Characterization of laboratory coagulation parameters and risk factors for intraventricular hemorrhage in extremely premature neonates
Manuscript handled by: David Lillicrap
Final decision: David Lillicrap, 04 May 2022
Abstract
Background
Extremely premature neonates have increased risk for bleeding, perhaps the most devastating version of which being intraventricular hemorrhage (IVH). Limited data are available for coagulation parameters in this vulnerable population.
Objectives
We conducted a prospective cohort study characterizing coagulation laboratory parameters in extremely premature neonates 23–30 weeks gestational age (GA) and determined coagulation parameters and clinical risk factors associated with IVH.
Patients/Methods
One hundred twenty neonates 23–30 weeks GA were enrolled, and umbilical cord blood samples were obtained and processed at the time of birth. Coagulation parameters including prothrombin time (PT), international normalized ratio (INR), activated partial thromboplastin time (APTT), and activity assays for factors II, VII, IX, X, XIII, and XIII subunit A antigen were performed by standard methods. Clinical risk factors were analyzed for association with IVH.
Results
Of the enrolled neonates, 29 (24.2%) experienced IVH. Persistent pulmonary hypertension (PPHN) independently predicted IVH risk with odds ratio (OR) 5.3 (95% confidence interval [CI] 1.1–24.3), P = .0338; and chronic lung disease (CLD) approached significance with OR 2.3 (95% CI 0.9–5.5), P = .0659. Coagulation parameters were evaluated for association with IVH, and there was no significant difference among coagulation tests in neonates with or without IVH or per GA. Reduced factor XIII subunit A showed significant association with death, P = .003.
Conclusions
We present a large, prospective study of laboratory coagulation parameters in extremely premature neonates, including factor X, factor XIII, and factor XIII subunit A not previously described in this population. These findings may impact clinical practice and should encourage additional study in this vulnerable population.
Essentials
- Premature neonates are at risk for intraventricular hemorrhage and coagulation norms are unknown.
- This prospective cohort study evaluated coagulation parameters and intraventricular hemorrhage risk.
- Coagulation parameters were defined for neonates <30 weeks gestational age.
- Persistent pulmonary hypertension independently predicted intracranial hemorrhage risk.
1 INTRODUCTION
Extremely premature neonates have an increased risk of bleeding, with development of intraventricular hemorrhage (IVH) as one of the most significant causes of morbidity, mortality, and long-term disability in this vulnerable population.1 Unfortunately, approximately 25% of very low birth weight (birth weight < 1500 grams) neonates experience IVH, with many occurring in the first 24 h of life, which contributes to mental retardation, developmental delay, and cerebral palsy.1, 2 The cause of IVH is multifactorial and thought due, in part, to variations in cerebral blood flow hemodynamics within immature germinal matrix microvasculature.1 Moreover, the concentration and function of coagulation proteins in extremely premature neonates remain largely unknown, including any specific association with IVH.
Previous investigation in healthy, older preterm infants, between 30–36 weeks gestational age (GA), showed differences in coagulant and anticoagulant factor activity compared to term infants in the landmark studies by Andrew et al.3, 4 Specifically, vitamin K-dependent factors were reduced compared to term infants, and vitamin K-dependent factors, contact factors, and anticoagulant factor activities were reduced compared to adults.3, 4 Neary et al. more recently reported a retrospective cohort study in which they determined values for prothrombin time (PT), activated partial thromboplastin time (APTT), and fibrinogen in infants <27 weeks GA on admission to the neonatal intensive care unit (NICU).5 Further, the same group completed a cross-sectional study of infants <30 weeks GA and additionally characterized values for PT, APTT, and fibrinogen on 106 neonates on the first day of life, with limited analysis of coagulation factor activities of factors II, VII, IX, protein C, free protein S, antithrombin, and tissue factor pathway inhibitor, as well as thrombin generation.6 In their analysis, there was no correlation with PT or APTT and IVH on either day 1 or during the first week of life. However, further investigation of IVH’s correlation with other coagulation parameters was not reported.6 Additional coagulation parameters in premature neonates of <30 weeks GA are unknown.
Despite this work, little is known about specific hemostatic disruptions that may contribute to IVH, or what normal coagulation parameters are in neonates <30 weeks GA. To address this issue, we have sought to characterize normal coagulation parameters in <30 weeks GA neonates and evaluate clinical risk factors that may be associated with IVH.
2 METHODS
2.1 Patients
This investigation was a prospective cohort study completed with the University of Illinois College of Medicine at Peoria (UICOMP) and the OSF St. Francis Children’s Hospital of Illinois (CHOI) NICU in Peoria, Illinois, USA. The CHOI NICU is a tertiary care center with a large level 3 nursery, accounting for several thousand deliveries per year, and more than 250 neonates born <30 weeks GA annually. Study approval was granted from the UICOMP Institutional Review Board. Parental consent was obtained, and neonatal patient data were de-identified.
