International Society on Thrombosis and Haemostasis core curriculum project: Core competencies in laboratory thrombosis and hemostasis
Abstract
Background
Laboratory analyses of blood samples are essential for diagnostics and therapy monitoring of patients with bleeding and thromboembolic diseases. Following publication of the core curriculum for clinical thrombosis and hemostasis, the International Society on Thrombosis and Haemostasis (ISTH) recognized that thrombosis and hemostasis laboratory specialists require distinct competencies that differ from medical doctors working clinically with patients. To address this gap the ISTH formed a working group of international hemostasis and thrombosis laboratory specialists to develop an evidence-based core curriculum for laboratory specialists.
Objective
This research sought consensus from the international community on core competencies required for laboratory specialists in thrombosis and hemostasis.
Methods
A draft list of 64 competencies was developed and an online stakeholder survey was circulated electronically to 15 302 ISTH members and contacts in the wider international community. The results were analyzed and used to develop the final approved core curriculum.
Results
Three hundred and thirty responses contained meaningful data, with broad international representation of specialists. No draft competencies were excluded, and 58 were rated as “does” or “shows how.” The Leik measure of consensus for most competences was “moderate” (n = 30) or “fair” (n = 32).
Conclusions
The development of an international core curriculum for laboratory specialists provides a foundation for the development and enhancement of education and quality management of the laboratory. Although there is no formal designation for laboratory specialists, international governing bodies and regulatory organizations are encouraged to consider the diagnostic core curriculum for development and accreditation of more standardized educational programs and formal assessment across jurisdictions.
Essentials
- To develop a global core curriculum for laboratory specialists in thrombosis and hemostasis.
- Core competencies developed by experts from literature and through an international survey.
- First evidence-based framework of global core competencies for laboratory specialists in this area.
- A reference for curriculum mapping, and educational and strategic development across jurisdictions.
1 INTRODUCTION
The International Society of Thrombosis and Haemostasis (ISTH) continually promotes high quality standards within the field of thrombosis and hemostasis through standardization and extensive collaboration among clinicians, researchers, and staff of diagnostic coagulation laboratories. With the growth and interest in this area, numerous regional societies have evolved to standardize practice within specific jurisdictions. These regional societies strive to standardize practice within a specific jurisdiction, which include national regulations that may not be shared or followed by other jurisdictions. Furthermore, the diagnostic portfolio and specific competencies of a clinical coagulation laboratory reflect local requirements; medical laboratory practices; as well as available equipment, reagents, and technology.
The development and use of core curricula are an accepted means of defining what individuals should learn and be able to do, and have been successfully implemented in many countries in all fields of health care and scientific education and practice.1-4 Competencies were felt to be the most appropriate format for defining the ISTH core curriculum in clinical thrombosis and hemostasis, because they refer to what an individual should be able to do, irrespective of how, when, or through what training program or experience they learned how to do it.5 The ISTH recognized a need and an opportunity to provide guidance to the international community for a continuum and standardization of the diagnostics in thrombosis and hemostasis. To this end, the ISTH originally undertook an initiative to draft and then gain consensus from the international community on a set of core competencies for clinical specialists taking care of patients with disorders of thrombosis and hemostasis.5 This published curriculum provides an international consensus framework for the minimum level of knowledge required for independent practice of clinical specialists. The completion of this project met the stated 2013 ISTH Council's priority for the development and application of core clinical competencies to ensure good standard practice and harmonization of training internationally.
While undertaking the clinical core curriculum project, it became apparent that specialists involved in laboratory diagnostic hemostasis and thrombosis require a different set of competencies from their clinical colleagues. It was evident that there are significant regional differences in diagnostic pathways, competencies, and available tests, and a gap in the literature in terms of defining and seeking international consensus on these areas. The current research seeks to address these shortcomings.
