Epidemiology, Treatment Patterns and Survival in Canadian Patients With Chronic Hepatitis B-Related Hepatocellular Carcinoma
Funding: The authors received no specific funding for this work.
ABSTRACT
Chronic hepatitis B (CHB) is the leading cause of hepatocellular carcinoma (HCC) globally. We described and evaluated the outcomes of patients with CHB-HCC in Canada. In this retrospective cross-sectional cohort study, data were analysed from CHB mono-infected subjects seen between 1 January 2012 and 31 December 2022, and entered the Canadian Hepatitis B Network Registry. Descriptive analysis and chi-squared modelling were used to compare cohorts, followed by multivariable survival analysis regarding survival post-diagnosis. Statistical analyses were completed in R version 2.2. Of the 6711 patients with CHB who met inclusion criteria, 232 (3.5%) developed HCC. Compared with the CHB cohort, the majority of CHB-HCC cohort were male, SEA and HBeAg negative and born in endemic area (80% vs. 56%, 73% vs. 55%, 84% vs. 54%, 64% vs. 40% and all p < 0001). Overall, median HBV DNA level was log 2.54 (IQR: 0–4.04). Advanced liver disease, defined as minimum Fibrosis stage F3, was seen in 9.4% of overall cohort, but 92% of HCC cohort. At diagnosis, median tumour size was 2.5 cm (IQR: 1.7–4.0) and mean tumour number was 1.33 (SD: 1.33), with 81% of patients BCLC 0-A. Fifty-three per cent of patients were diagnosed with HCC as part of surveillance protocols. The survival rate after HCC diagnosis was 78.7%, during the median follow-up of 52.9 months (IQR: 17–90). In multivariable analysis, survival was significantly correlated with diagnosis through the screening programme. In this large cohort of patients with CHB-HCC, the majority of patients were detected with early-stage HCC and received treatment with curative intent, resulting in strong survival rates.
1 Introduction
Chronic hepatitis B (CHB) infection affects over 250 million people worldwide and accounts for 55% of hepatocellular carcinoma (HCC) diagnoses [1]. HCC is the fifth most common cancer worldwide and the third most common cause of cancer-related mortality with disease burden expected to grow by 55% from 2020 to 2040 [2]. The diagnosis of HCC can cause significant human and financial cost to patients, families and the healthcare system, with the 5-year net cost of care for per patient with HCC being higher than per patient lifetime costs for lung, breast or colon cancer in Canada [3].
Although the burden of CHB is skewed towards Southeast and East Asia, there has been a gradual shift of prevalence towards increasing disease in India, the Americas, Oceania and Europe [4-6]. Rates of CHB infection in endemic areas, especially in Asia, reach as high as 8%, while in developed countries, such as Canada, the rates of infection rarely exceed 1.0% [7, 8]. However, the Canadian CHB population is primarily Asian, signifying a focus of disease on immigrant populations [9]. These populations are expected to continue growing as immigration remains Canada's primary driver of population growth [10]. Although there is an appreciation for the impact of CHB on the Canadian population [9], there is a gap in understanding the national impact of HCC within the context of CHB. Thus, the objective of this study was to identify the epidemiological characteristics and trends of CHB-HCC in Canada.
2 Methods
2.1 Study Design and Dataset From the Canadian Hepatitis B Network
The Canadian Hepatitis B (CanHepB) network includes primarily academic care centres for patients with CHB supported by the National Reference Laboratory (National Microbiology Laboratory, Public Health Agency of Canada, PHAC). The CanHepB registry is a web-based portal for data entry following local site chart review. Each centre is responsible for inputting anonymous, de-identified data using a unique code associated with each patient, thus enabling regional classification. All patients were seen in participating clinics between 1 January 2010 and 31 August 2021. Data submitted to the CanHepB network registry include pertinent demographics, past medical history, HBV infection course, treatment and progression (including HCC and liver decompensation). A waiver of consent was obtained from local research ethics board at each institution. All data received were anonymous and collected under an approved University of Calgary (U of C) Conjoint Ethics Research Board Approved Protocol (Ethics ID # REB16-0041), following consultation with the U of C privacy office, with sub-site ethics and legal agreements for data sharing between sites. Inclusion criteria in the current study included CHB mono-infection (HBsAg positive for greater than 6 months) and a diagnosis of HCC (AASLD criteria) [11, 12]. Exclusion criteria included viral co-infection with human immunodeficiency virus (HIV), hepatitis C (HCV) or hepatitis Delta (HDV), previous neoplastic diagnosis and/or concomitant neoplasm of extrahepatic origin.
