Heparin-induced thrombocytopenia complicating extracorporeal membrane oxygenation support: Review of the literature and alternative anticoagulants
Abstract
Heparin-induced thrombocytopenia (HIT) is a life-threatening prothrombotic, immune-mediated complication of unfractionated heparin and low molecular weight heparin therapy. HIT is characterized by moderate thrombocytopenia 5-10 days after initial heparin exposure, detection of platelet-activating anti-platelet factor 4/heparin antibodies and an increased risk of venous and arterial thrombosis. Extracorporeal membrane oxygenation (ECMO) is a form of mechanical circulatory support used in critically ill patients with respiratory or cardiac failure. Systemic anticoagulation is used to alleviate the thrombotic complications that may occur when blood is exposed to artificial surfaces within the ECMO circuit. Therefore, when HIT complicates patients on ECMO support, it is associated with a high thrombotic morbidity and mortality. The risk for HIT correlates with the accumulative dosage of heparin exposure. In ECMO patients receiving continuous infusion of heparin for circuit patency, the risk for HIT is not neglected and must be thought of in the differential diagnosis of the appropriate clinical and laboratory circumstances. The following article reviews the current knowledge in HIT complicating ECMO patients and the alternative anticoagulation options in the presence of HIT.
1 INTRODUCTION
Heparin-induced thrombocytopenia (HIT) is an important and potentially life-threatening antibody mediated reaction, caused by exposure to unfractionated heparin (UFH) and low molecular weight heparin (LMWH), that continues to cause considerable patient harm worldwide.1
In adult patients receiving heparin, the prevalence of HIT is reported to be 0.5%-5%.2, 3 Studies of adults have noted thrombotic complications at the time of the diagnosis of HIT in 30%-60% of patients.4, 5 The risk of thrombosis continues for several days after heparin withdrawal, with 50% of the patients diagnosed with HIT subsequently developing a thrombotic event.5 Prospective data on the prevalence of HIT and HIT with Thrombosis (HITT) in pediatric patients are lacking; however, published data of HIT in children suggest that the prevalence of HIT may be lower than in adults (1.5%-3.7%) and as low as 0.33% outside the subgroup of neonates receiving cardiopulmonary bypass.6-8 The highest incidence of pediatric HIT has been found in pediatric cardiac intensive care units.9, 10
Extracorporeal membrane oxygenation (ECMO) is a form of mechanical circulatory support used in critically ill patients with respiratory or cardiac failure. Systemic anticoagulation is used to alleviate the thrombotic complications that may occur when blood is exposed to artificial surfaces within the ECMO circuit.11 UFH is the most common anticoagulant used to prevent the formation of thrombus within the ECMO circuit.11
A nonmethodological review of English-language reports of HIT in ECMO patients, both pediatric and adult, was performed, searching four databases, MEDLINE, GOOGLE SCHOLAR, CINAHL, and EMBASE, in an attempt to find all reports.
2 HEPARIN-INDUCED THROMBOCYTOPENIA
HIT is a prothrombotic disorder caused by immunization against platelet factor 4 (PF4) after complex formation with heparin or other polyanions. A subset of anti-PF4/heparin antibodies is capable of intravascular activation of platelets and monocytes by cross-linking Fcγ receptors IIA (FcγRIIA), leading to platelet count decrease (thrombocytopenia) with or without thrombosis. HIT is often associated with devastating complications such as life- and limb-threatening thrombosis, making it one of the most serious adverse drug reactions in medicine.1, 4, 12
An important but less known presentation of HIT is the heparin-induced skin lesions (cutaneous HIT), defined as painful or pruritic inflammatory (erythematous) or necrotic lesions either localized to the site of injection or distal to it. This phenomenon begins on day 5 or later of UFH or LMWH use and concomitant thrombocytopenia is not mandatory.13
The diagnosis of HIT is complicated and based on both clinical suspicion and pathology confirmation. Clinical suspicion of HIT typically occurs with declining platelet counts in the setting of active heparin use. Standardized assessment of the clinical probability of HIT is a crucial step in the diagnosis of a patient with suspected HIT. Two major tools have been developed: the 4Ts score and the HEP (HIT expert probability) score.14 The 4Ts score, the most extensively studied assessment tool, incorporates four typical clinical features of HIT: (a) thrombocytopenia, (b) characteristic timing of thrombocytopenia, (c) presence of thrombosis or other clinical sequelae, and (d) the absence of other causes of thrombocytopenia. The pretest probability is estimated to be low (0-3 points), intermediate (4-5 points), or high (6-8 points).14 Although not validated for the pediatric population, Obeng et al15 retrospectively identified 155 patients <21 years old with sufficient data for 4Ts scoring out of 176 patients with anti-PF4/heparin antibody testing between 2007 and 2011. After using the 4Ts score, HIT was confirmed in 0/38 patients with low-risk 4Ts scores, 2/114 patients with intermediate-risk 4Ts scores and all three patients who had high-risk 4Ts scores presented with HIT with thrombosis. Of 12 positive HIT screening tests, results were falsely positive in 66.6% of patients with intermediate risk 4Ts scores and 100% of patients with low risk 4Ts scores. The prevalence of HIT was .058% and HIT with thrombosis was .046% in pediatric patients on UFH.