The use and interpretation of coagulation laboratory results are an integral part of the diagnosis and treatment of disorders affecting hemostasis and thrombosis and laboratory specialists provide practitioners with clinically meaningful laboratory interpretations. There is, however, minimal guidance on the international training of the individuals producing coagulation measurements and providing diagnostic interpretations. The European Federation of Clinical Chemistry and Laboratory Medicine has established a working group on Patient Focused Laboratory Medicine to determine a strategy to provide appropriate interpretative comments for all laboratory results to allow for patient understanding.6 The German-speaking Society of Thrombosis and Haemostasis Research (GTH) 7 also established a permanent working group to standardize and improve technology and interpretation of coagulation analyses. Moreover, the Argentine Cooperative Group on Haemostasis and Thrombosis (CAHT Group)8 created a Laboratory Working Group to identify difficulties and needs faced by hemostasis laboratories, as well as discuss and agree on actions to improve performance. International harmonization also improves quality of laboratory diagnostics.9, 10 Strategies for selecting appropriate tests and performing state-of-the-art diagnostic procedures to include appropriate and meaningful interpretation of hemostasis and thrombosis laboratory results are essential to ensure that referring clinician and patients understand the results, especially as the selection of optimized diagnostic pathways and reporting of laboratory results to patients will become more commonplace moving forward.6, 11
While some medical regulatory bodies identify a limited number of thrombosis and hemostasis laboratory competencies for medically trained individuals, non-physician trained specialists are not regulated through the same governing bodies as physicians, thus minimal guidance is provided by regional and national governing bodies on expectations for a thrombosis and hemostasis laboratory specialist. Published literature is limited related to competencies in the area of thrombosis and hemostasis, and there is a need for collaborative approaches with professional organizations, academia, and policy makers to build leadership and competencies within laboratory medicine, especially given the continual introduction of novel technologies into laboratories.12 Furthermore, rational and harmonized selection of tests and diagnostic pathways is economically advisable and provides opportunity to compare diagnostic outcome and patient care.
The aim of this project was to collect data on the international perspective, obtain consensus, and inform the international community on the core competencies for a diagnostic thrombosis and hemostasis laboratory specialist.
2 METHODS
This study was undertaken by the Laboratory Core Curriculum Working Group of the ISTH. The Working Group members (co-authors on this paper) were invited by the ISTH Education Committee. The Committee reviewed recommendations of laboratory specialists and chose global representatives to allow for an international perspective in the area of laboratory thrombosis and hemostasis. Members included PhDs, MDs, and laboratory technical specialists in the area of diagnostic thrombosis and hemostasis. A modified version of the methodology used in the Tuning Project (Medicine) was used to successfully gain consensus in the ISTH Clinical Core Curriculum and other previous studies.5, 13, 14 A review of current literature was undertaken, and a roundtable draft framework of core competencies was compiled. An electronic survey was drafted and distributed electronically to ISTH members using SurveyGizmo® (SurveyGizmo, Bolder, CO, USA). The proposal was discussed with the ISTH Ethics Committee, who concluded formal ethics approval for the questionnaire was not required as no personal identification information was collected or required to complete the survey.
2.1 Literature review and synthesis
The Working Group members were asked to identify existing curricula that encompassed laboratory testing in the area of thrombosis and hemostasis, which were reviewed and critiqued. The documents reviewed included various national guidelines and documentation from the Royal College of Physicians and Surgeons of Canada, the Royal College of Pathologists of Australasia, the Canadian Academy of Clinical Biochemistry, the British Society of Haematology, the Japanese Society for Laboratory Hematology,15-20 as well as various local institutional guidelines and training programs that included McMaster University's (Hamilton, Canada) General Pathology, Pediatric Hematology and Oncology, and Medical Biochemistry fellowship programs. In addition, a PubMed search was undertaken using the search terms: thrombosis AND hemostasis OR haemostasis OR laboratory OR competencies.
2.2 Stakeholder survey
At a face-to-face meeting in Berlin, Germany in July 2017, using the regional-/country-specific guidelines and brainstorming sessions, a draft competency framework consisting of 64 discrete competencies was developed. This survey was created in SurveyGizmo® and distributed to ISTH membership and community contacts (n = 15 302) by e-mail, through a collaborative member society—the International Society of Laboratory Hematology (ISLH)—and advertised on the ISTH website (www.isth.org).
Respondents were asked to indicate to what extent they considered that each competency should have been achieved by a laboratory specialist in thrombosis and hemostasis, who had completed their training and was ready to practice independently in the field. A rating scale was developed based on Miller's triangle,21 in which respondents indicated:
- Not learned—does not need to achieve this by the end of specialist training
- Knows—knows about it and is able to demonstrate their understanding of the appropriate basic sciences/is aware of the issues
- Knows how—is able to explain how and why they would do this/understands the principles
- Shows how—is able to demonstrate their competence in this in a simulated situation or artificial scenario
- Does—is able to demonstrate mastery of this in a real situation/does this consistently
Demographic information was also collected from respondents, including country of practice; type of workplace; primary area of focus; years in thrombosis and hemostasis field; and primary academic discipline. This survey was open between December 2017 and January 2018. Responses were analyzed statistically using Excel and SPSS, and free-text comments were analyzed thematically.