2.2 Statistical Analysis
The primary objective of this study was to describe the characteristics of the CHB-HCC population enrolled from tertiary referral clinics in Canada. This involved a descriptive analysis demographics, risk factors associated with CHB and liver disease course prior to HCC diagnosis. Data collected for comparison between cohorts were extracted from the first documented visit and survival post-diagnosis was calculated in patient's that developed HCC. Continuous variables were summarised as means or medians with categorical values summarised as proportions/percentages and counts. Characteristics were compared with null hypothesis testing between patients diagnosed with HCC and those not diagnosed during follow-up was conducted using unpaired t-test, chi-squared test and Mann–Whitney test based on the type of data being utilised, with a significance threshold of p < 0.05. The secondary objective of this study focused on evaluating survival outcomes in those with CHB-HCC [13]. Multivariable analysis was done to identify variables associated with a significant impact on mortality after diagnosis of HCC, followed by survival analysis involving significant variables. The endpoint for survival was defined as either the last date of follow-up, or the date of mortality if identified. Data analysis was conducted using R software, version 2.2.
3 Results
3.1 Baseline Demographics of CHB and CHB-HCC Cohort
Demographic data for the overall CHB cohort and individuals with CHB and HCC are summarised in Table 1. The overall cohort (n = 6711) was mainly male (56.1%) and of Southeast Asian (55.6%) background. The median age was 49 years (IQR: 39–60), 45.6% were HBeAg positive at the time of enrolment, the median HBV DNA level was log 2.54 (IQR: 0–4.04), 9.4% of patients were diagnosed with advanced liver disease, defined as fibrosis stage F3 or higher (based on transient elastography/FibroScan or biopsy). Of the patients who were treated with antivirals in the overall cohort, the majority received Tenofovir (21.1%), followed by Lamivudine (12.5%), and Entecavir (6.4%), with 7.4% on a combination therapy involving a minimum of two agents. The most common risk factor for the development of Hepatitis B was birth in an endemic country (reported in 40.8%).
| Total (N = 6711) | HCC (N = 232) | Non-HCC (N = 6479) | p | |
|---|---|---|---|---|
| Age (median [IQR]) | 49 (39, 60) | 65 (56.5, 73.0) | 49 (39, 60) | <0.001 |
| Male N, (%) | 3752 (56.1) | 183 (79.6) | 3569 (55.2) | <0.001 |
| Ethnicity N, (%) | <0.001 | |||
| Black | 899 (16.2) | 18 (7.9) | 881 (16.6) | |
| Other | 410 (7.4) | 9 (4.0) | 401 (7.5) | |
| SEA | 3735 (67.4) | 165 (72.7) | 3570 (67.1) | |
| Unknown | 144 (2.6) | 20 (8.8) | 124 (2.3) | |
| White | 356 (6.4) | 15 (6.6) | 341 (6.4) | |
| Risk factor N, (%) | ||||
| Born in endemic area | 2739 (40.8) | 147 (63.4) | 2592 (40.0) | <0.001 |
| Vertical transmission | 739 (11.0) | 26 (11.2) | 713 (11.0) | 1.000 |
| Childhood exposure | 658 (9.8) | 0 (0.0) | 658 (10.2) | <0.001 |
| Healthcare exposure | 887 (13.2) | 13 (5.6) | 874 (13.5) | 0.001 |
| High-risk behaviour | 265 (3.9) | 3 (1.3) | 262 (4.0) | 0.052 |
| Liver function and biochemistry median (IQR) | ||||
| HBeAg positive | 3061 (45.6) | 37 (15.9) | 3024 (46.7) | <0.001 |
| HBV DNA (viral load) log (10) IU/mL (median (IQR)) | 2.54 (0.00, 4.04) | 3.67 (1.86, 5.57) | 2.51 (0.00, 4.01) | <0.001 |