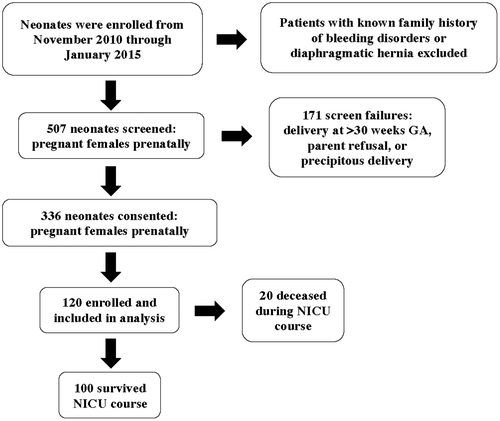
Patients were enrolled from November 4, 2010 through January 14, 2015 through screening and consent of pregnant females prenatally. Inclusion criteria was delivery at CHOI NICU, GA from 23 to 30 weeks, and ability to obtain umbilical cord blood at the time of birth. Exclusion criteria included delivery >30 weeks GA, a known diagnosis of diaphragmatic hernia or family history of a known bleeding disorder. Multiple pregnancies were not excluded. Five hundred seven patients were screened, 336 patients were consented, 171 patients were screen failures, and 120 patients were enrolled (Figure 1). The most common causes of screen failure were delivery at >30 weeks GA, parent refusal, or precipitous delivery. Screen failures >30 weeks GA occurred when pregnant females progressed beyond 30 weeks of pregnancy prior to delivery who were previously eligible for the study. The most common causes for consented patients to not be enrolled were clotted or insufficient umbilical cord blood sample at the time of birth.
Demographic and clinical variable data considered clinical risk factors thought to be potentially associated with IVH were collected on all infants. Clinical variables were defined as reported to the Vermont Oxford Network (VON) and are outlined in Table 1. The VON is an international, nonprofit collaboration of >1000 centers to which data is submitted on extremely premature neonates and all NICU admissions with the aim to standardize quality benchmarks and improve quality of NICU practice.7 These variables were determined and reported to the VON by clinical neonatologists providing care for the neonates during their hospitalization.
| Clinical variable | Definition |
|---|---|
| Congenital heart disease | Any congenital heart defect excluding PDA |
| Coagulopathy | Prolonged PT and/or APTT for GA +/− low fibrinogena |
| Chronic lung disease | Bronchopulmonary dysplasia as a result of severe RDS and subsequent mechanical ventilation support; receipt of supplemental oxygen at postmenstrual age of 36 weeks |
| Gastrointestinal perforation | Perforation in the gastrointestinal tract of a newborn with no demonstrable cause |
| Hyperbilirubinemia | Elevation of the bilirubin level above the 95th percentile adjusted for age of the infant during the first week of life |
| Necrotizing enterocolitis | Ischemic necrosis of the intestinal mucosa associated with severe inflammation, invasion of enteric gas forming organisms, and dissection of gas into the bowel wall and portal venous system with at least one of the following clinical signs: bilious gastric aspirate or emesis, abdominal distention, occult or gross blood in stool without fissure; and one of the following radiographic findings: pneumatosis intestinalis, hepato-biliary gas, pneumoperitoneum |
| Patent ductus arteriosus | Failure of the ductus arteriosus to close within 72 h after birth, presenting with at least one of the flowing findings: left to right or bidirectional ductal shunt on Doppler echo, systolic or continuous murmur; and at least two of the following findings: hyperdynamic precordium, bounding pulses, wide pulse pressure |
| Pulmonary interstitial emphysema | Evidence of air entrapment along pulmonary bronchovascular bundles |
| Pneumonia | Radiographic and clinical evidence of a focal infectious process of lung parenchyma |
| Persistent pulmonary hypertension | Failure of the normal circulatory transition after birth characterized by marked pulmonary hypertension causing hypoxemia secondary to right-to-left shunting of blood at the foramen ovale and ductus arteriosus |
| Pneumothorax | Extraplural collection of air or gas diagnosed by chest radiograph or needle aspiration |
| Periventricular leukomalacia | An ischemic lesion leading to areas of coagulation necrosis in the periventricular white matter at the external angles of the lateral ventricles, in the watershed areas of the deep penetrating arteries of the middle cerebral artery, noted on a cranial ultrasound, CT, or MRI scan obtained at any time |
| Respiratory distress syndrome | PaO2 50 mmHg, or a requirement for supplemental oxygen to maintain a pulse oximeter saturation over 85% within the first 24 h of life, and a chest radiograph consistent with RDS (reticulogranular appearance to lung fields with or without low lung volumes and air bronchograms) within the first 24 h of life |
| Seizure | Clinical evidence of subtle seizures or focal or multifocal clonic or tonic seizures within the first 3 days after birth |
| Surgery | Surgical procedure other than for PDA ligation, retinopathy of prematurity, NEC, or bowel perforation |
| Anemia of prematurity | Hematocrit <30% |
| Thrombocytopenia | Platelet count <100 000/mcL |
| Chest compressions | External cardiac massage given in the delivery room or during the initial resuscitation performed immediately after birth |
| Intubation | Ventilation through an endotracheal tube in the delivery room or during the initial resuscitation performed immediately after birth |
