Incidence and prevalence of hepatitis B in patients with diabetes mellitus in the UK: A population-based cohort study using the UK Clinical Practice Research Datalink
Funding information
GlaxoSmithKline Biologicals S.A. was the funding source and was involved in all stages of the study conduct and analysis. GlaxoSmithKline Biologicals S.A. also took charge of all costs associated with the development and publishing of the manuscript. All authors had full access to the data and agreed with the submission of the publication.
Summary
We assessed the incidence and prevalence of hepatitis B (Hep B) in patients with or without diabetes mellitus (DM) using the UK Clinical Practice Research Datalink (CPRD). This was a retrospective, observational study of diabetic and nondiabetic cohorts aged 0-80 years using CPRD (NCT02324218). Incidence rates (IR) for each cohort were calculated. Crude and adjusted (Poisson regression) IR ratios (IRR) were estimated with 95% confidence intervals (CI) to compare the cohorts. Hep B prevalence stratified by age, and hospitalization related to Hep B was also calculated. Of 7 712 043 subjects identified, 4 839 770 were included (DM: 160 760; non-DM: 4 679 010). Mean ages were 54.4 and 32.0 years, and 57.20% and 50.14% were male in the diabetic and nondiabetic cohorts, respectively. Hep B was identified in 29 diabetic and 845 nondiabetic subjects; IR was 4.03 per 100 000 person-years and 2.88 per 100 000 person-years, respectively. The adjusted IRR was 1.00 (95% CI: 0.70-1.50) between diabetic and nondiabetic cohorts. Hep B prevalence was higher in the diabetic cohort (0.01%-0.26%) than in the nondiabetic cohort (0.00%-0.03%) across the different age groups. Hep B-associated hospitalization IR was higher in the diabetic cohort (4.98-10.91) than the nondiabetic cohort (0.26-2.44). The Hep B IR, hospitalization and prevalence were higher in males in both cohorts. In conclusion, the risk of incident Hep B diagnosis in the diabetic cohort was not different from that in the nondiabetic cohort. However, prevalence of Hep B and Hep B-associated hospitalization rate was higher in the diabetic than in the nondiabetic cohort.
Abbreviations
-
- ACIP
-
- Advisory Committee on Immunization Practices
-
- CPRD
-
- Clinical Practice Research Datalink
-
- DM
-
- diabetes mellitus
-
- EIP
-
- Emerging Infection Program
-
- GP
-
- general practitioner
-
- HBV
-
- hepatitis B virus
-
- Hep B
-
- hepatitis B
-
- HES
-
- Hospital Episodes Statistics
-
- IR
-
- incidence rate
-
- IRR
-
- IR ratio
-
- NHANES
-
- National Health and Nutrition Examination Survey
-
- NOS
-
- not otherwise specified
-
- UK
-
- United Kingdom
Focus on the Patient Section (FPS)
- Hepatitis B virus (HBV) infection is the most common infection affecting the liver worldwide, and one-third of the global population is thought to have been infected anytime during their life. As a result of not recovering completely from the infection, chronic HBV infection is estimated to affect 240 million persons worldwide. HBV infections in adults can occur via sexual transmission, exposure to contaminated blood products or needles. Hepatitis B (Hep B) outbreaks have occurred including in adults with diabetes through contamination of blood glucose testing devices.
- The Hepatitis Vaccines Work Group of the Advisory Committee on Immunization Practices (ACIP) in the United States evaluated the risk of HBV infection among adults diagnosed with diabetes and recommended that all individuals aged 19-59 should be vaccinated with Hep B vaccine “as soon as feasible after a diagnosis of diabetes.”
- Hepatitis B virus adult vaccination is recommended on a risk-based approach, with varying degrees of success and vaccination coverage in different countries.
- In this study, we evaluated the incidence and prevalence of Hep B diagnosis in patients with or without diabetes using a large database of anonymous patient's medical records from general practitioners (Clinical Practice Research Datalink) and hospitalization records (Hospital Episode Statistics) in England.
- The investigators concluded that the risk of Hep B new diagnosis in diabetic patients is similar to the one in nondiabetic ones. However, the prevalence of Hep B and hospitalization associated with it is higher in the diabetic group than that the nondiabetic one. The strengths, limitations and potential methodological biases of the study are discussed.