2.3 Statistical analysis
The Likert scores were converted from words to numbers (1-5 as above) and the mean rating and standard deviation were calculated for each competency and for large subgroups of respondents (clinical/diagnostic lab versus other primary focus; hospital-based versus non-hospital-based; European versus non-European; and up to 20 years versus more than 20 years working in this field). The subset of respondents who answered all questions were used to compare the subgroup ratings using the intraclass correlation coefficient (ICC), to determine whether different groups rated the competencies similarly and whether any individual group could have skewed the overall ratings. The subset who answered all questions was also used to calculate the Leik measure of consensus for each competency, to indicate the level of agreement on their rating.22
2.4 Final analysis and framework development
Competencies were first arranged according to the mean rating of the Likert scores to identify whether any at the bottom should be omitted from the final framework, and according to the percentage of “does” responses to determine the cut-off between “does” and “shows how.” Finally, they were arranged according to the percentage of responses which were either “does” or “shows how,” to determine the cut-off between “shows how” and “knows how.” The Working Group reviewed each of these lists, and selected a cut-off, which seemed to have face validity, with particularly careful consideration of those competencies which borderline on either side of these cut-offs. The Working Group then considered the sequence of all the competencies and decided they should be left in the systematic order in which they had been presented for the survey. Finally, the proposed framework and findings were reviewed and approved by the ISTH Council.
3 RESULTS
3.1 Demographics
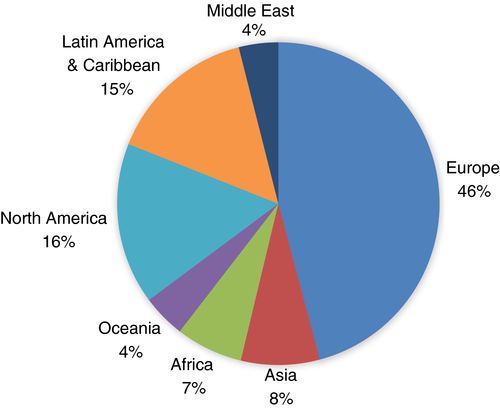
A total of 330 surveys that contained meaningful data were received from around the world. An equal number of respondents (both n = 141) indicated they primarily worked in a clinical/diagnostic laboratory compared to those primarily working in other areas (eg research or clinical/patient care). Approximately one third (35%; n = 99 of 283) of responders were hospital-based. The majority of participants had up to 20 years of experience in the field of laboratory thrombosis and hemostasis (n = 159), and a significant proportion had more than 20 years of experience (n = 122). Breakdown of the respondents by geographical region of practice is shown in Figure 1.
3.2 Quantitative data analysis
The mean Likert scores for all competencies were similar across the large subgroups of respondents that had been identified (Table 1). Europeans had a slightly higher mean rating across most competencies compared to non-Europeans, although for a single competency the largest difference between them was only 0.49 points on the Likert scale. The largest difference in mean ratings for a competency between those working in a clinical/diagnostic laboratory and those working elsewhere was 0.39; between those not based in a hospital and those hospital-based was 0.37; and between those with up to 20 years and those with more than 20 years of experience in the field was 0.32.
| Group | Number of respondents | Combined means and (SD) of all item ratings | Minimum-maximum of item mean ratings |
|---|---|---|---|
| All | 330 | 4.11 (1.07) | 3.27-4.58 |
| Clinical/diagnostic lab | 141 | 4.19 (0.31) | 3.32-4.69 |
| Primary focus not clinical/diagnostic lab | 141 | 4.05 (0.25) | 3.25-4.61 |
| Hospital-based | 99 | 4.12 (0.32) | 3.28-4.68 |
| Not hospital-based | 184 | 4.12 (0.26) | 3.22-4.60 |
| European | 116 | 4.23 (0.31) | 3.34-4.81 |
| Non-European | 137 | 4.06 (0.26) | 3.21-4.52 |
| 0-20 years in field | 159 | 4.07 (0.27) | 3.25-4.58 |
| >20 years in field | 122 | 4.18 (0.28) | 3.34-4.69 |
- Abbreviation: SD, standard deviation.
The ICC was calculated for each pairing using the subset of respondents (n = 234) who rated every single competency, Table 2. Values of > 0.9 indicate “strong” agreement, and 0.8-0.89 indicate “high” agreement. The findings demonstrate the different subgroups responded similarly to the survey and no subgroup significantly skewed the overall ratings.
| Comparison of ratings of individual subgroups | Intraclass correlation coefficient – absolute agreement (95% CI) |
|---|---|
| Clinical/diagnostic lab vs other primary focus | 0.88 (0.21-0.96) = “High” agreement |
| Hospital-based vs non-hospital-based | 0.91 (0.83-0.95) = “Strong” agreement |
| European vs non-European | 0.80 (0.16-0.94) = “High” agreement |
| Up to 20 years vs more than 20 years in field | 0.97 (0.94-0.98) = “Strong” agreement |
- Abbreviation: CI, confidence interval.