| Continuous positive airway pressure | Non-invasive respiratory support delivered without endotracheal intubation by providing a continuous level of positive pressure to the airways which distends the lungs, overcomes collapse, and improves ventilation |
| Oxygen therapy | Any supplemental oxygen in the delivery room or during the initial resuscitation performed immediately after birth. |
| Positive pressure ventilation | Airway pressure applied at the airway causing gas to flow into the lungs until the ventilator breath is terminated |
| Retinopathy of prematurity | Any retinopathy of prematurity noted on ophthalmologic exam |
| Peripherally inserted central catheter | Peripherally inserted central catheter placement during hospitalization |
| Central venous catheter | Central venous catheter placement during hospitalization |
| Umbilical arterial or venous catheter | Umbilical arterial or venous catheter placement during hospitalization |
- Abbreviations: APTT, activated partial thromboplastin time; CT, computed tomography; GA, gestational age; MRI, magnetic resonance imaging; NEC, necrotizing enterocolitis; PDA, patent ductus arteriosus; PT, prothrombin time; RDS, respiratory distress syndrome.
- a Per institutional age adjusted norms for infants.
2.2 Blood sample collection and processing
Following delivery of the neonate, clamping and severing of the umbilical cord and prior to parturition umbilical cord blood samples were collected. The umbilical cord was clamped at two points and the umbilical vein was prepped and entered with a large-bore needle according to usual institutional procedure. Approximately 3 ml of blood was collected into citrated tubes, with a final 9:1 ratio of blood to anticoagulant and labeled with study subject identifiers. Routine clinical practices such as milking the umbilical cord and placental inversion were avoided to prevent maternal/fetal mixing and platelet/coagulation activation prior to blood collection.
Blood samples were gently, manually inverted for mixing citrate with blood and delivered by hand to the laboratory following sample acquisition. Platelet-poor plasma (PPP) was prepared by 3000 × g centrifugation for 10 min at room temperature per usual protocols. Plasma was then aliquoted, labeled, and stored at −80°C within 4 h of collection.
2.3 Coagulation assays
Prothrombin time, international normalized ratio (INR), APTT, and activity assays for factors II, VII, IX, X, were performed by standard methods on the Behring Coagulation Timer (BCT) analyzer (Siemens Healthineers). Factor XIII A subunit antigen was assessed using the Zymutest Factor XIII-A assay (Aniara), which is a manual microplate enzyme-linked immunosorbent assay (ELISA), according to the manufacturer’s protocol. Factor XIII activity was assessed using the Berichrom FXIII assay (Siemens Healthineers), which is a fully automated immunoassay.8 Additional coagulation factors VIII, XI, and von Willebrand factor, whose deficiencies are well known to be associated with bleeding, would have been evaluated; however, extremely limited cord blood sample volume on this patient population limited the ability to complete additional factor assays.
2.4 Clinical assessment and IVH
Clinical data were abstracted from the medical record including aforementioned VON clinical variables and presence or absence of IVH. Severity of IVH was graded via head ultrasound according to criteria described by Papile et al. per standard of care by clinical radiologists.9 However, statistical evaluation of IVH by clinical grade (1–4), location (unilateral or bilateral), and timing was unable to be performed due to limited numbers in each category. VON clinical variables were defined as outlined in Table 1. Presence of IVH was determined by pediatric radiologists as part of the clinical care of neonates per institutional protocol, and it is routine that all neonates <30 weeks GA have a head ultrasound as standard of care.1, 10
2.5 Statistical analysis
Normality distribution was checked for continuous variables. When investigating the relationships among IVH, VON clinical variables, and coagulation parameters, most did not meet normality assumptions; thereby, Wilcoxon two-sample test was used to compare differences in outcomes between presence or absence of IVH. For categorical variables, Chi-square or Fisher’s exact test was used to assess differences between two groups of neonates. Multivariate logistic regression included those VON clinical variables with P values of <.05 from univariate analyses results. Continuous variables are presented as means (standard deviations) and median (range), categorical variables as frequency and percentages. For analyses of death with coagulation parameters, we adjusted for type 1 error and used a more constrictive P value of .01 to account for the many comparisons. SAS 9.4 (SAS Institute Inc.) was used for all data analyses.