1 INTRODUCTION
Hepatitis B virus (HBV) infection is a global public health issue with increasing complication and mortality rates.1 The prevalence of Hep B ranges from 5% to 10% in adults in sub-Saharan Africa and East Asia to <1% of in the population of Western Europe and North America.2 Acute infections progress to chronic infections in 80%-90% of children infected in their first year of life, 30%-50% of children infected before the age of 6 and <5% of people infected as adults. About 20%-30% of chronically infected adults develop cirrhosis and/or liver cancer.2 As of 2015, 185 countries vaccinate infants against Hep B as part of their national immunization programmes and 83% of children received the complete Hep B vaccine course.3 Nonetheless, global disease burden estimates indicate that nearly 240 million people are chronically infected with Hep B,2 suggesting that innovative strategies to address HBV infection are needed.4 Although the prevalence of HBV in the UK is low, an increasing trend has been recently observed.5 As HBV is stable for long periods of time on surfaces and is highly transmissible,6 many Hep B outbreaks have occurred particularly in adults with diabetes who receive assisted blood glucose monitoring.7
The Center for Disease Control and Prevention in the USA has received many reports of Hep B outbreaks in long-term care facilities, including nursing homes and assisted-living facilities since 1996. These outbreaks prompted the Hepatitis Vaccines Work Group of the Advisory Committee on Immunization Practices (ACIP) to evaluate the risk of HBV infection among adults diagnosed with diabetes mellitus (DM).7
Several studies were carried out to understand the burden of Hep B among diabetics. Data from the National Health and Nutrition Examination Survey (NHANES) showed a higher prevalence of HBV infection among noninstitutionalized individuals diagnosed with diabetes as compared to nondiabetics.8, 9 Data from the Emerging Infections Program (EIP) database showed that diabetes was independently associated with an increased risk for acute Hep B among adults without HBV risk behaviours.10 The vaccination of adult diabetics (20-59 years) with Hep B vaccine was found to be cost-effective.11 Therefore, ACIP recommends that all individuals aged 19-59 should be vaccinated with Hep B vaccine “as soon as feasible after a diagnosis of diabetes”.7
To our knowledge, studies investigating the incidence and prevalence of Hep B in DM patients, rather than the general population, are scarce outside the United States. According to WHO estimates, the global prevalence of diabetes was 9% in 2014.12 UK data published in 2013 reported a significant increase in the incidence and prevalence of type 2 DM.5 It has been further projected that by 2030, the total number of adults with diabetes will increase to 9.5% in England.13
This study was therefore undertaken to assess the incidence and prevalence of HBV diagnosis in DM and non-DM cohorts in the UK using the Clinical Practice Research Datalink (CPRD) database and hospitalization records in England.
2 MATERIALS AND METHODS
2.1 Data source, population and setting
Clinical Practice Research Datalink, an ongoing primary care database of anonymized medical records from general practitioners (GPs), covers over 11 million patients from more than 670 practices in the UK.14 Primary care data from CPRD GOLD is in part available for linkage with Hospital Episode Statistics (HES) admitted patient care data for approximately 50% of subjects.15 The validity of the diagnostic and prescription data in the CPRD for pharmacoepidemiological research has been well established.16, 17 Additionally, data from a preliminary feasibility assessment for this study showed that the incidence rate (IR) of Hep B recorded in the CPRD is comparable to published estimates (data not shown).
This was a retrospective, observational, cohort study, carried out from October 2014 to January 2015. The study population included male and female subjects aged between 0 and 80 years, with or without a diagnosis of DM (types 1 and 2). Subjects were included if classified as CPRD acceptable for research,14 if the subjects were registered in practices classified as up to standard by the CPRD for the time period 2000-2012 and had been registered in the CPRD database for at least 12 months at the date of inclusion (except infants below 1 year of age). For the analysis of HES data, records linked to more than one CPRD patient were excluded from the study. The study is registered on ClinicalTrials.gov (NCT02324218).
2.2 Study population and cohorts
Subjects were included in and could only contribute to either one of the two cohorts: DM and non-DM.
The DM cohort included subjects diagnosed with type 1 or type 2 DM, as confirmed by diagnosis READ codes (Table S1), reporting at least one DM diagnosis code during the study period, with or without a prescription for an antidiabetic drug.