| Platelets (IQR) | 206 (80) | 211 (78) | 139 (71) | <0.001 |
| Advanced liver disease | 632 (9.4) | 213 (91.8) | 419 (6.5) | <0.001 |
| HBV treatment | ||||
| Tenofovir (TDF) | 1416 (21.1) | 142 (61.2) | 1274 (19.7) | <0.001 |
| Lamivudine | 839 (12.5) | 46 (19.8) | 793 (12.2) | 0.001 |
| Entecavir | 430 (6.4) | 54 (23.3) | 376 (5.8) | <0.001 |
| Combination therapy | 493 (7.4) | 46 (19.8) | 444 (6.5) | <0.001 |
In total, 232/6711 (3.45%) of the patients who entered the CanHepB registry were identified with HCC, with a median age of 65 (IQR: 56.5–73.0). Of these patients, 79.6% (n = 183) were male, 72.7% (n = 165) were Southeast Asian, 7.9% (n = 18) were Black, 6.6% (n = 15) were White and 4.0% (n = 9) were of other ethnicities, with remainder unknown. Like the non-HCC cohort, the most common risk factor for HBV acquisition was birth in an endemic region (63.4%). At the time of HCC diagnosis, 60.2% had no antiviral treatment documented, although the majority had advanced liver disease (91.8%, 213/232). Patients with HCC were more likely to be HBeAg negative and had a higher median HBV viral load compared with those without liver cancer (3.67 vs. 2.51 log IU/mL).
In summary, compared to patients with CHB without HCC, individuals with liver cancer were older (median age: 65 vs. 49; p < 0.05), more often male (79.6% vs. 55.2%; p < 0.05) with a greater proportion of SEA descent (72.7% vs. 54.5%; p < 0.05) and had developed advanced fibrosis (91.8% vs. 6.5%, p < 0.05).
3.2 Outcome in Patients With CHB-HCC
In 232 patients diagnosed with CHB-HCC, 153 (63%) patients had adequate registry data for Barcelona Clinic Liver Cancer (BCLC) staging [14]. For the overall cohort, the median tumour size was 2.5 cm (IQR: 1.7–4.0) and mean tumour number was 1.33 (SD: 1.33).
Comparing stages, 36.5% (58) patients were staged as BCLC 0, 44.0% (70) were BCLC A, 15.1% (24) were BCLC B, and 4.4% (7) were BCLC C. In the CanHepB network registry, patients with CHB-HCC were found to be skewed towards early-stage disease, with the majority of patients (81%) identified as BCLC Criteria of 0-A. Regarding treatment, surgical intervention was provided to 32.7% of patients, with 13% receiving resection and 24.5% receiving liver transplant. Trans arterial chemoembolisation (TACE) was completed in 40 (17.2%) of patients and radiofrequency ablation (RFA) occurred in 90 (38.8%) patients. Direct palliation or palliative chemotherapy was provided in 31 (13.4%) of cases, and 7.8% of patients received a systemic agent, Sorafenib. Treatment adherence was compared relative to the BCLC guidelines. The first choice of treatment being adherent to BCLC criteria was most commonly seen in the early-stage BCLC-A cohort (85.7%), and least seen in BCLC-C cohort (16.7%). Patients with CHB-HCC had a 78.7% survival rate through a median follow-up of 61 months (IQR: 19–101).
Given a large portion were diagnosed through screening programmes (ultrasound follow-up every 6 months), patients with CHB with HCC were differentiated on the basis of diagnoses as part of screening (n = 133) and those outside of screening (n = 109; Table 2). Significant differences were found between those diagnosed through formal screening programme versus incidental/non-screening imaging with more vertical transmission of hepatitis B, and lower mortality (14.6% vs. 27.5%).