3 RESULTS
3.1 Demographics
One hundred twenty neonates were enrolled and included in analysis (Table 2). Of those, only two neonates were 30 weeks GA and the remainder were < 30 weeks GA. Throughout their NICU clinical course, 100 neonates survived, and 20 neonates died (Figure 1).
| VON clinical variable | Total | No IVH | + IVH | P value |
|---|---|---|---|---|
| N = 120 (%)a | N = 91 (75.8) | N = 29 (24.2) | ||
| Gestational age (weeks) mean (SD), median (range) | 27.1 (1.6), 27.1 (23–30) | 27.2 (1.6), 27.3 (23.4–30) | 26.8 (1.6), 26.6 (23.3–29.6) | .403 |
| Weight (grams) mean (SD), median (range) | 933.8 (281.7), 920 (375–1800) | 946.6 (292.7), 940 (375–1800) | 894.1 (245.1), 850 (460–1426) | .5978 |
| Sex, male | 60 (50) | 42 (70) | 18 (30) | .1355 |
| Sex, female | 60 (50) | 49 (81.7) | 11 (18.3) | .1355 |
| Congenital heart disease | 36 (30) | 30 (83.3) | 6 (16.7) | .209 |
| Anemia of prematurity | 97 (80.8) | 70 (72.2) | 27 (27.8) | .0539 |
| Chronic lung disease | 46 (38.3) | 30 (65.2) | 16 (34.8) | .0322 |
| Coagulopathy | 13 (10.8) | 8 (61.5) | 5 (38.5) | .3 |
| Gastrointestinal perforation | 7 (5.8) | 5 (71.4) | 2 (28.6) | .6751 |
| Hyperbilirubinemia | 104 (86.7) | 79 (76) | 25 (24) | 1 |
| Necrotizing enterocolitis | 5 (4.2) | 4 (80) | 1 (20) | 1 |
| Patent ductus arteriosus | 42 (35) | 29 (69.1) | 13 (30.9) | .2026 |
| Pulmonary interstitial emphysema | 5 (4.2) | 4 (80) | 1 (20) | 1 |
| Pneumonia | 12 (10) | 7 (58.3) | 5 (41.7) | .1592 |
| Persistent pulmonary hypertension | 8 (6.7) | 3 (37.5) | 5 (62.5) | .0197 |
| Pneumothorax | 9 (7.5) | 6 (66.7) | 3 (33.3) | .45 |
| Periventricular leukomalacia | 3 (2.5) | 2 (66.7) | 1 (33.3) | .5674 |
| Respiratory distress syndrome | 98 (81.7) | 73 (80.2) | 25 (86.2) | .4681 |
| Retinopathy of prematurity | 84 (70) | 60 (71.4) | 24 (28.6) | .0851 |
| Seizure | 7 (5.8) | 5 (71.4) | 2 (28.6) | .6751 |
| Surgery | 22 (18.3) | 17 (77.3) | 5 (22.7) | .8615 |
| Thrombocytopenia | 49 (40.8) | 35 (71.4) | 14 (28.6) | .3491 |
| Chest compressions | 4 (3.3) | 2 (50) | 2 (50) | .2458 |
| Intubation | 69 (57.5) | 49 (71) | 20 (29) | .1515 |
| Continuous positive airway pressure | 79 (65.8) | 58 (73.4) | 21 (26.6) | .3909 |
| Oxygen therapy | 55 (45.8) | 40 (72.7) | 15 (27.3) | .4647 |
| Positive pressure ventilation | 30 (25) | 23 (76.7) | 7 (23.3) | .902 |
| Peripherally inserted central catheter | 100 (83.3) | 75 (75) | 25 (25) | .779 |
| Central venous catheter | 13 (10.8) | 8 (61.5) | 5 (38.5) | .3 |
| Umbilical arterial catheter | 56 (49.1) | 38 (67.9) | 18 (32.1) | .0646 |
| Umbilical venous catheter | 90 (75) | 68 (75.6) | 22 (24.4) | .902 |
- Abbreviations: IVH, intraventricular hemorrhage; SD, standard deviation; VON, Vermont Oxford Network.
- Bold indicates statistical significant value.
- a Number in brackets is percent of total number of neonates for a particular clinical variable unless otherwise indicated, as in rows for gestational age and weeks of age.
