubulointerstitial damage.14 In both normotensive and hypertensive humans, there is a significant association between vitamin D status and the RAAS, including higher concentrations of plasma renin and AngII in patients with low concentrations of vitamin D and a significant inverse association of 25(OH)D and plasma renin activity (PRA).15-17 To our knowledge, no relationship between RAAS and vitamin D metabolism has been established in dogs.
Calcifediol, or 25-hydroxyvitamin D (25[OH]D) has been used in human medicine for the treatment of hypovitaminosis D associated with CKD and for the management of renal secondary hyperparathyroidism.18, 19 Calcifediol concentrations are correlated with calcitriol concentrations in dogs and significant increases in serum 25(OH)D can be achieved by providing additional substrate in the form of calcifediol.20, 21 Calcifediol significantly increases serum vitamin D metabolite concentrations, including 25(OH)D and 1,25-dihydroxyvitamin D (1,25[OH]2D; calcitriol) in dogs.22 The primary aim of this study was to determine if calcifediol supplementation has an effect on concentration of circulating mediators associated with the RAAS. We hypothesize that supplementation with calcifediol will decrease serum RAAS mediators in dogs with CKD.
2 MATERIAL & METHODS
2.1 Case selection criteria
Samples were retrospectively evaluated from a subset of client-owned dogs with CKD that were previously prospectively recruited from dogs examined at The Ohio State University's Veterinary Medical Center (OSU-VMC) for a study examining calcifediol supplementation.22 Dogs were diagnosed with either International Renal Interest Society (IRIS) CKD Stage 2 or 3 based on serial evaluations demonstrating abnormally low urine concentrating ability (urine specific gravity <1.030) and azotemia with no evidence of other diseases processes likely to cause these abnormalities. Dogs were required to be >1 year of age and have a body weight >10 kg. Dogs were excluded from the study if they were receiving medications known to affect calcium homeostasis and vitamin D metabolism, including corticosteroids and calcitriol.
2.2 Study design
At baseline, each dog had a complete physical examination performed, including body weight, body condition score (BCS) and muscle condition score (MCS). All BCS and MCS scoring were done by 1 author (Valerie Parker). A Doppler systolic blood pressure (average of 5 measurements) was measured. Blood was collected via jugular venipuncture and urine was collected via cystocentesis for CBC, serum biochemistry profile, urinalysis, and urine protein-to-creatinine ratio. Additional serum was stored at −80°C for analysis of vitamin D metabolites (25[OH]D, 1,25[OH]2D, 24,25[OH]2D), and RAS Fingerprint analysis, including equilibrium concentrations of angiotensin I/II/III/IV/1-5/1-7/aldosterone, the ratio of aldosterone to AngII (AA2), surrogate serum renin activity (PRA); determined by the sum of equilibrium concentrations of angiotensin I (AngI and AngII), surrogate angiotensin-converting enzyme (ACE) activity (as calculated by the ratio of equilibrium concentrations of AngII to AngI), and surrogate ACE2 activity (determined by the ratio of angiotensin 1-5 [Ang1-5] to AngII).23-25 The overall balance between the classical and alternative RAAS (ALT) was calculated as (Ang1-5 + Ang1-7)/(Ang1-5 + Ang1-7 + AngI + AngII). The adrenal function surrogate (AA2), which reflects the responsiveness of the adrenal gland to AngII, was calculated as aldosterone/AngII.
Dogs were prescribed an extended-release 25(OH)D medication. Dosage was based on body weight to provide approximately 2.0 μg/kg/day. Pre-made capsules were available in 30 and 60-μg capsules, and dogs received either 1 or 2 capsules per day, in the evening, with food. Dogs received the supplement for 3 months. After preliminary review of data from the first 4 dogs enrolled, based on the degree and rapid nature of the rise in serum 25(OH)D concentrations, calcifediol dosing for the next 6 dogs was modified to be provided on a Monday-Wednesday-Friday schedule. After 3 months of calcifediol supplementation, the supplement was discontinued. A complete physical examination was performed at baseline and at the monthly rechecks while dogs were receiving the calcifediol supplement. Blood pressures were measured via Doppler. Blood and urine were collected at each visit for serum biochemistry profile, serum 25(OH)D concentration, and urinalysis. Extra serum aliquots were frozen at −80°C for 1,25(OH)2D, 24,25(OH)2D, and RAS Fingerprint metabolite concentrations. Study dogs were fasted overnight for all visits, and each visit was completed at the same time of day, usually in the morning. CBC and chemistry profiles were also rechecked 56 days after discontinuation (month 5).