The HIT expert probability score is another clinical assessment tool that incorporates more clinical features than the 4Ts score (magnitude of platelet count fall, timing of platelet count fall, nadir platelet count, thrombosis, skin necrosis, acute systemic reaction, bleeding, and other causes of thrombocytopenia). Each of these features is evaluated using a score ranging from −3 (inconsistent with a HIT diagnosis) to +3 (consistent with a HIT diagnosis).14
Laboratory testing for suspected HIT includes immunologic assays such as the PF4 ELISA, Particle Gel Immunoassay, Stic expert, Hemosil HIT-Ab, and Accustar HIT-immunoglobulin G (IgG) that detect circulating anti-PF4/heparin antibodies. Immunoassays are available in most medical centers.16, 17 Unfortunately, immunoassays have a poor specificity (74%-86%) and anti-PF4/heparin antibodies can be detected in patients without HIT,2, 18 especially within the cardiac surgery setting and after ECMO, where repeated and prolonged exposures to UFH are common.19 Nevertheless, increasing the optical density (OD) threshold of the immunoassays enhances specificity without compromising sensitivity and could prevent unnecessary testing and/or treatment with non-heparin-based anticoagulants in patients with possible HIT.20 Functional assays, including the gold standard 14C-serotonin release assay (SRA), measure platelet-activating effects of anti-PF4/heparin antibodies with >95% sensitivity and specificity for HIT.21 However, these assays are technically complex to perform and are not routinely available at most medical centers.
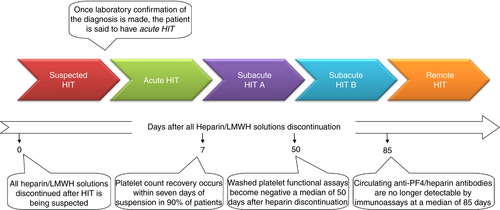
The management of patients with suspected HIT includes immediate discontinuation of all sources of heparin and the initiation of an alternative anticoagulant. The clinico-immunologic response to discontinuation of heparin in a patient with acute HIT follows a predictable pattern. This stereotyped pattern allows HIT to be conceptually separated into phases (Figure 1).22 Further detailed aspects of HIT are beyond the scope of this manuscript and have been thoroughly outlined in the theme issue of Thrombosis and Haemostasis, HIT in 2017 and beyond, 2016: 116/5 (Nov).
3 HIT AND ECMO
The diagnosis of HIT in ECMO patients is even more challenging and requires awareness and a high index of suspicion. The use of mechanical circulatory support, such as ECMO circuit, can simultaneously result in thrombocytopenia and thrombosis through platelet activation and consumption, mimicking the presentation of HIT.11, 23 Disseminated intravascular coagulation or sepsis can complicate patients on ECMO support and share clinical characteristics with HIT. In addition, mechanical circulatory support may influence the development of HIT as a result of continued platelet activation and ongoing release of PF4.24, 25 Any unexpected clotting of the system's oxygenator should raise the suspicion of HIT. It is important to mention that many modern ECMO circuitry components are heparin-coated (such as Bioline, Maquet Cardiopulmonary AG, Hirrlingen, Germany) in an effort to ameliorate the immune response to circuit components.26-28 Heparin coatings were originally designed for application in cardiac surgery bypass circuitry for devices that come into contact with blood. These components include tubing, oxygenators, centrifugal pumps, filters, bubble traps, catheters, and miscellaneous tubing set accessories. The heparin in these systems is covalently bonded to albumin in the coating.29 As an alternative to heparin, phosphorylcholine coating has a major role in the improvement of biocompatibility, durability, and antithrombogenicity of the circuit for ECMO. Circuits such as Eurosets A.L. One ECMO (phosphorylcholine) (Eurosets), Terumo X Coating™ (poly-2-methoxylacrylate) (Terumo Cardiovascular Systems Corporation), Maquet Safeline® (synthetic immobilized albumin), and Softline® (a heparin-free biopassive polymer) are widely available in the case of confirmed HIT.
The incidence of HIT in patients receiving ECMO is not well characterized and with conflicting reports, especially in the pediatric population.30-32 There is accumulating data, especially case reports, indicating cases of both adult (Table 1) and pediatric (Table 2) patients who developed HIT while on ECMO support. It is important to clarify that not all cases were confirmed by functional assays.29, 30, 32-49, 31, 50-61 Kimmoun et al62 retrospectively reviewed 5797 adult patients under venoarterial (VA) ECMO from 20 French centers between 2012 and 2016. Of 39 patients with suspected HIT and positive anti-PF4/heparin antibodies, 21 had confirmed HIT by functional assay (0.36% [95% confidence interval] [0.21-0.52]). The platelet course was similar between confirmed and excluded HIT (P = 0.65). Mortality rate was 33.3% [13.2-53.5] in confirmed and 50% [23.8-76.2] in excluded HIT (P = 0.48). The authors concluded that prevalence of HIT among patients under VA ECMO is extremely low at 0.36% with an associated mortality rate of 33.3%, which appears to be in the same range as that observed in patients treated with VA ECMO without HIT. The authors outlined a central limitation to their study where various diagnosis algorithms and tools were used in recruiting centers, potentially leading to an underestimation of HIT prevalence among patients under VA ECMO.