3.2 Characterization and analysis of coagulation parameters
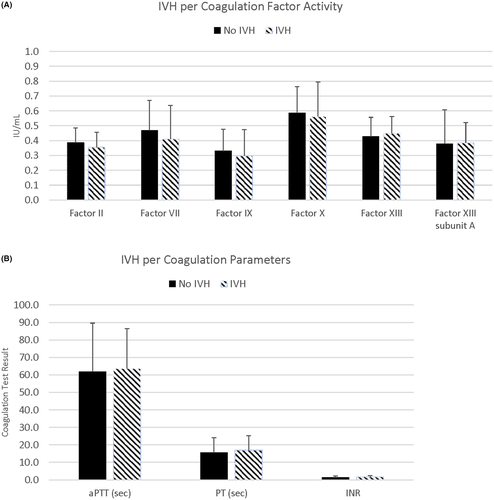
Non-clotted blood samples were obtained on enrolled patients, and prepared platelet poor plasma was assayed for PT; INR; APTT; factor II, VII, IX, X, and XIII activities; and factor XIII A subunit antigen, and were analyzed via GA (Table 3) and presence or absence of IVH (Table 4). Though 120 neonates were enrolled in the study, the number of individual coagulation tests performed on each neonate varied, as the blood sample volume was limited on very low birth weight, extremely premature neonate umbilical cord blood. Additionally, umbilical cord blood acquisition was prioritized for the clinical management of the neonate, with study sample blood obtained from residual cord blood available, which further limited sample available for analysis. Each individual coagulation parameter was evaluated for association with IVH, and there was no significant difference among coagulation tests in neonates with or without IVH (Figure 2A,B; Figure S1 in supporting information). Further, there was no significant difference within coagulation tests per GA.
| Coagulation test | 23–30 weeks GA | 23–24 weeks GA | 25–27 weeks GA | 28–30 weeks GA | ||||
|---|---|---|---|---|---|---|---|---|
| # neonates | Mean (SD) | # neonates | Mean (SD) | # neonates | Mean (SD) | # neonates | Mean (SD) | |
| Median (Range) | Median (Range) | Median (Range) | Median (Range) | |||||
| PT (sec) | 79 | 16 (8.2) | 10 | 13.86 (2.04) | 38 | 17.6 (10.5) | 31 | 14.7 (5.6) |
| 14 (10.2–56.6) | 13.35 (10.5–17) | 14.5 (10.2–56.6) | 13.8 (10.9–44.2) | |||||
| INR | 79 | 1.53 (0.77) | 10 | 1.35 (0.22) | 38 | 1.67 (1) | 31 | 1.41 (0.5) |
| 1.3 (0.9–5.6) | 1.3 (1–1.7) | 1.4 (0.9–5.6) | 1.3 (1–4) | |||||
| APTT (sec) | 77 | 62.7 (26.24) | 10 | 59.2 (17.9) | 36 | 60.7 (21.93) | 31 | 66.13 (32.65) |
| 58 (29–192) | 61.5 (30–89) | 57.5 (29–135) | 58 (41–192) | |||||
| Factor II IU/ml | 75 | 0.38 (0.1) | 9 | 0.38 (0.06) | 34 | 0.37 (0.13) | 32 | 0.38 (0.07) |
| 0.37 (0.05–0.69) | 0.40 (0.31–0.45) | 0.36 (0.05–0.69) | 0.38 (0.23–0.57) | |||||
|
Factor VII IU/ml |
83 | 0.45 (0.21) | 10 | 0.44 (0.14) | 38 | 0.46 (0.26) | 35 | 0.45 (0.15) |
| 0.43 (0.05–1.32) | 0.40 (0.26–0.75) | 0.42 (0.05–1.32) | 0.43 (0.20–0.93) | |||||
|
Factor IX IU/ml |
68 | 0.32 (0.15) | 9 | 0.33 (0.15) | 31 | 0.32 (0.19) | 28 | 0.31 (0.11) |
| 0.27 (0.00–0.86) | 0.33 (0.19–0.67) | 0.26 (0.00–0.86) | 0.32 (0.05–0.53) | |||||
|
Factor X IU/ml |
69 | 0.58 (0.19) | 9 | 0.54 (0.16) | 31 | 0.62 (0.23) | 29 | 0.55 (0.15) |
| 0.54 (0.23–1.20) | 0.51 (0.35–0.89) | 0.61 (0.23–1.20) | 0.54 (0.29–0.93) | |||||
|
Factor XIII IU/ml |
81 | 0.43 (0.12) | 10 | 0.46 (0.10) | 37 | 0.42 (0.11) | 34 | 0.44 (0.14) |
| 0.42 (0.08–0.76) | 0.44 (0.36–0.72) | 0.42 (0.19–0.76) | 0.45 (0.08–0.70) | |||||
| Factor XIII | 79 | 0.38 (0.20) | 9 | 0.40 (0.13) | 36 | 0.39 (0.23) | 34 | 0.36 (0.20) |
|
subunit A IU/ml |
0.34 (0.03–1.23) | 0.46 (0.23–0.57) | 0.36 (0.03–1.23) | 0.33 (0.03–0.74) | ||||
- Note: All factor values in IU/ml, where normal pooled plasma contains 1.0 IU/ml.