2.3 Vitamin D metabolite analysis
Serum 25(OH)D, 1,25(OH)2D, and 24,25(OH)2D concentrations were measured by liquid chromatography-mass spectrometry by a Vitamin D External Quality Assessment Scheme (DEQUAS)-certified laboratory (Heartland Assays, Inc, Ames, Iowa).
2.4 Renin-angiontensin-aldosterone system metabolite analysis
Equilibrium analysis of RAAS metabolites was performed by a commercial laboratory (Attoquant Diagnostics, Vienna, Austria). Equilibrium angiotensin peptide concentrations were measured in blood collected in serum tubes after an equilibration procedure at 37°C, followed by the addition of an inhibitor cocktail to prevent angiotensin metabolism. This cocktail contains ethylenediaminetetraacetic acid, pepstatin A, p-hydroxymercuribenzoic acid, phenanthroline and specific inhibitors for renin and aminopeptidases to a final concentration of 5% v/v. Further information is gained during the equilibration process because of the enzymatic cascade of RAAS being allowed to continue. This includes the calculation of angiotensin ratios that allows the estimation of RAAS enzymes, including AngII/AngI as a surrogate of ACE activity, AngI + AngII as a surrogate of renin activity, and Ang1-5/AngII as a surrogate of ACE2 activity. Following centrifugation, samples were spiked with stable isotope-labeled internal standards for each angiotensin metabolite at a concentration of 200 ng/L. Following C18-based solid-phase-extraction, samples were subjected to LC-MS/MS analysis using a reversed-phase analytical column (Acquity UPLC C18, Waters) operating in line with a XEVO TQ-S triple quadrupole mass spectrometer (Waters) in MRM mode. Internal standards were used to correct for peptide recovery of the sample preparation procedure for each angiotensin metabolite in each individual sample. Angiotensin peptide concentrations were calculated considering the corresponding response factors determined in appropriate calibration curves, on condition that integrated signals exceeded a signal-to-noise ratio of 10. The lower limit of quantification in ng/L for the individual peptides are: 3.0 pM for angiotensin I, 2.0 pM for angiotensin II, 3.0 pM for angiotensin 1-7, 2.0 pM for angiotensin 1-5, 2.5 pM for angiotensin III, 2.0 pM for angiotensin IV, and 15.0 pM for aldosterone.
2.5 Data analysis
Statistical analysis was performed using a commercial statistics software package (GraphPad Prism 7). Descriptive statistics were summarized using median and range for numeric variables. Changes in vitamin D metabolite and RAAS mediators over time were evaluated using Friedman's repeated-measures test and Dunn's post-hoc analysis. For values that were below the level of quantification of the assay, the value was set at ½ the lower limit of quantification. P-values < .05 were considered significant. Adjusted P values were used based on Graph Pad software for multiple comparison testing.
3 RESULTS
Ten dogs were initially evaluated for inclusion, with 4 dogs being excluded because of the usage of RAAS-inhibiting medications (n = 3) and incomplete data (n = 1). Six dogs with IRIS Stage 2 and 3 CKD were enrolled. Of these 6 dogs, 2 dogs received calcifediol daily and 4 received it on a Monday-Wednesday-Friday schedule. The median dose provided was 2.1 μg/kg per dose (range, 1.5-2.7 μg/kg). Median age was 6 years (range, 1-13 years). Breeds represented included German shepherd dog (n = 2), mixed breed (n = 2), Bernese mountain dog (n = 1), and golden retriever (n = 1). Median body weight was 26.7 kg (range, 15.3-40.1 kg). Median BCS was 5/9 on a 9-point scoring system (range, 5-6). Muscle condition score was normal in 3/6 dogs, with the remaining 3 dogs having mild muscle atrophy. There were 4 female spayed dogs, 1 castrated male, and 1 intact male.