The Leik measure of consensus was also calculated for each of the 64 competencies using the subset of respondents who rated every one of them (n = 234), and is shown in Table 3. Of these, 30 showed “moderate” consensus (Leik 0.41-0.60), and 32 “fair” consensus (0.21-0.40). Two showed “poor” consensus (≤0.20), which were “Use baseline laboratory tests including full/complete blood count (FBC/CBC), platelet count (PC), blood film, prothrombin time (PT), activated partial prothrombin time (APTT), fibrinogen, and thrombin time (TT) to screen for coagulation disorders” and “Explain screening test results and advise on further testing if appropriate.”
| Competency | Mean all ratings (1-5) | Does % | Shows + does % | Category | Leik consensus | Likert graph (ratings 1-5) |
|---|---|---|---|---|---|---|
| 1. The role of the laboratory specialist in thrombosis and hemostasis | ||||||
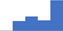
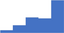
| Apply knowledge of the biochemistry, physiology and pathophysiology of the coagulation, fibrinolytic, platelet and vascular systems. | 3.93 | 46.6% | 62.7% | Shows how | 0.49 |
|
| Demonstrate competencies in areas with implications for thrombosis and hemostasis such as inflammation, imaging and genetics. | 3.27 | 18.6% | 39.8% | Knows how | 0.44 |

|
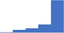
| Recommend appropriate tests in the thrombosis and hemostasis laboratory and point of care testing for diagnosis or therapeutic monitoring in clinical settings. | 4.47 | 68.4% | 85.1% | Does | 0.21 |
|
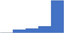
| Provide a clinically useful interpretation of the laboratory and point of care results. | 4.46 | 69.4% | 83.6% | Does | 0.22 |
|
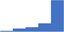
| Provide an interface between the laboratory and the clinical coagulation service. | 4.40 | 66.0% | 83.3% | Does | 0.25 |
|
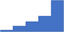
| Provide leadership, training and mentorship. | 4.06 | 48.3% | 72.4% | Shows how | 0.43 |
|
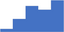
| Undertake basic, applied and/or diagnostic research related to the scope of a clinical thrombosis and hemostasis laboratory. | 3.70 | 33.1% | 56.3% | Knows how | 0.47 |
|
| Manage a diagnostic laboratory including budget, prioritization of testing and resources, laboratory workflow. | 3.93 | 45.0% | 65.0% | Shows how | 0.46 |
|
| 2. Basic laboratory principles | ||||||
| Incorporate and manage an internal quality control system of a laboratory. | 4.39 | 66.1% | 80.9% | Does | 0.26 |
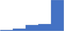
|
| Incorporate and manage an external quality control/assessment (proficiency testing) program of a laboratory. | 4.23 | 57.7% | 74.9% | Does | 0.32 |

|
| Manage the development of, implementation of, and adherence to laboratory standard operating procedures. | 4.34 | 62.9% | 81.1% | Does | 0.27 |
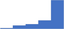
|
| Seek and maintain laboratory accreditation. | 4.10 | 51.3% | 73.1% | Shows how | 0.41 |
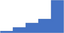
|
| Manage health and safety in laboratory practice. | 4.29 | 58.3% | 79.3% | Does | 0.34 |
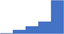
|
| Develop and apply appropriate reference intervals, to include pediatric, adult and gender specific as appropriate for thrombosis and hemostasis laboratory testing. | 4.22 | 55.8% | 75.7% | Does | 0.32 |
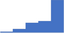
|
| Demonstrate understanding of the strengths and limitations, including approaches for troubleshooting, of laboratory tests in the diagnosis and therapeutic monitoring in patients with thrombotic and hemostatic disorders. | 4.29 | 58.2% | 82.3% | Does | 0.26 |
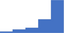
|
| Manage the installation and validation of new diagnostic instruments. | 3.97 | 43.0% | 68.7% | Shows how | 0.42 |
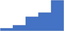
|
| Manage the installation and validation of new diagnostic tests including evaluations of new reagent charges. | 4.12 | 51.3% | 74.0% | Shows how | 0.40 |
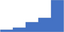
|
| Perform appropriate validation studies to ensure clinically relevant reagent sensitivities such as APTT reagent with sensitivity to intrinsic factors and Lupus Anticoagulant and monitoring heparin; PT reagent for accurate ISI determination and geometric mean calculation (mean normal PT). | 4.21 | 53.8% | 77.2% | Does | 0.36 |
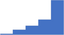
|
| 3. Pre-analytical variables | ||||||
| Apply an understanding of the impact of how conditions of sample collection (eg sample type, appropriate anticoagulant, venipuncture procedure, sampling time, sequence of sampling and contamination issues, tube volume, effects of hematocrit) and transport (temperature, time, activation) influence laboratory tests. | 4.36 | 62.6% | 80.8% | Does | 0.28 |