- Abbreviations: APTT, activated partial thromboplastin time; GA, gestational age; INR, international normalized ratio; PT, prothrombin time; SD, standard deviation; sec, seconds.
| Coagulation test | No IVH | + IVH | p value | ||
|---|---|---|---|---|---|
| # neonates | Mean (SD), median (Range) | # neonates | Mean (SD), median (Range) | ||
| PT (sec) | 55 | 15.6 (8.4), 13.6 (10.5–56.6) | 22 | 16.9 (8.3), 14.8 (10.2–44.2) | .3787 |
| INR | 55 | 1.5 (0.8), 1.3 (1–5.6) | 22 | 1.6 (0.8), 1.5 (0.9–4.0) | .3562 |
| APTT (sec) | 55 | 61.8 (27.8), 57 (29–192) | 20 | 63.6 (22.8), 59 (30–135) | .4634 |
|
Factor II IU/ml |
53 | 0.39 (0.1), 0.38 (0.05–0.69) | 21 | 0.36 (0.11), 0.34 (0.21–0.65) | .0915 |
|
Factor VII IU/ml |
59 | 0.47 (0.20), 0.45 (0.05–1.32) | 22 | 0.41 (0.23), 0.35 (0.05–0.96) | .1828 |
|
Factor IX IU/ml |
50 | 0.33 (0.15), 0.3 (0.05–0.86) | 17 | 0.30 (0.18), 0.26 (0.00–0.67) | .3669 |
|
Factor X IU/ml |
50 | 0.59 (0.18), 0.55 (0.27–1.02) | 18 | 0.56 (0.23), 0.55 (0.23–1.20) | .4267 |
|
Factor XIII IU/ml |
58 | 0.43 (0.13), 0.43 (0.08–0.76) | 21 | 0.45 (0.11), 0.42 (0.29–0.72) | .6781 |
|
Factor XIII subunit A IU/ml |
55 | 0.38 (0.23), 0.34 (0.03–1.23) | 22 | 0.39 (0.13), 0.40 (0.03–0.64) | .4521 |
- Note: All factor values in IU/ml, where normal pooled plasma contains 1.0 IU/ml.
- Abbreviations: APTT, activated partial thromboplastin time; INR, international normalized ratio; IVH, intraventricular hemorrhage; PT, prothrombin time; SD, standard deviation; sec, seconds.
One neonate was noted to have factor IX activity of 0.00 IU/ml, or <1%. The accuracy of this factor IX activity was confirmed with a low curve analysis (factor IX activity <0.10 IU/ml). This individual was a female born 25 4/7 weeks GA, had sepsis with development of chronic lung disease (CLD), and subsequently died of progressive CLD. A left grade 1 IVH was noted that resolved on subsequent imaging. There was no documentation of additional coagulopathy or bleeding complications throughout her 3 months of life, and an APTT and factor IX activity were not obtained in the clinical hospital laboratory. Unfortunately, factor IX activity in our study was determined after acute hospitalization and the family was lost to follow-up, making additional laboratory investigation of the patient or family impossible. There were no neonates in the study who were subsequently diagnosed with a congenital disorder of hemostasis or thrombophilia per our knowledge.
3.3 Mortality and coagulation parameters
Analysis for association of death from all causes and coagulation parameters was completed. Only reduced factor XIII subunit A showed significant association with death, P = .003. Those neonates that died during their NICU course had a factor XIII subunit A of 0.26 IU/ml interquartile range (IQR) [0.20, 0.31] versus those that were alive has a factor XIII subunit A of 0.40 IU/ml IQR [0.29, 0.51]. There was no statistical significance found between death and IVH.
3.4 Clinical variable analysis for association with IVH
3.4.1 IVH severity grade
Severity of IVH was graded via head ultrasound according to standard criteria.9 In total, 14 neonates experienced grade 1 IVH, 5 neonates experienced grade 2 IVH, 7 neonates developed grade 3 IVH, and 3 neonates suffered grade 4 IVH. The small number of specific severities of IVH limited the ability to perform further statistical analysis associated with IVH severity grade.