| Reference | Age/sex | Reason for ECMO | HIT diagnosis | Anticoagulant | Initial bolus (mcg/kg) | Infusion dose (mcg/kg/min) | Target aPTT (s) | Duration of ECMO | Complications |
|---|---|---|---|---|---|---|---|---|---|
| Balasubra-manian33 | 53 y/M | Respiratory failure secondary to Wegener's granulomatosis | ELISA | Lepirudin with Plasmapheresis | Not given | 0.083 (renal failure) | 1.5-2.5 times the baseline | 7 d | None |
| Koster32 | 40 y/F | Myocardial infarction caused by graft thrombosis |
PaGIA HIPA |
Bivalirudin | 500 | 8.3 | 200-220a | 8 d |
None . Switched to Berlin Heart RVAD |
| Beiderlinden34 | 26 y/F | ARDS d/t aspiration | Proven (NFS) | Argatroban | Not given | .2 | 50-60 | 13 d |
N/A Survived |
| 26 y/F | ARDS d/t pneumonia | Proven (NFS) | Argatroban | Not given | .2 | 50-60 | 6 d |
N/A Survived |
|
| 42 y/F | ARDS d/t Wegener's granulomatosis | Proven (NFS) | Argatroban | Not given | .2 | 50-60 | 6 d |
N/A Survived |
|
| Pappalardo29 | 71 y/F | Myocardial dysfunction after cardiac surgery |
ELISA PaGIA HIPA |
Bivalirudin (heparin-coated ECMO) | 500 | 8.3-25 | 180-220a | 6 d | Thrombi in oxygenator and mitral valve prosthesis |
| Dolch30 | 40 y/M | ARDS d/t Herpes simplex virus pneumonitis | ELISA | Argatroban | Not given | .35 (reduced to 0.02 d/t hepatic failure) | 45-60 | 131 d | Multiorgan failure after bilateral lung transplant; death |
| Bergh35 | 69 y/F | Myocardial dysfunction after cardiac surgery | ELISA | Bivalirudin with plasmapheresis | N/A | N/A | N/A | >7 d | None |
| Kuhl36 | 49 y/M | Myocardial dysfunction after cardiac surgery | Proven (NFS) | Argatroban Aspirin | N/A | N/A | N/A | N/A | Lt ventricular thrombus; death |
| Phillips 37 | 44 y/F | Acute hypoxic respiratory failure | HIT Panel (NFS) | Argatroban | Not given | 0.1-0.65 | 170-200a | 7 d | None |
| Garland38 | 27 y/F | Myocardial dysfunction after cardiac surgery |
ELISA SRA |
Bivalirudin | N/A | N/A | N/A | 17 d |
None. Switched to Thoratec HeartMate II LVAD |
| Parlar39 | 22 y/F | Myocardial dysfunction after cardiac surgery | Clinically | Fondaparinux | 2.5 mg daily SC injection | None | N/A | 7 d | ECMO system replacement d/t inadequate oxygenation |
| Glick40 | N/A | N/A | SRA | Argatroban | N/A | N/A | N/A | N/A | N/A |
| Koster41 | 58y/M | Respiratory failure secondary to COPD; awaiting double LTX | IgG-specific chemiluminescent immunoassay |
Argatroban (preoperative) Bivalirudin (intra- and postoperative) |
N/A N/A |
N/A .003 |
160-180a | 21 d | None |
| Natt42 | 59 y/M | ARDS |
ELISA SRA |
Argatroban; non heparin-coated circuit | N/A | N/A | N/A | 22 d | Treatment withdrawn; death |
| 41 y/M | Respiratory failure secondary to pneumonia |
ELISA SRA |
Bivalirudin; non heparin-coated circuit |
N/A | N/A | N/A | 30 d | N/A Survived | |
| 26 y/W | Respiratory failure secondary to pneumonia; SLE; CRF | Previous diagnosis |
Bivalirudin; non heparin-coated circuit |
N/A | N/A | N/A | 9 d | None | |
| 41 y/M | ARDS d/t pneumonia |
ELISA positive SRA negative |
Bivalirudin; non heparin-coated circuit Fondaparinux |
N/A | N/A | N/A | 13 d | N/A (survived to decannulation) | |
| 32 y/W | Respiratory failure secondary to H1N1 |
ELISA positive SRA negative |
Bivalirudin; non heparin- coated circuit |
N/A | N/A | N/A | 50 d | Treatment withdrawn; death | |
| Rouge43 | 49 y/M | Cardiogenic shock with dilated cardiomyopathy and pulmonary embolism |
ELISA positive HIPA positive |
Argatroban | Not given | 0.2 | N/A | 12 d | None survived |
| 69 y/M | Respiratory failure secondary to pneumococcal pneumonia |
ELISA positive HIPA positive |
Argatroban | N/A | 1 | N/A | 20 d | Survived to decannulation Multiorgan failure after colectomy d/t ischemic colitis; death | |
| Chen44 | 55 y/M | Cardiogenic shock secondary to infected internal defibrillator and endocarditis |
ELISA positive SRA positive |
Bivalirudin | 0.75 mg/kg | 1.75 mg/kg/h | 2.5 × baselinea | 13 d | Orthotopic heart transplantation; survived |
| Ito45 | 62 y/W | Cardiac arrest d/t refractory ventricular fibrillation |
Enzyme immunoassay positive Functional assay positive |
Argatroban | N/A | N/A | N/A | 4 d | Bilateral arm gangrene; intra-aortic balloon pump; survived |
| Ratzlaff46 | 58 y/M | Respiratory failure secondary to H1N1 | ELISA positive |
Argatroban; non heparin-coated circuit |
Not given | 0.1 | 60-90 | 28 d | Treatment withdrawn; death |
| Gellatly47 | 56 y/M | Cardiogenic shock secondary to ischemic cardiomyopathy | ELISA positive | Lepirudin | N/A | N/A | N/A | N/A | Switched to BiVAD; survived |
| Sandoval48 | 77 y/M | Cardiac arrest d/t right coronary emboli |
ELISA positive SRA positive |
Argatroban | N/A | N/A | N/A | N/A | N/A |
| Sin47 | 27 y/M | ARDS |
ELISA positive SRA positive |
Argatroban; non heparin-coated circuit |
Not given | .2 | 50-60 | N/A (over 79 d) | Transferred for lung transplant evaluation |
| Welp31 | 62 y/F | Cardiogenic shock d/t Myocardiac infarction |
ELISA positive SRA positive |
Argatroban; non heparin-coated circuit |
N/A | N/A | N/A | N/A | N/A |
- Abbreviations: ARDS, acute respiratory distress syndrome; d/t, due to; ELISA, H/PF4 antibodies enzyme linked immunosorbent assay; HIPA, heparin induced platelet aggregation; N/A, not available; NFS, not further specified; PaGIA, particle gel immune assay; SRA, 14C serotonin release assay. aACT (s) rather than aPTT.