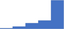
|
| Apply an understanding of how factors that impact sample preparation and storage prior to analysis (eg stability of analyte, centrifugation, impact of temperature on storage) influence laboratory tests. | 4.36 | 61.9% | 81.4% | Does | 0.29 |
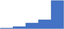
|
| Apply an understanding of the patient factors that influence laboratory testing (eg age, gender, race, blood group, pregnancy, fasting, exercise, storage time, impact of/effect of medication, time from event such as deep vein thrombosis or bleeding). | 4.29 | 58.5% | 78.3% | Does | 0.34 |
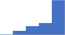
|
| 4. Initial assessment of hemostasis | ||||||
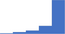
| Use baseline laboratory tests including FBC/CBC, PC, blood film, PT, APTT, fibrinogen, and TT to screen for coagulation disorders. | 4.58 | 76.1% | 87.7% | Does | 0.16 |
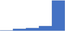
|
| Perform a basic laboratory assessment of primary hemostasis (eg bleeding time or platelet function assay). | 4.40 | 63.9% | 83.2% | Does | 0.27 |
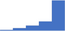
|
| Demonstrate understanding of the limitations for clinical use of the hemostasis analyses results, eg the limitations of the derived fibrinogen. | 4.29 | 59.5% | 79.4% | Does | 0.31 |
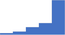
|
| Explain screening test results and advise on further testing if appropriate. | 4.55 | 71.9% | 88.6% | Does | 0.20 |
|
| 5. Specialized testing for bleeding disorders | ||||||
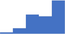
| Refer to the ISTH SSC for recommendations for all specialized hemostasis testing for inherited and acquired bleeding disorders. | 3.99 | 43.6% | 66.2% | Shows how | 0.42 |
|
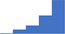
| Formulate follow-up testing as appropriate based on the results of specialized testing for bleeding disorders. | 4.24 | 51.9% | 80.2% | Does | 0.34 |
|
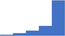
| Perform appropriate and validated mixing studies in patients with a prolonged PT, APTT, and/or TT to distinguish between factor deficient state or the presence of an inhibitor. | 4.42 | 66.1% | 84.3% | Does | 0.28 |
|
| Carry out appropriate tests to distinguish between a specific or non-specific inhibitor (eg Lupus Anticoagulant) and quantify a Bethesda titre. | 4.29 | 57.2% | 80.7% | Does | 0.32 |
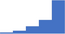
|
| Investigate for specific factor deficiencies using a systematic approach that takes into consideration reagent factor and Lupus Anticoagulant sensitivities (eg elevated APTT, normal PT). | 4.40 | 62.7% | 83.8% | Does | 0.28 |
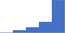
|
| Apply understanding of the difference between one-stage and chromogenic (or two-stage) factor (F) VIII and FIX assays in the diagnosis and monitoring of patients with a deficiency state and substitution therapies. | 4.11 | 46.1% | 74.6% | Shows how | 0.40 |
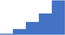
|
| Determine activity and antigen level in the setting of fibrinogen or FII deficiency. | 3.92 | 36.3% | 64.8% | Shows how | 0.39 |

|
| Test for rare bleeding disorders and abnormalities not detected during screening (eg FXIII deficiency, FXI deficiency/Hemophilia C or fibrinolytic abnormalities) in patients with clinically significant bleeding. | 4.05 | 41.1% | 72.3% | Shows how | 0.37 |
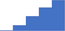
|
| Perform and interpret comprehensive qualitative and quantitative testing for VWD, including antigen and activity analyses (eg Ristocetin-dependent and independent functional von Willebrand factor assays). | 4.26 | 54.7% | 78.2% | Does | 0.35 |
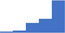
|
| Perform appropriate aggregation mixing studies to differentiate between type 2B VWD and platelet type VWD. | 3.86 | 36.5% | 64.2% | Shows how | 0.42 |
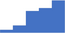
|
| Assess platelet function (aggregation, secretion, flow cytometry analysis). | 3.99 | 41.6% | 70.8% | Shows how | 0.40 |
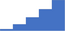
|
| Carry out appropriate tests for monitoring of platelet inhibitor therapies. | 3.84 | 36.4% | 62.6% | Shows how | 0.43 |

|
| Apply understanding of genetic testing in diagnosis of bleeding disorders (eg congenital hemophilia, VWD). | 3.68 | 30.3% | 55.6% | Knows how | 0.44 |

|
| Apply understanding of the function, limitations and applications of global tests for bleeding states (eg thromboelastography/-metry, thrombin generation). | 3.88 | 36.5% | 63.5% | Shows how | 0.41 |

|
| 6. Specialized testing for thrombotic disorders | ||||||