Pertinent laboratory values for enrolled dogs are listed in Table 1. Dogs were classified as having IRIS Stage 2 (n = 4) or Stage 3 (n = 2) CKD, based on 2019 IRIS Staging of CKD guidelines. There was a significant increase in phosphorus concentration at month 5 compared to baseline. Otherwise, there were no significant differences in any laboratory values at any time points. Dogs were considered either non-proteinuric (n = 4) or borderline proteinuric (n = 2). Five dogs were eating a veterinary therapeutic renal diet while 1 dog was eating a commercially available complete and balanced diet. Dogs received: gabapentin (n = 3), aluminum hydroxide (n = 2), trazodone (n = 2), maropitant (n = 2), ketorolac ophthalmic solution (n = 2), amlodipine (n = 1), diphenhydramine (n = 1), fenbendazole (n = 1), fluoxetine (n = 1), flurbiprofen ophthalmic (n = 1), omeprazole (n = 1), phenylpropanolamine (n = 1), or sucralfate (n = 1).
| Variable | Baseline | Month 3 | Month 5 |
|---|---|---|---|
| Hematocrit (%) | 39 (30-44) | n/a | 37 (32-41) |
| BUN (mg/dL) | 33 (8-70) | 31.5 (12-85) | 34.5 (14-86) |
| Creatinine (mg/dL) | 2.3 (1.6-3.8) | 2.5 (1.6-4.7) | 2.9 (1.6-4.8) |
| Phosphorus (mg/dL) | 3.6 (2.7-6.3)a | 4.4 (2.7-6.9)ab | 4.5 (3.1-7.1)b |
| Total Calcium (mg/dL) | 10.8 (9.2-11.6) | 10.8 (9.5-12.5) | 10.7 (9.4-13.2) |
| Ionized Calcium (mg/dL) | 5.08 (4.95-5.31) | 4.98 (4.7-5.71) | n/a |
| Sodium (mEq/L) | 149 (146-152) | 147 (144-148) | 148 (143-152) |
| Potassium (mEq/L) | 4.75 (4.3-5.2) | 4.74 (4.0-5.34) | 4.72 (4.33-5.67) |
| Ionized Magnesium (mg/dL) | 1.36 (1.16-1.65) | 1.29 (1.08-1.75) | n/a |
| USG | 1.014 (1.005-1.020) | n/a | n/a |
| UPC | 0.14 (0.06-0.4) | n/a | n/a |
| BP (mm Hg) | 144 (109-154) | 133 (126-140) | n/a |
- Note: Values are displayed as median (range). Statistically significantly different results are bolded and denoted by differing superscripts.
- Abbreviations: UPC, urine protein-to-creatinine; USG, urine specific gravity.
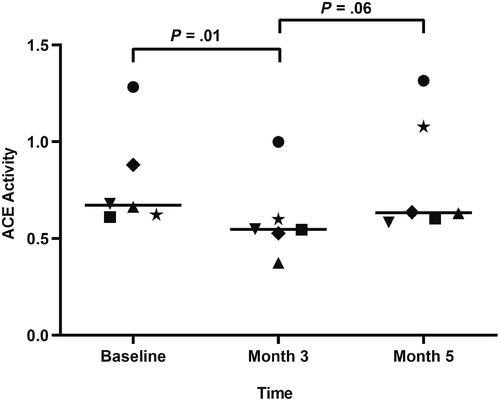
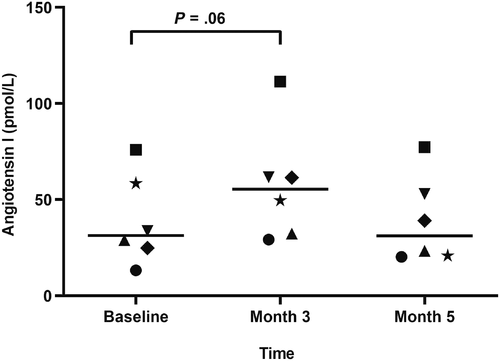
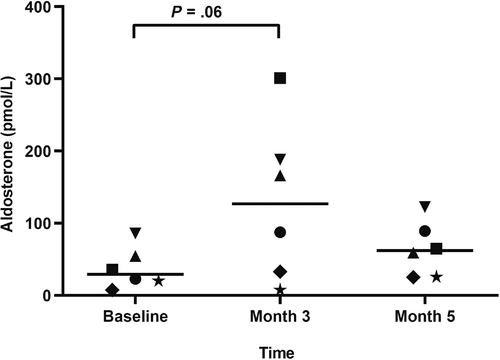
Serum vitamin D metabolite concentrations are listed in Table 2. All vitamin D metabolites were significantly higher at month 3 compared to baseline. Serum concentrations of RAAS mediators are listed in Table 3 (median; range). Surrogate ACE activity, as calculated by the ratio of AngII to AngI, was significantly lower at month 3 (0.5; 0.4-1) compared to baseline (0.7; 0.6-1.3; P = .01; Figure 1). There was no significant difference in ACE activity between either month 3 and month 5 (0.6; 0.6-1.3; P = .06) or baseline and month 5 (P ≥ .99). No significant differences were noted at any time point for angiotensin I/II/IV/1-5/1-7, aldosterone, PRA, AA2, ACE2, nor ALT (Figures 2 and 3). AngIII values could not be assessed because of the majority (>75%) were below the limit of quantification.