| Reference | Age/sex | Reason for ECMO | HIT diagnosis | Anticoagulant | Initial bolus (mcg/kg) | Infusion dose (mcg/kg/min) | Target aPTT (s) | Duration of ECMO | Complications |
|---|---|---|---|---|---|---|---|---|---|
| Deitcher50 | 4 y/F | DCM bridge to transplant | ELISA | Lepirudin | 400 | N/A | 1.5-2.5 times the baseline | 13 d | Uncontrolled bleeding after transplant, death |
| Mejak51 | 2 wk/F | Myocardial dysfunction after cardiac surgery | HIT positive (NFS) | Argatroban | N/A | N/A | N/A | N/A | N/A |
| Tcheng52 | 8 y/M | DCM | ELISA | Argatroban | 0.5 | 0.5-1.5 | 2 times the baseline | 6 d | None |
| 16 y/M | OHT d/t ICM, acute rejection | ELISA (history of HIT) | Argatroban | 2 | 0.6-2 | 2 times the baseline | 10 d | None | |
| 8 y/F | Complex congenital heart disease, heart failure | Clinical | Argatroban | 2 | 1.5-2 | 2 times the baseline | 1 d | None | |
| 15 mo/F | DCM, heart failure | ELISA | Argatroban | 2 | 2-24 | 2 times the baseline | 39 d | None | |
| Alsoufi53 | 1 wk/F | Myocardial dysfunction after cardiac surgery | ELISA | Argatroban | 50 (ECMO priming) | 0.15-1.8 | 200a | N/A | Pulmonary embolism, death |
| Dager54 | 17 y/F | Respiratory failure d/t bilateral pulmonary contusions | ELISA | Lepirudin | 100 | 120 | 2 times the baseline | 11 d | Respiratory failure, Death |
| Scott55 | 17 mo/M | Respiratory Failure | ELISA | Argatroban | None | 1-2 | 180-200a | 9 d | Renal failure |
| Knoderer56 | 21 mo/M | Myocardial dysfunction after cardiac surgery | ELISA | Lepirudin | N/A | N/A | N/A | N/A | N/A |
| Potter57 | 1 y/F | MI post cardiac surgery, bridge to transplant | ELISA | Argatroban | 7 (ECMO priming) | 1 | 250-300a | >13 d | None |
| 11 d/F | Myocarditis | Clinical | Argatroban | 11 (ECMO priming) | .1 | N/A | 10 d | Disseminated thrombi formation, Death 2 min after ECMO started | |
| 6 d/F | Myocardial dysfunction after cardiac surgery | Clinical | Argatroban | 10 (ECMO priming) | .5 | 200-220a | 7 d | Disseminated thrombi formation, death | |
| Pollak58 | 1 d/F | CDH | ELISA | Bivalirudin | 400 | 1-2.8 | 180-200a | 21 d | Disseminated thrombi formation, death |
| Nagle59 | 15 mo/F | ARDS | ELISA | Bivalirudin | 100 | 50-300 | N/A | 10 d | None |
| Preston60 | 8 y/M | Bridge to lung transplantation |
ELISA SRA |
Bivalirudin with Plasmapheresis | 750-1600 | 20-30 | 60-80 | N/A | Allosensitization |
| Obeng61 | 4 y/F | Myocardial dysfunction after cardiac surgery | ELISA | N/A | N/A | N/A | N/A | N/A | None |
- Abbreviations: ARDS, acute respiratory distress syndrome; CDH, congenital diaphragmatic hernia; DCM, dilated cardiomyopathy; d/t, due to; ELISA, H/PF4 antibodies enzyme linked immunosorbent assay; ICM, idiopathic cardiomyopathy; MI, myocardial infarction; N/A, not available; NFS, not further specified; OHT, orthotropic heart transplant; SRA, 14C serotonin release assay.aACT (s) rather than aPTT.