| Refer and employ, where appropriate, ISTH-SSC and other international guidelines and recommendations for all specialized coagulation testing for thrombotic disorders. | 4.09 | 50.2% | 70.5% | Shows how | 0.41 |
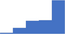
|
| Identify the limitations (eg strengths, weaknesses) of specialized testing for thrombotic disorders. | 4.19 | 54.2% | 75.9% | Does | 0.34 |
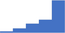
|
| Formulate follow-up testing as appropriate based on the results of specialized testing for initial and recurrent thrombotic events. | 4.21 | 53.2% | 77.8% | Does | 0.33 |
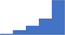
|
| Interpret functional and genetic tests used for the diagnosis of acquired and congenital inhibitor deficiencies (eg antithrombin, protein C, protein S, tissue factor pathway inhibitor). | 4.15 | 50.3% | 74.1% | Shows how | 0.39 |
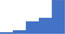
|
| Perform functional and genetic testing of congenital and acquired prothrombotic factors (eg FV Leiden, Prothrombin G20210A, JAK2). | 3.78 | 34.5% | 60.2% | Knows how | 0.43 |

|
| Interpret functional and genetic testing of congenital and acquired prothrombotic factors. | 4.07 | 47.0% | 72.1% | Shows how | 0.43 |
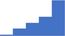
|
| Perform and interpret laboratory tests used for the diagnosis of the antiphospholipid syndrome. | 4.42 | 63.4% | 84.5% | Does | 0.26 |
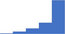
|
| Perform and interpret laboratory tests used for the therapeutic monitoring in patients taking vitamin K antagonists, heparins, antiplatelet drugs and DOACs. | 4.40 | 63.7% | 82.0% | Does | 0.26 |
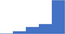
|
| Apply knowledge on the use and limitations of tests for activated coagulation status (eg D-Dimer, prothrombin fragment 1 + 2, Thrombin-Antithrombin complex). | 4.18 | 49.3% | 76.2% | Shows how | 0.38 |
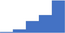
|
| Apply understanding of the function, limitations and applications of global tests for thrombotic and fibrinolytic disorders (eg thromboelastography/-metry, thrombin generation). | 3.90 | 36.2% | 66.0% | Shows how | 0.40 |
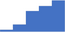
|
| Perform and interpret laboratory tests for the assessment of the fibrinolytic system (eg plasminogen, plasminogen activator inhibitor-1, alpha-2-antiplasmin, thrombin activatable fibrinolysis inhibitor), and for monitoring fibrinolytic therapy. | 3.74 | 31.3% | 59.5% | Knows how | 0.42 |

|
| Apply knowledge of new developments eg flow-based systems, flow cytometry, to diagnostic practice in thrombosis and hemostasis. | 3.49 | 27.1% | 47.5% | Knows how | 0.51 |

|
| 7. Apply understanding of the changes to coagulation, including laboratory testing and monitoring therapeutic management, in the following special situations: | ||||||
| Liver disease. | 4.08 | 50.2% | 70.3% | Shows how | 0.46 |
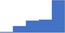
|
| Autoimmune diseases that impact on thrombosis and hemostasis, eg Lupus Anticoagulants, Systemic Lupus Erythematosus. | 4.07 | 47.5% | 70.4% | Shows how | 0.43 |
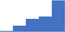
|
| Trauma associated coagulopathies and massive transfusion. | 4.04 | 44.5% | 69.0% | Shows how | 0.44 |
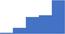
|
| HIT (4 T-score, screening and confirmation tests). | 4.10 | 48.6% | 71.8% | Shows how | 0.43 |
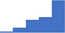
|
| Pregnancy (during the pregnancy and postpartum). | 4.07 | 46.5% | 70.1% | Shows how | 0.44 |
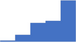
|
| Neonates. | 3.75 | 32.5% | 61.1% | Shows how | 0.44 |

|
| Pediatrics. | 3.82 | 35.0% | 62.5% | Shows how | 0.43 |

|
| Cancer and hematology malignancies. | 3.95 | 41.1% | 64.9% | Shows how | 0.43 |
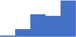
|
| Thrombotic microangiopathies. | 3.96 | 42.0% | 68.2% | Shows how | 0.43 |
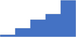
|
| Inflammation, infection associated coagulopathies, disseminated intravascular coagulation. | 4.08 | 46.6% | 72.0% | Shows how | 0.42 |
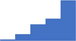
|
| Acquired coagulopathies including heart valve diseases, Extracorporeal Membrane Oxygenation or acquired specific factor inhibitors (eg anti-FVIII antibodies). | 3.84 | 36.3% | 63.0% | Shows how | 0.44 |

|
| Perioperative management of thrombotic and hemostatic disorders. | 4.01 | 43.6% | 69.5% | Shows how | 0.43 |
|
| Renal disorders. | 3.91 | 39.2% | 65.5% | Shows how | 0.43 |
|
- Abbreviations: APTT, activated partial thromboplastin time; DOAC, direct oral anticoagulants; FBC/CBC, full/complete blood count; HIT, heparin induced thrombocytopenia; ISI, international sensitivity index; ISTH, International Society on Thrombosis and Haemostasis; PC, platelet count; PT, prothrombin time; SSC, Scientific and Standardization Committee; TT, thrombin time; VWD, von Willebrand disease.