The non-DM cohort included subjects without personal history of DM diagnosis, antidiabetic drug therapy, records of DM related clinical events or non DM-related drug prescription before the end of the study period. Female patients with gestational diabetes were also included in the non-DM cohort. Subjects reporting any DM-related diagnosis event or DM-related drug prescription before study inclusion date, or a DM-related drug prescription without a DM-related diagnosis event, or only potentially DM-related diagnostic events with or without a DM-related drug prescription were classified as prevalent DM patients and excluded from the main analyses of Hep B IR.
Ethnicity (established by post hoc analysis, Table 1) from HES records was considered first, and if it was missing or unknown, then the ethnicity recorded during the GP visit was considered.
| Characteristics | Categories | DM cohort (N = 160 760) | Non-DM cohort (N = 4 679 010) |
|---|---|---|---|
| n (%) | n (%) | ||
| Age at inclusion (y) | [0-20] | 4193 (2.61) | 1 531 899 (32.74) |
| [21-40] | 19 246 (11.97) | 1 516 032 (32.40) | |
| [41-60] | 77 575 (48.26) | 1 070 263 (22.87) | |
| [61+] | 59 746 (37.16) | 5 608 161 (11.99) | |
| Follow-up duration from inclusion date (y) | [0-3] | 7444 (4.63) | 2 540 346 (33.64) |
| [3-5] | 11 926 (7.42) | 1 122 016 (14.86) | |
| [5-10] | 57 030 (35.48) | 2 115 689 (28.02) | |
| [10+] | 84 360 (52.48) | 1 773 232 (23.48) | |
| Gender | Female | 68 807 (42.80) | 2 333 186 (49.86) |
| Male | 91 953 (57.20) | 2 345 824 (50.14) | |
| Region | East Midlands | 6844 (4.26) | 190 846 (4.08) |
| East of England | 14 080 (8.76) | 461 798 (9.87) | |
| London | 14 689 (9.14) | 572 459 (12.23) | |
| North East | 3059 (1.90) | 78 799 (1.68) | |
| North West | 21 800 (13.56) | 523 962 (11.20) | |
| Northern Ireland | 5048 (3.14) | 131 935 (2.82) | |
| Scotland | 14 975 (9.32) | 436 339 (9.33) | |
| South Central | 15 557 (9.68) | 507 485 (10.85) | |
| South-East Coast | 15 397 (9.58) | 458 836 (9.81) | |
| South West | 13 472 (8.38) | 379 709 (8.12) | |
| Wales | 15 268 (9.50) | 356 661 (7.62) | |
| West Midlands | 14 804 (9.21) | 398 439 (8.52) | |
| Yorkshire & The Humber | 5767 (3.59) | 181 742 (3.88) | |
| DM type | NOS | 14 (0.01) | - |
| Type 1 | 6659 (4.14) | - | |
| Type 2 | 154 087 (95.85) | - |
| DM Cohort | Non-DM cohort | ||||
|---|---|---|---|---|---|
| Hep B (N = 29) | No Hep B (N = 155 938) | Hep B (N = 845) | Hep B (N = 4 678 165) | ||
| n (%) | n (%) | n (%) | n (%) | ||
| Ethnicity | Asian or Asian British | 6 (20.69) | 3497 (2.24) | 75 (8.88) | 92 041 (1.97) |
| Black | 2 (6.90) | 1442 (0.92) | 80 (9.47) | 53 641 (1.15) | |
| Mixed | 0 | 356 (0.23) | 12 (1.42) | 30 556 (0.65) | |
| White | 13 (44.83) | 90 020 (57.73) | 315 (37.28) | 2 120 067 (45.32) | |
| Other ethnic groups | 1 (3.45) | 1067 (0.68) | 39 (4.62) | 46 026 (0.98) | |
| Unknown | 7 (24.14) | 59 556 (38.19) | 324 (38.34) | 2 335 834 (49.93) | |
- The ethnicity analysis presented in this table was a post hoc analysis. N, number of subjects with available data; n, number of subjects in each category; %, percentage of subjects; DM, diabetes mellitus; NOS, not otherwise specified, [0-3], follow-up duration is ≥0 and ≤3 y; [3-5], follow-up duration >3 and ≤5 y; [5-10] follow-up duration >5 and ≤10 y; [10+], follow-up duration >10 y; Hep B, hepatitis B infection diagnosis during follow-up; No Hep B, no hepatitis B infection diagnosis during follow-up; y, years.