| Variable | Overall | Diagnosed outside of surveillance (i.e., incidental) | Diagnosed by surveillance | p |
|---|---|---|---|---|
| N | 232 | 133 | 109 | |
| Age (median, IQR) | 65 (56.5, 73) | 65.00 (56.00, 70.00) | 65.50 (57.00, 76.00) | 0.304 |
| Male (N, %) | 183 (79.6) | 89 (81.7) | 94 (77.7) | 0.561 |
| Race | 0.553 | |||
| Black | 18 (7.9) | 8 (7.6) | 10 (8.2) | |
| Other | 9 (4.0) | 5 (4.8) | 4 (3.3) | |
| SEA | 165 (72.7) | 80 (76.2) | 85 (69.7) | |
| White | 15 (6.6) | 6 (5.7) | 9 (7.4) | |
| Risk factors N, (%) | ||||
| Endemic area | 147 (63.4) | 75 (68.8) | 72 (58.5) | 0.138 |
| Vertical transmission | 26 (11.2) | 7 (6.4) | 19 (15.4) | 0.049 |
| Childhood exposure | 232 (100.0) | 109 (100.0) | 123 (100.0) | NA |
| Healthcare exposure | 13 (5.6) | 3 (2.8) | 10 (8.1) | 0.136 |
| High-risk behaviour | 3 (1.3) | 1 (0.9) | 2 (1.6) | 1.000 |
| Liver function and biochemistry median (IQR) | ||||
| HBeAg positive N, (%) | 37 (27.6) | 24 (31.2) | 13 (22.8) | 0.382 |
| HBV (viral load) (log (10) IU/mL) | 3.67 (1.86, 5.57) | 3.11 (1.68, 5.65) | 3.87 (2.17, 5.35) | 0.841 |
| ALT | 42 (26.0, 62.0) | 42.0 (26.0, 67.0) | 41.5 (27.7, 59) | 0.590 |
| AFP | 5.6 (2, 42) | 8 (3106) | 4 (1, 14) | 0.007 |
| Platelets | 211 (78) | 144 (75) | 158 (84) | 0.540 |
| Advanced liver disease | 213 (91.8) | 101 (92.7) | 112 (91.1) | 0.838 |
| HCC characteristics N, (%) | ||||
| Lesion number (mean, SD) | 1.33 (1.33) | 1.37 (1.56) | 1.30 (1.11) | 0.713 |
| Average lesion size (median, IQR) | 2.50 (1.70, 4.00) | 2.50 (1.60, 4.05) | 2.60 (1.80, 4.00) | 0.547 |
| Diagnosed on CT | 61 (33.5) | 27 (31.0) | 34 (35.8) | 0.602 |
| Diagnosed on MRI | 75 (41.2) | 35 (40.2) | 40 (42.1) | 0.916 |
| BCLC staginga | 0.365 | |||
| 0 | 58 (36.5) | 23 (41.1) | 35 (34.0) | |
| A | 70 (44.0) | 20 (35.7) | 50 (48.5) | |
| B | 24 (15.1) | 11 (19.6) | 13 (12.6) | |
| C | 7 (4.4) | 2 (3.6) | 5 (4.9) | |
| HCC treatment N, (%) | ||||
| Ablation | 90 (38.8) | 46 (42.2) | 44 (35.8) | 0.385 |
| Transplant | 30 (12.9) | 12 (11.0) | 18 (14.6) | 0.414 |
| Resection | 63 (27.2) | 32 (29.4) | 31 (25.2) | 0.574 |
| TACE | 40 (17.2) | 21 (19.3) | 19 (15.4) | 0.552 |
| Sorafenib | 18 (7.8) | 9 (8.3) | 9 (7.3) | 0.791 |
| Palliative | 13 (5.6) | 6 (5.5) | 7 (5.7) | 0.951 |
| Appropriate treatment | 111 (81.6) | 40 (87.0) | 71 (78.9) | 0.254 |
| Follow-up time (months) | 52.9 (17.0, 89.6) | 42.9 (15.3, 84.8) | 59.3 (21.4, 96.8) | 0.280 |
| Deceased | 48 (21.3) | 30 (28.6) | 18 (15.0) | 0.021 |
- a As per the Barcelona Clinic Liver Cancer staging system, with associated treatment recommendations [14].