| Variable | Baseline | Month 3 | Month 5 |
|---|---|---|---|
| 25(OH)D (ng/mL) | 43.2 (33.8-58.3)a | 250 (204-310)b | 81.4 (49.4-112)a |
| 1,25(OH)2D (pg/mL) | 35.2 (29.3-56.7)a | 66.1 (57.3-88.1)b | 31.6 (25.6-50.5)ac |
| 24,25(OH)2D (ng/mL) | 14.4 (9.0-21.3)a | 68.4 (22.1-142)b | 38.3 (16.9-104)a |
- Note: Values are displayed as median (range). Significantly different values are denoted by differing superscripts.
| Variable | Baseline | Month 3 | Month 5 |
|---|---|---|---|
| AngI (pM) | 31.3 (13.2-75.9) | 51.2 (29.2-111) | 31.1 (20.2-77.3) |
| AngII (pM) | 22.4 (17-46.3) | 31 (12.1-60.7) | 25.7 (14.7-46.6) |
| Ang IV (pM) | 2.5 (1-13.4) | 4.4 (1-18) | 3 (1-15.4) |
| Ang1-5 (pM) | 20.6 (6-39.3) | 19.8 (11.9-32.7) | 14.9 (12.9-19.2) |
| Ang1-7 (pM) | 9.3 (1-16) | 8.2 (1-17.8) | 9.3 (1-13.7) |
| PRA | 52.4 (30.2-122) | 86.5 (44.3-172) | 55.3 (38-124) |
| ACE | 0.7 (0.6-1.3)a | 0.5 (0.4-1)b | 0.6 (0.6-1.3) |
| AA2 | 1.1 (0.6-3.7) | 4 (0.5-13.8) | 2.4 (1-4) |
| ACE2 | 0.7 (0.3-1.2) | 0.7 (0.3-2.2) | 0.6 (0.3-1.1) |
| ALT | 0.3 (0.2-0.4) | 0.3 (0.2-0.5) | 0.3 (0.2-0.4) |
| Aldosterone (pM) | 29.2 (7.5-85.8) | 105 (7.5-301) | 62 (25-123) |
- Note: Values are displayed as median (range). Significantly different values are bolded and denoted by differing superscripts.
- Abbreviations: AA2, aldosterone to AngII; ACE, angiotensin-converting enzyme; ALT, alternative RAAS; PRA, plasma renin activity; RAAS, renin angiotensin aldosterone system.
4 DISCUSSION
This small study revealed that calcifediol supplementation for 3 months resulted in a significant decrease in surrogate ACE activity, as determined by the ratio of AngII to AngI. This finding could indicate a relationship between the RAAS and vitamin D metabolites in dogs with CKD.