To estimate the prevalence of Hep B diagnosis among DM patients, the DM cohort was extended to include prevalent DM patients. DM type (1, 2 or unspecified) was based on an existing classification.16
2.3 Outcome definition
The primary study outcome was the occurrence of incident Hep B diagnosis after inclusion in the study, that is after the DM onset date in the DM cohort. A minimum of 12 months Hep B disease-free period was required for a case to be considered incident. Hep B cases were identified as patients with at least one recorded Hep B clinical diagnosis code, hospitalization records or confirmatory diagnostic laboratory test results (Table S2).
The secondary analyses included new-onset or prevalent Hep B diagnosis among new-onset or prevalent DM and non-DM patients regardless of the onset of Hep B in relation to the diagnosis of DM. The rate of Hep B-associated hospitalization among DM and non-DM patients was also assessed.
2.4 Data collection and case ascertainment
The final study database consisted of data extracted from CPRD GOLD primary care and HES inpatient hospitalization databases. All Hep B cases identified in the DM cohort and a subset of up to 100 cases identified in the non-DM cohort were ascertained through complete database profile review by two independent reviewers. This review included all available clinical, treatment and laboratory test histories available in CPRD to assess the positive predictive value (PPV) of the case classification algorithm.
For the calculation of the PPV, a true-positive (TP) and a false-positive (FP) value were first estimated. TP was the number of cases defined using the algorithm and confirmed by inter-reviewer consensus assessment. FP was the number of cases defined using the algorithm but not confirmed by inter-reviewer consensus assessment. PPV was calculated by dividing TP by TP + FP. The PPV of the case definition algorithm and the inter-reviewer agreement were estimated.
2.5 Sample size
A feasibility assessment conducted over 2000-2012 identified a DM cohort of 161 429 subjects (with 798 132 person-years) with a Hep B IR of 3.8 per 100 000 person-years. In addition, a cohort of 7 413 411 non-DM subjects (with 50 601 322 person-years) was identified. The statistical power was estimated according to the potential difference between the two cohorts, using parameters identified during the feasibility assessment. A random sample of the eligible non-DM subjects using a sampling ratio of 1:30 DM/non-DM was selected.
2.6 Statistical analysis
To account for differences in DM and non-DM cohort characteristics and to minimize the potential bias on the unadjusted incidence and prevalence estimates, the primary analyses were complemented by sensitivity analysis. To maintain confidentiality and individual data anonymization, the planned statistical analyses were conducted only if at least five cases were observed for a given strata or subgroup.
2.7 Primary analysis
The Hep B IRs were calculated as the total number of incident Hep B cases during the study period divided by the total number of person-years at risk in each cohort. The follow-up person-time at risk was calculated from the DM onset date (DM cohort), or from study inclusion date (non-DM cohort), until the earliest of the following events date: Hep B diagnosis; transfer out of the practice; death; 1 January of the year of the 81st birthday; and 31 December 2012 or last practice data collection.
The unadjusted IR ratio (IRR) between the DM and non-DM population was calculated as the IR in DM divided by IR in non-DM. The 95% confidence interval (CI) for unadjusted IRR was calculated using a Poisson regression model including DM cohort (yes/no) as covariate. The IRR was adjusted using a Poisson regression model, with DM cohort (yes/no), age at inclusion in the study, gender, geographical region of GP practice, calendar year of inclusion in the cohort and number of GP consultations in the 12 months before inclusion as covariates. The IRR was derived from the exponential of the coefficient associated with the exposure status and its 95% Wald.
Prevalence was estimated based on the total number of Hep B cases ever recorded in subjects’ medical histories (DM and non-DM subjects; not only new-onset Hep B during the study period). The prevalence was estimated as the proportion of Hep B cases among all subjects in the cohort.
Hospitalization rates were estimated for the subset of subjects in each cohort eligible for HES inpatient data linkage, as authorized by their GP practices. The incidence of hospitalization was estimated similarly to the IR in the primary analyses and was based on new-onset Hep B cases in the study follow-up period recorded in the HES inpatient discharge data recorded using ICD-10 diagnosis codes.
2.8 Sensitivity analysis
- Inclusion of HES inpatient cases: A sensitivity analysis was performed to evaluate the impact on IR estimates of including additional Hep B cases captured in the HES database but not recorded in CPRD.