Sokolovic et al63 reported that in a cohort of adult ECMO patients, eight of 96 patients were positive for HIT (positive SRA or H/PF4 ELISA with OD >1) and seven of those patients had documented thrombotic events (HITT) based on predefined criteria (HIT and HITT incidence in the study group of 8.3% and 7.3%, respectively). HIT-positive patients were significantly younger and had a trend toward higher average HIT ELISA OD. The alternative anticoagulation used was not mentioned. Functional assays for HIT confirmation were not done routinely performed, especially when ELISA OD was >1.0 or patients suffered from thrombosis, thus HIT was possibly overdiagnosed. Felli et al64 described three ECMO patients with clinical suspicion of HIT who were ELISA positive for H/PF4 antibodies. UFH was immediately discontinued with alternative argatroban treatment. A case-control study comparing bivalirudin and heparin treatment on ECMO patients revealed four more cases of HIT on ECMO patients.65 Vayne et al,66 in a single center prospective trial, reported of 57 adult patients (3.5%) who were supported by ECMO for at least 5 days. HIT was suspected in two patients with ECMO circuit dysfunction and unexpected platelet count decrease after day 5. High levels of PF4-specific IgG were detected in both patients, and HIT was confirmed by a serotonin release assay. The authors concluded that an abnormal platelet count evolution especially when associated with ECMO circuit clotting, rather than PF4-specific antibodies, should prompt the suspicion of HIT. Looking into different ECMO modalities, Choi et al67 identified 28 adult patients who developed HIT on ECMO in a systematic review aimed at comparing the characteristics and outcomes of HIT in patients undergoing VA ECMO and veno-venous (VV) ECMO. Out of these 28 patients, 53.6% underwent VA ECMO and 46.4% underwent VV ECMO. Patients on VA ECMO had a lower median platelet count nadir (VA ECMO: 26.0 vs VV ECMO: 45.0 per μL, P = .012) and were more likely to experience arterial thromboembolism (VA ECMO: 53.3% vs VV ECMO: 0.0%, P = .007). Kaplan-Meier survival plots including time from ECMO initiation revealed no significant differences in survival in patients supported on VA ECMO vs VV ECMO (P = .300).
4 TREATMENT OF HIT
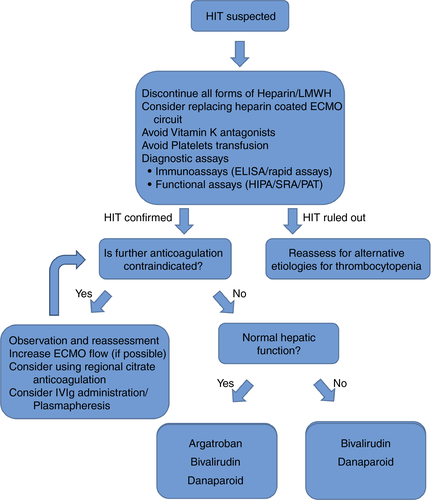
Management of HIT includes the immediate discontinuation of all forms of heparin exposure, including line flush solutions, and the administration of an alternative anticoagulant such as direct thrombin inhibitors (DTIs) and factor Xa inhibitors50 (Table 3) even without indication of the underlying condition. If another anticoagulant is not administered, there is a substantially increased risk of symptomatic or fatal thrombus formation. Twenty to 50% of patients will progress to a clinically significant thrombotic event without further anticoagulation therapy with a 30-fold increased risk of thrombosis than the normal populations.68, 69 Thus, in the presence of HIT, ECMO support is an absolute indication for an alternative anticoagulation agent. Because of their HIT-promoting properties, treatments with LMWH, vitamin K antagonists during the acute phase of HIT, platelets transfusions, and implantable vena cava filters should be avoided, at least until platelets count recovers.70 Intravenous immunoglobulins and plasmapheresis were also suggested as alternatives to anticoagulation or even in addition in severe cases.71, 72 Regional citrate anticoagulation for hemodialysis was first introduced in 1961. It is an alternative to heparin in patients who are at increased risk for bleeding. It permits effective anticoagulation across the dialysis circuit without affecting the patient's systemic coagulation. Citrate anticoagulation is currently used during renal replacement therapy (RRT), as well as in cases of ECMO combined with RRT as an additional local anticoagulant. There is one ongoing study regarding the use of regional citrate anticoagulation in ECMO patients less than 1 year of age (ClinicalTrials.gov Identifier: NCT00968565) and results are pending. However, to date, there is no evidence to support the use of citrate as a sole anticoagulant during ECMO.73 If a heparin-bonded circuit is used, it is prudent (but not often practical) to consider removal of any catheters or circuit components with heparin bonding if possible. Thus, if platelet recovery does not occur after withdrawal of heparin, it is possible that ongoing exposure from the heparin coating is a factor.29 The proposed HIT management algorithm for ECMO patients is presented in Figure 2.