3.4.2 Risk factors for IVH
VON clinical variables were analyzed for association with IVH (Table 2) and were analyzed by Fisher exact test and Chi square. For all neonates, CLD and persistent pulmonary hypertension (PPHN) were significantly associated with IVH, and anemia of prematurity approached significance. Univariate (unadjusted) logistic regression revealed for CLD, odds ratio (OR) 2.5 (95% confidence interval [CI] 1.1–5.9), P = .0322 and PPHN, OR 6.1 (95% CI 1.4–27.4), P = .0197; and anemia of prematurity, OR 4.1 (95% CI 0.9–18.5), P = .0539 approached significance (Table 5). Multivariate (adjusted) logistic regression included those VON clinical variables with P values of <.05 from univariate analyses results. Based on these criteria, CLD and PPHN were entered into the statistical model. We used Fisher's Exact test and p-value = .2573, which demonstrated there were not statistically significant associations between CLD and PPHN. The final analyses indicated that only PPHN independently predicted IVH risk with OR 5.3 (95% CI 1.1–24.3), P = .0338; and CLD approached significance with OR 2.3 (95% CI 0.9–5.5), P = .0659 (Table 5). Other clinical variables evaluated (Table 1) were not significantly associated with IVH.
| VON clinical variable | No IVH | + IVH | Univariate (unadjusted) results | Multivariate (adjusted) results | ||
|---|---|---|---|---|---|---|
| P value | OR (95% CI) | p value | OR (95% CI) | |||
| Persistent pulmonary hypertension | 3 (37.5%) | 5 (62.5%) | .0197 | 6.1 (1.4–27.4) | .0338 | 5.3 (1.1–24.3) |
| Chronic lung disease | 30 (65.2%) | 16 (34.8%) | .0322 | 2.5 (1.1–5.9) | .0659 | 2.3 (0.9–5.5) |
| Anemia of prematurity | 70 (72.2%) | 27 (27.8%) | .0539 | 4.1 (0.9–18.5) | N/A | N/A |
- Abbreviations: CI, confidence interval; IVH, intraventricular hemorrhage; OR, odds ratio; VON, Vermont Oxford Network.
4 DISCUSSION
The coagulation system begins to develop around 10 weeks GA in utero, and continues to progress and mature with advancing GA in neonates.3, 4, 11-13 This process continues postnatally in infants, until moving toward adult levels around 6 months of age.4, 12 Neonatal coagulation can be conceptualized as dynamic, as a majority of healthy term newborn infants do not experience spontaneous hemorrhagic or thrombotic complications. Therefore, though coagulation protein concentrations may be lower than those of adults, balanced hemostasis is usually maintained.
Nevertheless, it is recognized that neonates have increased bleeding vulnerability related to congenital or acquired hemostatic disorders.14 The process of labor and mode of delivery have been shown to contribute significant effects on the coagulation system with higher coagulant and anticoagulant protein levels in infants who were affected by the stress of labor versus those delivered per elective caesarian section.15 In the extremely premature neonate delivered via caesarian section, one may hypothesize that there would be increased risk in suffering a hemostatic insult due to coagulation system immaturity and lower coagulation factor levels; however, supporting data are lacking.
Herein, we report a large, prospective study of laboratory coagulation parameters on cord blood samples in extremely premature neonates. Interestingly, we observed that our data signaled generally reduced coagulation parameters compared to day 1 of life healthy premature infants 30–36 weeks GA reference values per Andrew et al.3 for coagulation factor activities: factors II, VII, IX, and factor XIII subunit A; and generally increased coagulation parameters for factor X activity, PT (sec) and APTT (sec) as outlined in Table S1 in supporting information. These differences may be explained by developmental hepatic and coagulation system immaturity, and possibly due to additional comorbidities experienced by our cohort of 23–30 week GA infants versus reportedly healthy 30–36 week GA infants.3 Further, our investigation evaluated cord blood samples at the time of birth compared to those obtained via peripheral blood by Andrew et al.3, 4 There may be additional coagulation activation and coagulation factor consumption at the time of birth, which may be missed if later sampling peripheral blood, further contributing to these differences. We observed median coagulation parameters in our cohort were comparable to those reported by Neary et al. in <28 week GA neonates6 (Table S1). Coagulation parameters for factor X, factor XIII, and factor XIII subunit A were not evaluated by Neary’s group, and they similarly evaluated umbilical cord blood drawn at birth.5, 6 Additional statistical evaluation of these observations was unable to be completed due to lack of available data and small sample sizes.