- Confirmatory and potential Hep B case definition: A more sensitive Hep B code list was used including Hep B diagnostic codes potentially related with Hep B (eg routine screening) and tests performed for which results were not available, to assess the impact of a more sensitive Hep B case definition in the IR estimates.
- Matched DM with non-DM analyses: A ratio of up to 10 matched non-DM subjects per DM subject was used to match the DM to non-DM subjects according to GP practice geographic area, age group at index date, gender and CPRD eligibility/follow-up period overlap. Unadjusted IRR was calculated comparing the two matched cohorts.
All statistical analyses were performed using the Statistical Analysis Systems (SAS) version 9.2 (SAS Institute Inc., Cary, North Carolina, USA).
3 RESULTS
3.1 Study population
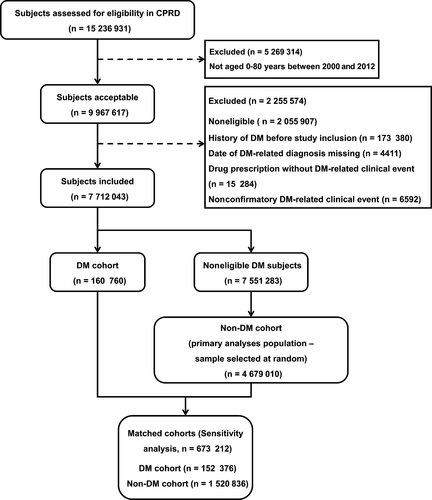
From a total of 7 712 043 study eligible subjects, the DM cohort included 160 760 patients. From the eligible 7 551 283 subjects, the non-DM cohort included a random sample of 4 679 010 subjects (Figure 1). The mean ages were 54.4 and 32.0 years, 57.20% and 50.14% were male, and ethnicity data were available for 62% and 50% of subjects in the DM and non-DM cohorts, respectively (Table 1). In the DM cohort, 95.85% of subjects had type 2 diabetes, 4.14% had type 1 diabetes, and 0.01% were nonspecified. For the sensitivity analyses, the DM cohort of 152 376 patients was matched with 1 520 836 subjects in the non-DM cohort.
3.2 Hep B incidence rate
Twenty-nine incident Hep B cases were identified in the DM cohort, corresponding to an IR of 4.03 per 100 000 person-years. There were 845 incident Hep B cases in the non-DM cohort corresponding to an IR of 2.88 per 100 000 person-years. The unadjusted IRR between DM and non-DM cohorts was 1.40 (95% CI: 0.97-2.03); the adjusted IRR was 1.00 (95% CI: 0.7-1.5; Table 2). The three IRR estimates showed no statistically significant differences between the Hep B IR in the DM and non-DM cohorts.
| Primary analyses | IRR per 100 000 PY (95% CI) |
|---|---|
| Unadjusted Hep B IRR | 1.40 (0.97-2.03) |
| Adjusted Hep B IRR | 1.0 (0.70-1.50) |
| Sensitivity analyses: matched cohorts | |
| Unadjusted Hep B IRRa | 1.2 (0.80-1.70) |
- IRR, incidence rate ratio; 95% CI, 95% confidence interval; PY, person-years.
- a Unadjusted IRR between DM and matched non-DM cohorts.
Across the age groups, the Hep B IR in the DM cohort ranged from 2.61 (95% CI: 1.05-5.38) in subjects aged 61 years or older to 5.53 (95% CI: 0.14-30.79) in the 0-20 years age group. In the non-DM cohort, the IR ranged from 0.81 (95% CI: 0.64-1.01) in the 0-20 years age group to 5.19 (95% CI: 4.72-5.69) in the 21-40 years age group (Table 3). In the DM cohort, the incidence of Hep B was lower in females (2.27 [95% CI: 0.91-4.67]) than in males (5.36 [95% CI: 3.36-8.11]; Table 3). Of the subjects with available ethnicity information, a higher proportion of Asian ethnics was observed among Hep B incident cases in the DM cohort (20.6% vs 2.2% among DM subjects without incident Hep B) and in the non-DM cohort (8.9% vs 2.0% among non-DM subjects without incident Hep B).