| Agent | Class | Dose | Monitor | Clearance | t1/2 | Antagonist | Comments |
|---|---|---|---|---|---|---|---|
| Lepirudin | Irreversible DTI |
0.4 mg/kg IV bolus (max 44 mg) .15 mg/kg/h (max 16.5 mg/h) |
aPTT ratio; target 1.5-2.5 |
Renal | 80-90 min (depends on renal function) | None | According to the US FDA, Baxter healthcare corporation has made a decision to discontinue Refludan® for injection on May 31, 2012 |
| Argatroban | Reversible DTI |
No bolus indicated 2 mcg/kg/min (max 10 mcg/kg/min) ELSO guidelines: 0.5-1 mcg/kg/min |
aPTT ratio; target 1.5-3 target 1.5-2.5 |
Hepatic | 30-50 min (depends on hepatic function) | None |
|
| Bivalirudin | Reversible DTI |
0.75 mg/kg IV bolus .2 mg/kg/h ELSO guidelines: 0.05-0.5 mg/kg IV bolus 0 .03-0.1 mg/kg/h |
aPTT ratio; target 1.5-2.5 |
Renal (20%) Proteolysis (plasma) (80%) |
25-30 min | None |
|
| Danaparoid | Heparinoid, selective inhibitor of anti-factor Xa |
30 units/kg IV bolus 1.2-2 units/kg/h |
Anti-Xa activity target 0.4-0.8 IU/mL |
Renal | 18-24 h | Protamine does not reverse the bleeding effects | Organon, Inc., discontinued manufacturing Orgaran® on August 14, 2002, resulting from a shortage in drug substance |
| Fondaparinux | Indirect factor Xa inhibitor |
5 mg (<50 kg) 7.5 mg (50-100 kg) 10 mg (>100 kg) SC qd |
Anti-Xa activity can be monitored but laboratory monitoring is not necessary | Renal | 18 h | None |
|
Note
- Data obtained from Truven health analytics Inc. MICROMEDEX® solutions; www.micromedexsolutions.com.
- ELSO, Extracorporeal Life Support Organization; refers to 2014 Anticoagulation guidelines. (https://www.elso.org/portals/0/files/elsoanticoagulationguideline8-2014-table-contents.pdf).
- Abbreviations: ACT, activated clotting time; aPTT, activated partial thromboplastin time; FDA, US Food and Drug Administration; INR, International Normalized Ratio; IV, intravenous; PO, per os; PT, prothrombin time; SC, Subcutaneous.
4.1 DTIs
DTIs are a relatively new class of short-acting anticoagulants that bind directly to the active sites on thrombin molecules, and express more predictable pharmacokinetics and greater reduction of thrombin generation compared with UFH. These anticoagulants have several theoretical advantages over UFH, especially in children.74 First, DTIs bind thrombin directly, independent of antithrombin 3, making them more reliable in patients with low or fluctuating antithrombin 3 activity. Second, DTIs do not bind to other plasma proteins or cells and as a result are not prone to daily changes in serum chemistry or cell counts. Therefore, DTIs may provide a more predictable dosing regimen that allows for a consistent anticoagulant effect with less bleeding compared with UFH, making them useful in ECMO patients.11 Finally, DTIs inhibit both clot-bound and circulating thrombin, which can lead to improved efficacy.22
Three synthetic DTIs, argatroban, bivalirudin, and lepirudin, have been used in ECMO patients. Argatroban has been most often cited in ECMO applications. Infusion of argatroban is started at 0.5-1 mcg/kg/min and adjusted to maintain aPTT 1.5-2.5 times baseline values, but anti-IIa levels can also be used if available.75 Including argatroban in the ECMO circuit prime and administering an initial bolus before starting the continuous infusion has also been described.76 Ten pediatric and 16 adult ECMO patients with HIT and argatroban treatment have been described to date; although most of them had no hemorrhagic or thromboembolic complications, three pediatric and four adult patients died. Postmortem revealed disseminated thrombi formation in the pediatric patients,53, 57 whereas in the adult patients, the causes of death were left ventricular thrombus36 and multiorgan failure after bilateral lung transplant30 and after ischemic colitis.41
Kawada et al77 reported two neonates undergoing ECMO for congenital diaphragmatic hernia associated with hypoplastic lung. Continuous intravenous administration of argatroban was initiated as the primary anticoagulant, without prior evidence of HIT. ECMO support was safely continued in these neonates for 6 and 78 days, respectively, without evidence of hemorrhagic or thromboembolic complication. Beiderlinden et al34 reported nine ECMO adult patients with argatroban anticoagulation, three of whom had proven HIT. The first patient received an argatroban starting dose of 2 mcg/kg/min. Because this dose resulted in severe bleeding, a 10-fold lower dose of 0.2 mcg/kg/min was used for the eight consecutive patients without significant bleeding. Phillips et al37 recommended a starting dose of argatroban between 0.1 and 0.2 mcg/kg/min and then titrating the dose based on a combination of aPTT, ACT, and clinical evidence of early thrombi formation. This pharmaco-therapeutic strategy resulted in sufficient anticoagulation without thrombotic or hemorrhagic complications of argatroban.