Factor XIII testing was included in our analysis as Andrew et al. described factor XIII subunit A and subunit B parameters in healthy full term and preterm 30–36 weeks GA, but not <30 weeks GA,3, 4 and since congenital absence of factor XIII leads to considerable bleeding diathesis with significant risk for intracranial hemorrhage.16 Factor XIII is critical in stabilizing the fibrin monomer by facilitating cross-linking and congenital absence of factor XIII was first described in the 1960s.17, 18 Our study was notable for lower factor XIII subunit A having significant association with death from all causes, but not associated with IVH specifically. The only evidence to date suggesting factor XIII may have a therapeutic role in the prevention of IVH was reported by Shirahata et al. in 1990.19 In a limited prospective study, premature neonates were randomized to standard care or standard care with a single dose of a plasma-derived FXIII concentrate (Behringwerke AG). Neonates that received factor XIII concentrate within 6 h of delivery had a lower incidence of IVH 15% (2 of 13) versus 75% (6 of 8) in control subjects. The authors demonstrated a consistent rise in factor XIII activity after infusion. In our analysis factor XIII activity was reduced compared to normal adult levels and factor XIII subunit A antigen was reduced compared to normal adult levels and those in premature infants 30–36 weeks GA reported by Andrew et al.,3 but no association with IVH was found. Potential therapeutic benefit of factor XIII concentrate administration to prevent IVH remains unknown. Additional pharmacological interventions aimed at reducing the incidence and severity of IVH have been investigated including: phenobarbital, pancuronium bromide, ethamsylate, vitamin E, indomethacin, ibuprofen, and factor VIIa.1, 20-22 Though these therapies may have seemed promising, none have become established agents in the prevention or reduction of severity of IVH in premature neonates.
Our investigation further evaluated clinical risk factors associated with IVH in this vulnerable population. We demonstrated that PPHN was independently and significantly associated with IVH. Neonates with PPHN were 5.3 times more likely to develop IVH. These data suggest that tenuous hemodynamic flow associated with this clinical condition may alert the clinician to the increased risk of IVH. Surprisingly, thrombocytopenia and chest compressions were not shown to be independently associated with IVH in our current study as we demonstrated previously in a retrospective cohort study; however, that investigation included >600 infants <30 weeks GA.23
4.1 Limitations
In total, our study included 120 extremely premature neonates; however, only 29 experienced IVH (Table 2). The number of neonates with IVH for each coagulation test analyzed ranged from 17 to 22 (Table 4), and the number of neonates with IVH for which VON clinical variables were associated with IVH—PPHN, CLD, and anemia of prematurity—were 5, 16, and 27, respectively (Table 5). Additionally, very low birth weight, extremely premature neonates have limited umbilical cord blood available, and sample acquisition was prioritized for neonate clinical management with extra blood utilized for study sample analysis. This reduced sample procurement for analysis narrowed abundance of coagulation tests performed and could lead to potential bias of coagulation tests assayed. Further, this limited number of IVH, and those individual coagulation tests performed in each developmental GA cohort and in the entire study population reduced the probability of detecting statistically significant associations with IVH and lower statistical power overall.
5 CONCLUSIONS
In summary, we present a large, prospective study of laboratory coagulation parameters on cord blood samples in extremely premature neonates <30 weeks GA. Furthermore, to our knowledge coagulation parameters for factor X, factor XIII, and factor XIII subunit A have not been previously described in this patient population. Though each individual coagulation parameter was evaluated for association with IVH, there was no significant difference among coagulation tests in neonates with or without IVH and per GA. However, evaluation of clinical risk factors was significant for PPHN independent association with IVH. Our results have potential to impact international neonatal clinical practice and should encourage additional study in this vulnerable population.
ACKNOWLEDGMENTS
The authors thank all the patients and their families, University of Illinois College of Medicine at Peoria (UICOMP) and the OSF St. Francis Children’s Hospital of Illinois (CHOI) neonatal intensive care unit staff and physicians. This work was supported by an investigator-initiated sponsored study research grant from Novo Nordisk. The sponsor had no role in study design, collection, analysis, interpretation of the data, writing of the manuscript, or decision to submit the article for publication.
AUTHOR CONTRIBUTIONS
JCR and MDT conceptualized and designed the research, collected data, and analyzed results. JCR drafted the initial article. RMB performed laboratory studies. HW performed statistical analysis. JCR, MJJ, MKL, RMB, HW, and MDT contributed to study design, reviewed and revised the manuscript, and approved the final article as submitted.
CONFLICTS OF INTEREST
JCR has received grant funding from Novo Nordisk for an investigator-initiated research grant and has served as a consultant and speaker for Novo Nordisk related to hemophilia and rare bleeding disorders. MDT has received grant funding from Novo Nordisk for an investigator-initiated research grant and has served as a consultant and speaker for Novo Nordisk. All other authors declare no competing financial interests.