| Category | DM cohort (N = 155 967) | Non-DM cohort (N = 4 679 010) | ||
|---|---|---|---|---|
| Cases (n) | IR per 100 000 PY (95% CI) | Cases (n) | IR per 100 000 PY (95% CI) | |
| Age (y) at inclusion | ||||
| [0-20] | # | 5.53 (0.14-30.79) | 75 | 0.81 (0.64-1.01) |
| [21-40] | # | 2.63 (0.32-9.50) | 445 | 5.19 (4.72-5.69) |
| [41-60] | 19 | 5.31 (3.20-8.29) | 281 | 3.49 (3.09-3.92) |
| [61+] | 7 | 2.61 (1.05-5.38) | 44 | 1.27 (0.93-1.71) |
| Gender | ||||
| Female | 7 | 2.27 (0.91-4.67) | 337 | 2.31 (2.07-2.58) |
| Male | 22 | 5.36 (3.36-8.11) | 508 | 3.43 (3.14-3.75) |
| DM type | ||||
| NOS | - | - | - | - |
| Type 1 | # | 3.29 (0.08-18.33) | - | - |
| Type 2 | 28 | 4.06 (2.70-5.87) | - | - |
- N, number of subjects with available data; n, number of hepatitis B cases in each category; #, denotes 1-4 cases, results not disclosed; IR, incidence rate; 95% CI, 95% confidence interval; PY, person-years; DM, diabetes mellitus; NOS, not otherwise specified; y, years.
3.3 Hep B prevalence
The prevalence of Hep B ranged between 0.01% and 0.26% across the different age groups in the DM cohort and between 0% and 0.03% in the non-DM cohort (Table 4). Hep B prevalence was highest in the 41-60 years age group in the DM cohort (0.26%), and in the 21-40 years and 41-60 years age group in the non-DM cohort (0.03%). The prevalence was higher in males in both cohorts (males: DM: 0.21%, non-DM: 0.02%; females: DM: 0.12%, non-DM: 0.01%).
| Category | DM cohort (N = 307 345) | Non-DM cohort (N = 4 679 010) | ||
|---|---|---|---|---|
| Cases (n) | % (95% CI) | Cases (n) | % (95% CI) | |
| Age (y) at inclusion | ||||
| [0-20] | # | 0.01 (0.00-0.08) | 75 | 0.00 (0.00-0.01) |
| [21-40] | 79 | 0.23 (0.18-0.28) | 445 | 0.03 (0.03-0.03) |
| [41-60] | 332 | 0.26 (0.23-0.29) | 281 | 0.03 (0.02-0.03) |
| [61+] | 122 | 0.09 (0.07-0.11) | 44 | 0.01 (0.00-0.01) |
| Gender | ||||
| Female | 161 | 0.12 (0.10-0.14) | 337 | 0.01 (0.01-0.02) |
| Male | 373 | 0.21 (0.19-0.24) | 508 | 0.02 (0.02-0.02) |
| DM type | ||||
| Type 1 | 57 | 0.17 (0.13-0.22) | - | - |
| Type 2 | 477 | 0.17 (0.16-0.19) | - | - |
- N, number of subjects with available data; n, number of hepatitis B cases in each category; #, denotes field where there were 1-4 cases or results not disclosed; %, percentage of prevalence of hepatitis B at each category; 95% CI, exact 95% confidence intervals; DM, diabetes mellitus.
3.4 Rate of hospitalization due to Hep B
The IR of Hep B-related hospitalizations in the DM cohort ranged from 4.98 (95% CI: 2.15-9.81) in the ≥61 years age group to 10.91(95% CI: 3.54-25.45) per 100 000 person-years in the 21-40 years age group. It ranged between 0.26 (95% CI: 1.14-0.43) in the 0-20 years age group and 2.44 (95% CI: 2.02-2.91) in 21-40 years age group in the non-DM cohort subset of subjects eligible for HES inpatient data (Table 5). The rate of hospitalization due to Hep B was higher in males (9.72%) compared to females (2.16%; Table 5). Hep B was frequently a secondary HES diagnosis in both DM (60.71% [17/28]) and non-DM cohorts (69.46% [166/239]).