- a Means, percentages, and graphs are derived from all 330 responses, whilst the Leik measure of consensus are from the 234 complete responses).
The mean ratings of each competency, together with a visual representation of responses, are shown in Table 3. The Working Group reviewed the list of competencies ranked by percentage of respondents who indicated “does” and agreed that a cut-off value at or above 51.9% had face validity to support a “does” competency in the proposed framework. Reviewing the competencies ranked by percentage of respondents who indicated either “does” or “shows how,” led the Working Group to suggest that a cut-off of less than 61.1% had face validity as a “knows how” competency in the proposed framework. The remaining competencies were placed in the “shows how” category. The Working Group reviewed the borderline competencies at each of these cut-off points and agreed that none needed to be moved to a different category. Based on the analysis of survey data, the Working Group proposed that laboratory specialists in thrombosis and hemostasis who had completed their training should be able to do 26 of the competencies, show how to do 32 of the competencies, and know how to do the remaining 6 competencies (Table 3).
3.3 Qualitative data analysis
The free-text responses were supportive of the survey and development of a core curriculum, and many commented that it seemed comprehensive. The Working Group reviewed all suggestions for additional competencies but agreed that the majority were already represented in the framework (eg differentiating types of thrombocytopenia, interpreting results in consumptive coagulopathy, the special situations of HIV and malnutrition, teaching competencies, and multidisciplinary team-working). Other suggestions seemed more appropriate for medical doctors rather than laboratory specialists (eg gene therapy, communication with patients, and the use of clinical probability scoring systems such as BATS—bleeding assessment tools). The Working Group agreed that adding a competency relating to the measurement of extended half-life products was appropriate, specifically, the laboratory specialist should be able to “Apply understanding of assays used for the measurement of extended half-life products” at the level of shows how. The Working Group noted that as of yet these products are not available internationally.
One response suggested rewording the Section 6 competency, “Perform functional and genetic testing of congenital and acquired prothrombotic factors (e.g., FV Leiden, Prothrombin G20210A, JAK2)” because there is currently no standardized hypercoagulation work-up to include these genetic tests. The Working Group supports this comment and therefore decided to add, “in collaboration with the medical service if clinically indicated” at the end of the competency.
Respondents also highlighted the importance of clarifying how this curriculum for laboratory specialists relates to, and differs from, the ISTH Clinical Curriculum; that some individuals work across the field and so need both clinical and laboratory competencies; and that laboratory specialists also need to be able to follow regional requirements and respond to local variation. It is also recognized that there may be variability in available thrombosis and hemostasis laboratory services and laboratory specialists’ ability to meet the core curriculum in relation to local requirements and practices.
4 DISCUSSION
The ISTH undertook this project to help address an identified gap in published guidance on core competencies for a thrombosis and hemostasis laboratory specialist for the international community. The aim was to develop a consensus-based core curriculum that all independent, expert clinical and non-clinical laboratory specialists in the area of hemostasis and thrombosis would be expected to demonstrate. In addition, it was anticipated that such a curriculum could be used as a blueprint for training programs, and would be a useful resource for international governing bodies and regulatory organizations to help guide expectations in areas of laboratory thrombosis and hemostasis.
This study used the same methodology as the ISTH clinical core curriculum in thrombosis and hemostasis,5 but this time with a carefully selected group of thrombosis and hemostasis laboratory specialists instead of clinicians.