Bivalirudin is a DTI with a unique pharmacologic profile: it undergoes predominant non-organ elimination (proteolysis), and has the shortest half-life (approximately 25 minutes). Its affinity for thrombin is intermediate between that of lepirudin (highest) and argatroban (lowest).78 Bivalirudin provides an interesting option for patients diagnosed with HIT because it is cleaved by thrombin. This avoids major drug accumulation in case of renal and/or hepatic impairment.79 Finally, ACT can be used with good correlation to monitor the clinical efficacy of anticoagulation.71, 72, 80
Published doses of bivalirudin used in pediatric ECLS include an initial bolus of 0.05-0.5 mg/kg followed by an infusion rate of 0.03-0.1 mg/kg/h that was subsequently adjusted to maintain aPTT 1.5-2.5 times baseline values or within the physician-defined range.59, 81 Dose escalations may be needed in accordance with longer time on ECMO and the use of continuous RRT. Three pediatric and 10 adult ECMO patients with HIT and bivalirudin treatment were published. We published a report of the first pediatric ECMO patient who developed HIT and switched to bivalirudin.58 Unfortunately, our patient died after disseminated thrombi formation following congenital diaphragmatic hernia repair. None of the adult patients suffered significant side effects or uncontrolled bleeding. An early case series82 reported four patients with suspected HIT who underwent coronary artery bypass grafting using CPB and bivalirudin anticoagulation. Anticoagulation was monitored using ACT. Anticoagulation during CPB was effective, and total operating times were acceptable. One patient experienced excessive postoperative bleeding. Gates et al71 reported a 5-month-old infant who had undergone the Norwood procedure subsequently complicated by bowel ischemia requiring resection. The patient then developed acute HIT and was referred for Glenn shunt with successful use of bivalirudin as anticoagulant on cardiopulmonary bypass. No clots were encountered on the circuit, and good hemostasis was obtained within 10 minutes after ultrafiltration. The patient had an uneventful postoperative course. Koster et al32 reported a 40-year-old woman who required ECMO for myocardial failure after cardiac surgery and developed HIT. She was treated with bivalirudin. No significant bleeding was mentioned. Chen et al44 reported a 55-year-old man who developed HIT while on ECMO because of cardiogenic shock. After HIT was suspected (and later confirmed), anticoagulation with bivalirudin was initiated without any adverse effects.
Lepirudin is the DTI less used and its availability is currently limited. Three pediatric and two adult cases of lepirudin use in this population have been described. Although a 21-month-old baby boy with myocardial dysfunction postcardiac surgery was successfully treated,56 the other two pediatric cases were reported dead, one of uncontrolled bleeding after heart transplant50 and the other secondary to pulmonary failure after ECMO was removed.54 One adult had no complications and was weaned after 7 days but treatment included plasmapheresis, which may have influenced the outcome.33 The other was successfully switched to biventricular assist device without complications.47
Plasma concentrations of DTIs considered useful, accurate, and most correlated with anticoagulation properties of all monitoring alternatives. Several assays are available for plasma concentrations of DTIs. Seidel et al83 compared aPTT, thrombin time (TT), and prothrombin time (PT) to drug levels obtained by the ecarin chromogenic assay (ECA). They analyzed 495 samples of patients with confirmed or suspected HIT on treatment with either argatroban (n = 37) or lepirudin (n = 80). Mean DTI levels ± standard deviation (SD) were .41 ± .36 μg/mL for argatroban and .20 ± .21 μg/mL for lepirudin. Results of aPTT were highly variable for both argatroban and lepirudin. Significant correlations (P < .01) were found between ECA-based DTI level and TT (argatroban, r = .820 and lepirudin, r = .830), PT (argatroban, r = −.544), and aPTT (lepirudin, r = .572). Multiple regression analyses revealed that the TT predicted 54% of argatroban and 42% of lepirudin levels, but no significant impact was seen for PT or aPTT. The aPTT-guided monitoring of DTI therapy leads to a high percentage of patients with inaccurate plasma levels, hence resulting with either undertreatment or overtreatment. Monitoring according to exact plasma concentrations as obtained by specific tests such as ECA may be more appropriate.
4.2 Parenteral factor Xa inhibitors
This is a class of anticoagulant drugs that directly inhibits factor X without using AT as a mediator. Several drugs with several routes of administration are available, amongst which are fondaparinux (subcutaneous) and danaparoid (subcutaneous/intravenous). Both fondaparinux and danaparoid have long half-lives (17-21 and 17-28 hours, respectively) and without available efficient antagonists, bleeding complications may be difficult to manage. Additional oral agents will be further described under direct oral anticoagulants. To date, there are no pediatric ECMO cases that were treated with F-Xa inhibitor. Parlar et al39 reported an ECMO patient who developed HIT and was switched to fondaparinux daily subcutaneous injections without significant adverse reactions.
4.3 Direct oral anticoagulants
Direct oral anticoagulants (DOACs) have gained interest for their potential usage in HIT patients. Two classes of anticoagulants comprise the DOACs, factor Xa inhibitors (rivaroxaban, apixaban, and edoxaban) and DTI (dabigatran) (Table 4). There is a great scarcity of data on the use of DOACs in HIT patients, especially in the pediatric population, and none of them have US Food and Drug Administration-approved pediatric labeling. Off-label use of DOACs in pediatrics is largely extrapolated from adult dosing guidelines. Preclinical data showed that rivaroxaban affects thrombin generation in umbilical cord blood at similar doses to those used in adults,84 suggesting that dosing may be both feasible and simpler in neonates than dosing with UFH. There are many clinical trials currently recruiting pediatric patients and are under way to assess their effects and efficacy in this special population.85
| Drug | Class | FDA-approved adult indications | Drug characteristics | Dosage | Monitoring | Antidote | Findings from pediatric trials |
|---|---|---|---|---|---|---|---|
| Rivaroxaban | Factor Xa inhibitor (first approved) |
|
Reversibly inhibits free and bound factor Xa as well as that in the prothrombinase complex ultimately preventing clot formation and growth |
20 mg OD 15 mg OD for CrCl 15-50 mL/min |
No monitoring needed |
Andexanet Aripazine |
|
| Apixaban | Factor Xa inhibitor |
|
Reversibly inhibits free and bound factor Xa as well as that in the prothrombinase |
Andexanet Aripazine |
|
||
| Edoxaban | Factor Xa inhibitor (most recently approved) |
|
Has the fastest time to maximum effect (1-2 h) Offers options for once-daily dosing and for patients with renal impairment Absorption not affected by the presence of food |
30 mg OD (low dose) 60 mg OD (high dose) |
No monitoring needed | Andexanet |
Phase I, open-label, preliminary PD/PK trial in patients 0-18 years old. The dose matched to low and high adult doses (30 and 60 mg, respectively) will be given to children requiring oral anticoagulation as a single dose Results have not been published yet |
| Dabigatran | DTI |
|
Transported via the Pg-p efflux protein Reaches Cmax in about 1 h if taken on an empty stomach. If given with a high-fat meal, the Cmax increases to 2 h, without any change in the bioavailability of the medication |
150 mg BID 75 mg BID for CrCl 15-30 mL/min |
No monitoring needed |
Idarucizumab Aripazine |
|
Note
- Data obtained from Truven health analytics Inc. MICROMEDEX® solutions; www.micromedexsolutions.com.