| Category | DM cohort (N = 92 626) | Non-DM cohort (N = 2 742 367) | ||
|---|---|---|---|---|
| Cases (n) | IR per 100 000 PY (95% CI) | Cases (n) | IR per 100 000 PY (95% CI) | |
| Age (y) at inclusion | ||||
| [0-20] | # | 10.17 (0.26-56.65) | 14 | 0.26 (0.14-0.43) |
| [21-40] | 5 | 10.91 (3.54-25.45) | 123 | 2.44 (2.03-2.91) |
| [41-60] | 14 | 6.50 (3.55-10.91) | 93 | 1.94 (1.57-2.38) |
| [61+] | 8 | 4.98 (2.15-9.81) | 9 | 0.44 (0.20-0.84) |
| Gender | ||||
| Female | # | 2.16 (0.59-5.54) | 99 | 1.16 (0.94-1.41) |
| Male | 24 | 9.72 (6.23-14.47) | 140 | 1.60 (1.34-1.89) |
| DM type | ||||
| NOS | - | - | - | - |
| Type 1 | # | 5.88 (0.15-32.74) | - | - |
| Type 2 | 27 | 6.51 (4.29-9.47) | - | - |
- N, number of subjects with available data; n, number of hepatitis B cases in each category; #, denotes field where there were 1-4 cases, results not disclosed; IR, incidence rate; 95% CI, 95% confidence interval; PY, person-years; DM, diabetes mellitus; NOS, not otherwise specified; HES, Hospital Episodes Statistics; y, years.
4 DISCUSSION
This retrospective database study is the first one evaluating the incidence and prevalence of Hep B and Hep B-related hospitalization rates among DM and non-DM populations in the UK using the CPRD data source. Studies from the United States have reported an increased risk for HBV among persons with diabetes regardless of HBV risk behaviours.7, 8, 10 HBV transmission among diabetics, especially in older populations, has resulted from lapses in infection control associated with blood glucose monitoring.18 Another study showed that patients with diabetes were involved in HBV outbreaks as a consequence of the habit of performing capillary blood sampling using nondisposable multipatient devices.19 Furthermore, an increased risk of HBV exposure has been reported in the United States.8, 10 A more severe chronic HBV infection and exacerbation of DM-associated liver disease in the DM population has been shown in several studies.10, 20-23 As a result, ACIP has recommended that individuals aged 20-59 years old should be vaccinated against HBV as soon as possible after the diagnosis of DM.7
In the present study, the risk for new-onset incident Hep B among patients with DM was not different from that in non-DM subjects after adjustment for age at inclusion, gender, geographical region of the GP, year of the inclusion in the cohort and number of consultations during the 12 months prior to inclusion. The IR of Hep B in the DM cohort was highest in the 0-20 years age group. Available literature on laboratory confirmed Hep B has shown that overall the incidence of Hep B in the UK is low.24, 25 In an earlier study conducted in the UK, the IR of Hep B among population ≥15 years of age was reported to be 1.6 per 100 000 person-year.26 However, the authors did not consider diabetes as a risk factor for Hep B infection. The IRR estimate was not adjusted for ethnicity, which is only partially documented in CPRD and is not recorded for 50% of the study subjects. The risk for type 2 diabetes is known to be higher in migrant populations from South-East Asia settling in western countries than in the local population.27, 28 In addition, Hep B infection and transmission are geographically more prevalent in the Asian regions.29, 30 A different proportion of Hep B cases in subjects of South-East Asian ethnicity among diabetics may have impacted the estimation of the IRR, as ethnicity may have been a potential confounder in the comparison between DM and non-DM cohorts.
The observed prevalence of Hep B during the follow-up period was higher in the DM cohort compared to the non-DM cohort in all age groups. Males had a higher prevalence of Hep B than females in both study cohorts. These findings are in line with previous studies where a higher HBV-diabetes prevalence ratio was observed for men.8, 26 Likewise, a study conducted in Turkey revealed higher prevalence of occult HBV infections in diabetic patients.31 The range of prevalence observed in this study is consistent with published prevalence estimates (HPE data: 0.1% and 0.5% of the UK population).32, 33 A higher Hep B-associated hospitalization IR was observed in the DM than that in the non-DM cohort in this study.