Specialists are required in all areas of laboratory medicine. This is, at least in part, due to the gap in the availability of specific objectives in clinical training programs and the continual technical advances in instrumentation, as well as an industry move to develop manufacturer-specific diagnostic test algorithms, and acknowledgment of the lack of technical laboratory specialists across disciplines. Laboratory thrombosis and hemostasis is an area that is highly specialized for the investigation of coagulopathies, including the selection and interpretation of laboratory tests and possibly advising on further testing, as this is often complex, diverse, and very patient-specific.
4.1 Limitations of the research
The results from the survey generated 330 meaningful responses, with 234 (70.9%) complete responses, representing a very low response rate. “Survey fatigue” and the length of the survey may have been factors influencing the completion rate. The majority of responses (62%) were from Europe (46%) and North America (16%), but the wider international community was represented in this study and no national or other large subgroup statistically skewed the overall findings. The survey was only available in English, which may have had a negative impact on the response rate, and even perhaps the reason for some of the incomplete surveys, but it did avoid issues of changes in meaning of competencies that can sometimes arise when they are translated. It is unclear why almost 30% of respondents who started the survey did not rate all competencies; however, the number received did allow for valid cohort and subgroup analysis (n = 330), as well as ICC and Leik calculations (n = 234).
4.2 Validity of survey results
There is recognition in the international community of the complexity and diversity in the area of laboratory hemostasis and thrombosis as respondents scored all 64 competencies as “does,” “shows how,” or “knows how.” As a diverse group of international diagnostic experts in this area developed the competencies, and no unimportant “distractors” were included, it was not unreasonable to expect that the criteria were also likely to be considered important by the survey participants. Further evidence of the face validity of the draft competencies was that the responses only demonstrated “poor” consensus for two competencies, with the other 62 competencies showing moderate (n = 30) or fair (n = 32) consensus as determined by the approach described by Leik.22
4.3 The implications and applications of the findings
Laboratory medicine has an important role in the diagnosis and management of patients. The core curriculum for a laboratory specialist in thrombosis and hemostasis provides an international perspective and framework in this field for clinical laboratories that provide specialized laboratory services in this area. This core curriculum is offered as a focus for education, training, and accreditation to promote a quality laboratory medicine service through consultation and interpretation of diagnostic laboratory test results in the area of hemostasis and thrombosis.
4.4 Areas of future work
The core curriculum provides ISTH and Scientific Subcommittees with a framework for the development of educational tools and resources for members interested in specializing in laboratory hemostasis and thrombosis. While there are currently no regulatory guidelines in this area, the development of the core curriculum will provide many countries with guidance that could potentially be used for accreditation purposes. ISTH will be able to monitor the uptake of the curriculum over time with the development of future education training projects through the ISTH Education and Outreach Committee. The ISTH also recognizes that periodic review of this and the clinical core curriculum by hemostasis and thrombosis expert panels will be required, eg every 5 years, as advancements in this laboratory and clinical subspecialty continue to evolve.
5 CONCLUSION
The draft ISTH core curriculum for laboratory specialists in hemostasis and thrombosis was accepted by the international community, as evidenced by the level of agreement and rating across all 64 competencies and positive free-text comments. Although there is no formal designation for thrombosis and hemostasis laboratory specialists, international governing bodies and regulatory organizations now have a consensus-based international core curriculum that will allow for the mapping, development, and accreditation of educational programs and formal assessment of laboratory specialists across jurisdictions.
ACKNOWLEDGMENTS
The authors would like to thank all members of the ISTH Council and the ISTH Education and Outreach Committee for their leadership and guidance for this project. The authors also acknowledge and are grateful to all the ISTH members for taking the time to review and complete this survey, as well as all other respondents in the wider thrombosis and hemostasis community. The project was led by an international committee with membership from ISTH members and leadership and was supported by funding from the ISTH. The authors would also like to thank Catherine Zimmer from the University of North Carolina, who undertook the calculation of ICC and the Leik measure of consensus.
CONFLICT OF INTEREST
No conflicts of interest were reported by any of the committee members.
AUTHOR CONTRIBUTIONS
Karen A. Moffat, Verena Kiencke, Alicia N. Blanco, Claire McLintock, Flora Peyvandi, Moniek P.M. de Maat, Murray J. Adams, Pantep Angchaisuksiri, Sukesh Nair, Hiroko Tsuda, Munif Haddad, Thomas Renne, R. Cary Clark, and Michael T. Ross contributed to the development of the draft competencies and the survey used to build the curriculum and provided feedback on various drafts. All these co-authors participated in the finalization of the curriculum and analysis of the survey results. Karen A. Moffat and Michael T. Ross wrote the draft manuscript and all authors reviewed and approved the manuscript.