3.3 Association Between HCC Screening and Survival After HCC Diagnosis
The primary outcome chosen for the secondary objective was survival post-HCC diagnosis. Baseline variables selected to assess association with survival included patient demographics, biochemical characteristics and HCC characteristics, as summarised in Table 3. On univariable analysis, diagnosis during screening was the only variable with a statistically significant hazard ratio (p < 0.05) of 0.378, thus a relative decrease in risk of mortality by roughly 62%. Multivariable analysis was limited to the patient cohort with complete data for all variables. Given there was a single variable with a statistically significant role in survival, age, gender and diagnosis during screening were considered to ensure appropriate multivariable analysis. Diagnosis during screening continued to demonstrate a statistically significant reduction in mortality, with HR of 0.077 (p < 0.05). A Kaplan–Meier diagram was also generated, which demonstrated a moderate positive signal, suggestive of improved survival post-HCC diagnosis in the screening cohort (Figure 1).
| Variable | Univariable coefficient estimate | CI | p | Multivariable coefficient estimate | CI | p |
|---|---|---|---|---|---|---|
| Age | 0.976 | (−0.05, 0) | 0.0526 | 0.979 | (0.96–1.00) | 0.096 |
| Male | 0.762 | (−1.02, 0.47) | 0.4759 | 0.681 | (0.318–1.45) | 0.328 |
| SEA | 0.910 | (−1.29, 1.1) | 0.8778 | — | — | — |
| Caucasian | 0.274 | (−3.57, 0.98) | 0.2638 | — | — | — |
| HbEAg positive | 1.137 | (−0.66, 0.92) | 0.7509 | — | — | — |
| HBV (viral load) | 1.209 | (0.02, 0.36) | 0.0303 | — | — | — |
| ALT | 1.002 | (0, 0) | 0.2342 | — | — | — |
| Lesion number | 1.157 | (−0.09, 0.38) | 0.2312 | — | — | — |
| Advanced Liver Diseasea | 1.588 | (−0.72, 1.64) | 0.4422 | — | — | — |
| Diagnosed during Screening | 0.378 | (−1.63, −0.31) | 0.0039 | 0.392 | (0.202–0.761) | 0.006 |
- a Defined as known biopsy-proven F3 fibrosis and beyond, or Fibroscan kPA >9.0.
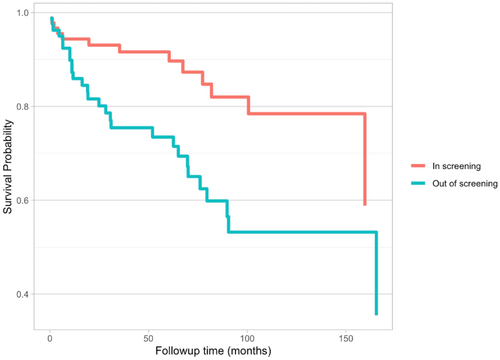
3.4 Interpretation
In this large multi-ethnic cohort of patients with CHB-HCC in Canada, the majority of patients were detected with early-stage HCC, received curative treatment, and had excellent survival rates driven by surveillance practices in tertiary centres.
The majority of patients with CHB, with or without HCC enrolled in the CanHepB registry were born in an endemic country. The current study highlights the disproportionate burden of disease in immigrant populations, particularly SEA populations, along with older and male patient, hence validates current international screening guidelines for high-risk CHB populations [15-18]. The identification of high-risk cohorts is crucial in efforts to address knowledge gaps for targeted populations, especially in medical settings where racial and ethnic data are not regularly collected. The findings we provide regarding the viral status of the overall cohort and patients with HCC in particular support the need for further consideration of these variables both in new patients and in future studies. Although HB antigen and HCV antibody screening is required for the Canadian immigration medical examination, hepatitis-related information is not transferred over and subsequently, and there remains a gap between when patients arrive and when they are referred for specialist care [19, 20]. Thus, the current research also emphasises the need for HBsAg screening for people born outside of Canada once they arrive, and to ensure high-risk patients are appropriately transferred and referred for ongoing care [21].