A similar study in the United States reported a higher prevalence of Hep B among adults with DM than in those without DM.18 Another study reported a 1.6-times (95% CI 1.3-1.9) higher prevalence of HBV infection among diabetics than nondiabetics.8 Differences in the data reported in our study and in the US studies can be attributed to differences in the study designs. NHANES: National health and nutrition examination survey is a program of studies designed to assess the health and nutritional status of adults and children in the United States and includes a representative sample of the noninstitutionalized US population. The survey is unique in that it combines interviews and physical examinations data; therefore, Hep B is laboratory confirmed. The Emerging Infectious program is a national resource for surveillance, prevention and control of emerging infectious diseases used to study specific infectious diseases and, similarly to NHANES, includes laboratory confirmed Hep B cases while in the CPRD laboratory confirmation of Hep B is not routinely reported.8, 10
The main strength of this study lies in the use of the CPRD database, a large population-based electronic medical record data set which can link primary and hospital data and provide a good representation of the UK population registered with a GP practice.14 Approximately half of the GP practices allowed linkage with the HES inpatient database. However, CPRD does not include data or recorded variables on populations at higher risk of Hep B, for example prisoners, injectable illegal drug users, homeless and temporary migrant workers. The study assumed that these populations would probably be equally distributed between the DM and non-DM cohorts, with minimal potential for differential information or selection bias.
As Hep B infection screening is often performed confidentially during sexually transmitted disease consultations or at walk-in clinics, the GP records may not have complete information on the dates of Hep B infection or the results and serological status. Therefore, the onset date of first diagnosis may be earlier than the first date of recorded diagnosis of Hep B in the GP medical records. We attempted to minimize this bias in the primary design and analyses by considering only new-onset DM during the study period together with new-onset Hep B diagnosis after the DM onset, but this would have reduced the number of eligible participants. Furthermore, Hep B laboratory testing results may not be systematically recorded in the CPRD, and negative Hep B screening results may be less likely to be recorded than positive results. In addition, some of the READ codes included in the case definition may require further validation on their predictive value when recorded for a single event in the patient's GP medical history records. However, we assumed that this was an unlikely differential between DM and non-DM subjects, unless DM subjects were subject to more intensive screening or testing than non-DM patients. HES inpatient subset data linked to CPRD were limited to England; therefore, outcomes involving hospital data were restricted to the English CPRD practices subset.
Hepatitis B vaccination coverage in the UK is very low, as until recently it was only recommended to those UK populations which are at increased risk of infection. As of 2017, Hep B vaccination will be introduced in the infant vaccination schedules through a combination vaccine.34 There is no recommendation for Hep B vaccination of DM patients. The likelihood of Hep B vaccination was assumed to be similarly distributed between DM and non-DM patients and was therefore not considered in the analyses.
In conclusion, we found that the risk of new Hep B diagnosis after DM onset was no different to the risk among patients without DM. However, prevalence of Hep B during the follow-up period was higher in subjects who developed DM in all age groups compared to those subjects without DM. The Hep B-associated hospitalization rate was also higher in the DM cohort than in the non-DM cohort.
ACKNOWLEDGEMENTS
The authors would like to thank the following from GSK: Laurence Baril for her contribution to the study design, Morgane Guennec for performing the feasibility assessment analyses and Pam Kalodimos for supporting the operational aspects of the study. Anchal Sood and Alpár Pöllnitz provided writing assistance and Lakshmi Hariharan, Angeles Ceregido and Susana Montenegro Gouveia (XPE Pharma and Sciences c/o GSK) provided publication coordination and editorial support.
AUTHORS CONTRIBUTIONS
CM, LDM, GLCF and YEH were involved in the conception or the design of the study. CM, YF, GLCF and YEH participated in the data collection. GLCF and YEH performed the study/project and participated. TVS contributed with materials or reagents. All authors had access to the data, participated in the analysis and interpretation of the data, were involved with developing this manuscript, gave final approval before submission and are accountable for all aspects of the work.
CONFLICT OF INTEREST
CM and LDM are employees of GSK and hold stock options/restricted shares from the sponsoring company. AD is an employee of GSK. GLCF was an employee of GSK at the time of the study. YF was an employee for GSK when the study started, and then she changed to a statistical consultant for GSK continuing to support this study from statistical point of view since 1 September 2014. YEH has declared no conflict of interest. TVS reports a research grant for the epidemiological study of COPD from GSK and personal fees from NovoNordisk and Sanofi during the conduct of the study.
DISCLOSURE
This study is based in part on data from the CPRD obtained under licence from the UK Medicines and Healthcare Products Regulatory Agency. However, the interpretation and conclusions contained in this report are those of the authors alone.