Humans with varying degrees of hypovitaminosis D have significantly higher concentrations of AngII as compared to a healthy controls,15 and there is a significant inverse association between concentrations of 25(OH)D and 1,25(OH)2D and both PRA and AngII.16 The mechanism of vitamin D-related suppression of the RAAS is thought to occur through its effects on renin transcription.12, 13 This effect is likely due, in part, to the effects of vitamin D on the cyclic AMP response element, in particular impacting its ligand-binding domain.26 While renin is integral in converting angiotensinogen to AngI, no direct action of renin on the activity of the ACE has been established. The effects of therapies on the RAAS must also consider the balance between the classical (AngI, AngII, aldosterone, ACE, and angiotensin II type-1 receptor) and the ALT (Ang1-5, Ang1-7, ACE2, and Mas receptor) RAAS. The peptides, enzymes, and receptors of the ALT RAAS counteract the effects mediated by the classical RAAS and a shift toward a higher ALT RAAS activity is thought to be beneficial. No significant change was detected in this study in either the ACE2 surrogate or the ratio of ALT to classical RAAS activity, although this could be because of the low statistical power of this study and a type II error.
The results contained within this study could indicate an alternative role for vitamin D in the negative endogenous regulation of the RAAS, warranting further evaluation on a larger cohort of dogs. Given the small sample size, it is possible that these results are not reflective of the true influence of vitamin D on the RAAS.
One other significant finding noted in this study was that phosphorus changed over time. At month 5, phosphorus concentrations were significantly higher compared to baseline, with no significant difference between other timepoints. It seems most likely that this is related to progression of kidney disease over the study period. Additional markers of CKD-mineral and bone disorder (eg, parathyroid hormone, fibroblast growth factor-23) were evaluated previously in these dogs.22 While there was no significant difference in parathyroid hormone over the 5-month study, fibroblast growth factor-23 concentrations did increase over time, most likely consistent with disease progression.
There were limitations to this study. As this was a small study performed on a subset of samples from a prior study involving calcifediol supplementation,22 the study was likely underpowered to detect differences in some variables. As such, future studies with a larger cohort may detect differences that were not significant in this study. While dogs did receive different dosing regimens of calcifediol, the serum 25(OH)D concentrations at month 3 of supplementation did not differ between the dogs.
Equilibrium analysis of RAAS mediators is becoming more common in scientific research, but differences exist between circulating RAAS and equilibrium concentrations of RAAS, though these have shown good correlations.27-29 Additionally, results for angiotensin IV, angiotensin 1-7, and aldosterone all included concentrations below the lower level of quantification; thus these concentrations were set at ½ of the lower level of quantification. Greater than 50% of the concentrations for angiotensin III were below the level of quantification, precluding final evaluation. Our assessment of ACE and PRA activity are calculated from measured angiotensin peptide concentrations, instead of being directly measured themselves. That said, the renin surrogate is strongly correlated to PRA measurement, the ACE surrogate has been shown to correlate to ACE activity measurement in functional ACEi studies, and these and related surrogates are now being used in human studies of hypertensive heart disease and heart failure.23-25, 30
It is possible that some of the other medications that dogs received could have impacted results (eg, amlodipine; n = 1). Hypertensive cats with CKD that receive amlodipine have increased concentrations of PRA.31 Healthy research dogs receiving amlodipine had higher aldosterone concentrations.32 We did not appreciate any other obvious patterns in the results in this small group of dogs after removing the 4 dogs that received RAAS inhibitors.
In conclusion, supplementation with calcifediol in dogs with CKD resulted in a significant decrease in surrogate ACE activity, as determined by the ratio of equilibrium concentrations of AngII to AngI.
ACKNOWLEDGMENT
Funding provided by VCA Animal Hospitals.
CONFLICT OF INTEREST DECLARATION
Dr. Quimby has received grant/research support from EveryCat Health Foundation, Morris Animal Foundation, Nestle Purina, Trivium Vet, and Zoetis. She has consulted for Boehringer Ingelheim, Dechra, Elanco, Gallant, Hill's, IDEXX, Nestle Purina, Royal Canin, Vetoquinol, and Zoetis. Dr. Parker has received grant/research support from Waltham Foundation, Nestle Purina, American Kennel Club, and OPKO Health, Inc. She has received speaker honoraria from Antech, Royal Canin, Nestle Purina, the American College of Veterinary Internal Medicine, and Veterinary Information Network, Inc. She has consulted for Elanco. No other authors declare a conflict of interest.
OFF-LABEL ANTIMICROBIAL DECLARATION
Authors declare no off-label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
This study used samples from a previous study at The Ohio State University (IACUC # 2013A00000122).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.