Our CHB-HCC cohort also demonstrated strong survival rates. The majority of patients were diagnosed with early-stage disease (BCLC 0-A) and treated with curative intent with a high overall survival rate throughout follow-up. Our patients with HBV experienced better prognostic outcomes in comparison with other large cohorts such as a 2887 patient cohort established by Wang et al., which reported a 5-year survival of 19.5% over a median follow-up time of 9 months [21]. When reflecting on screening adherence, although 91.8% of our patients with HCC were identified as possessing advanced liver disease, only 57% were diagnosed as part of a screening protocol. As expected with an early-stage disease population, the HCC treatment goals were primarily curative in intent. Our results support that patient outcomes regarding survival could be maximised by improving current screening engagement and by increasing adherence to BCLC treatment guidelines. There are multiple barriers to this across each level of healthcare and future studies should look to better understand the Canadian experience with HCC screening, such as understanding the role of immigrant perspectives, the impact of the COVID-19 pandemic, and how screening is distributed between primary and specialist care. Ultimately, gaining an improved understanding of the system-level and centre-level barriers to screening and treatment adherence is required to help inform future HCC detection and management initiatives.
The identification of patients diagnosed as part of a screening protocol enabled the collection of granular data into the practical utility of screening for a high-risk population. Our data suggest important long-term benefits of adherence to HCC screening in patients with CHB. Patients diagnosed through screening demonstrated a significant decrease in mortality during follow-up, and improved survival based on univariable and multivariable analysis. The current study showed a high rate of diagnosis via a screening programme (57%). By comparison, Davila et al. (2007) found only 28% of the patients with HCC in their cohort had received a screening test, whereas 42% of HCC diagnoses were identified through surveillance in a study by El-Serag et al. (2016) [22, 23]. Thus, a more robust screening programme likely contributed to overall improved survival post-HCC diagnosis, highlighting the utility of consistent practices. Interestingly, there was no significant difference in tumour size or number between patients diagnosed in screening versus outside of screening, suggesting there may be other factors beyond the early diagnosis that impacted survival, such as potentially ease of mobilising appropriate care given patients were already well integrated into the medical system. However, consistent surveillance is difficult to execute, with some studies observing the rates of complete surveillance (one ultrasound every 6-month interval) in the non-cirrhotic CHB population to be as low as 6.7%. [24] Our study supports the development of novel programmes to address the challenges of screening adherence, such as the use of a dedicated automatic recall HCC surveillance programme implemented at the University of Calgary [25].
The strengths of this study include the heterogenous nature of the population sample, representing a large cohort of an ethnically diverse CHB population in a country with a publicly funded healthcare system. Collection of racial/ethnic data itself is a strength, as Canadian healthcare systems often do not collect this data, and important to address disparities in care for minorities and foreign-born Canadians. Additionally, granular data regarding the adherence to surveillance at the time of HCC diagnosis allowed assessment of screening systems effectiveness. The data is limited by it's retrospective nature and inherent selection bias. The data was collected exclusively from tertiary care centres, and the majority of patients were from centres in Alberta and Ontario, with fewer patients from British Columbia and Quebec, two of the three most populous provinces in Canada [26]. Thus, data regarding involvement beyond tertiary care sites is limited, such as whether the patient had any additional screening ultrasounds. Furthermore, there are certain limitations based on the initial dataset as well, such as no genotype data to further classify patients and BCLC classification was not done in one-third (32%) of patients with HCC. Most patients who present with advanced-stage HCC are referred for management by medical oncologists, rather than hepatology in Canada; thus, most of our data were on patients eligible for curative therapy (i.e., RFA, resection or transplant). However, our study of >200 patients with CHB with HCC is one of the largest and most comprehensive cohort studies showing the importance of HCC surveillance, identification of racial and clinical risk factors, treatment outcomes and long-term survival in tertiary referral centres.
4 Conclusion
In this large retrospective multi-ethnic cohort of adult patients with CHB followed in tertiary referral centres, HCC was more common in older individuals with HBV of SEA background, and those with advanced liver disease. Patients diagnosed with early HCC through screening programmes and treated with curative intent had >80% 5-year survival rates. The study highlights the ongoing need for improved patient-centred healthcare policies for CHB diagnosis, treatment and screening of high-risk populations.
Author Contributions
Y. Sachar and M. Brahmania were involved in study conception, data collection, data analysis and interpretation, protocol drafting and editing. M. Brahmania was involved in protocol editing. M. Brahmania is acting as the article guarantor. The remaining authors were all involved in data collection, as well as manuscript revision. All authors have approved the final version of the protocol.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Research data are not shared.