- Abbreviation: aPTT, activated partial thromboplastin time; BID, twice daily; Cmax, maximum concentration; DTI, direct thrombin inhibitor; DVT, deep vein thrombosis; dTT, dilute thrombin time; ECT, ecarin clotting time; FDA, US Food and Drug Administration; NVAF, nonvalvular atrial fibrillation; OD, once daily; PD, pharmacodynamic; PE, pulmonary embolism; PK, pharmacokinetic; PT, prothrombin time; TT, thrombin time; VTE, venous thromboembolism.
Shatzel et al86 summarized the published reports of patients with HIT treated with DOACs, which in total encompassed 54 patients (mean age 68.4 years, 68% male), of which 94% had laboratory proven HIT by either PF4 ELISA or SRA, and 48% of reported cases had thrombosis present at the diagnosis of HIT. The most commonly used DOAC was rivaroxaban (59%), followed by apixaban (28%) and dabigatran (13%). In total, 78% of patients received a non-heparin anticoagulant considered appropriate for use in HIT (argatroban, bivalirudin, or fondaparinux) before initiation of DOAC. Twenty-two percent of patients received DOAC alone. In total, only 1 of 54 reported cases had thrombus progression after the initiation of DOAC (a catheter-related thrombus that expanded despite DOAC use, and responded to catheter removal and continued DOAC administration). In total, 3 of 54 reported cases described instances of clinically relevant bleeding, with no reported bleeding-related mortality. There was no reported HIT-related mortality, and only one reported case of thrombosis progression despite DOAC administration. Previous in vitro data have suggested that DOACs do not cause platelet activation or aggregation in the presence of HIT antibodies, implying DOACs to be efficacious therapeutics for patients with HIT,87 and, indeed, evidence supporting efficacy and safety of DOACs for acute HIT is increasing, with the most experience reported for rivaroxaban. Warkentin et al88 reviewed the literature of DOACs for the treatment of HIT. They reported of a thrombosis rate of 1 of 46 patients (2.2%; 95% CI 0.4%-11.3%) in patients treated with rivaroxaban during acute HIT (group A, n = 25; group B, n = 21); major hemorrhage was seen in 0 of 46 patients. Similar outcomes in smaller numbers of patients were observed with apixaban (12 patients) and dabigatran (11 patients). DOACs offer simplified management of selected patients, as illustrated by a case of persisting (autoimmune) HIT (>2-month platelet recovery with inversely parallel waning of serum-induced heparin-independent serotonin release) with successful outpatient rivaroxaban management of HIT-associated thrombosis.
4.4 Nafamostat mesylate
Nafamostat mesylate (NM) is a synthetic serine-protease inhibitor with very short half-life used for acute pancreatitis, shock and in hemodialysis and plasmapheresis to reduce hemorrhagic complications. NM and its metabolites inhibit amiloride-sensitive sodium conductance in the renal cortical collecting ducts leading to impaired urinary potassium excretion and potential hyperkalemia.89 Controversial results of its use during ECMO application, mostly from Korea and Japan, were reported. Lim et al90 found that NM was associated with a higher risk of bleeding than heparin in ECMO patients. In contrast, Park et al91 and Han et al92 reported that NM was a safe alternative anticoagulant for ECMO patients with a high risk of bleeding. Nagaya et al93 reported the use of NM in 12 neonates on ECMO who had a high risk of bleeding that was well controlled in eight of these 12 neonates. The average dose used was 0.48 ± 0.22 mg/kg/h and were monitored by measuring ACT. However, no large study of NM use has yet been reported, especially in the pediatric population. Even more, accurate dosage and titration method principles have not yet been established in the pediatric population and further studies are needed.94
4.5 Factor XIIa inhibitors
While insignificant for hemostasis, the contact system is essential for thrombus stabilization and growth. As the primary reason for anticoagulation in ECMO patients is the prevention of thrombi formation, generated by the blood-circuit interactions, the identification and inhibition of key elements in this process is of great value. Thrombosis on artificial surfaces like medical devices or ECMO circuits is triggered by factor-XII (FXII) activation.95 Therefore, coating catheters and circuits with the potent FXIIa-directed corn trypsin inhibitor allow them to remain patent longer than uncoated catheters when inserted in the jugular veins of rabbits.96 Similarly, in FXII knockout rabbits, the time to occlusion of uncoated catheters is prolonged by more than twofold,97 and an FXIIa-directed antibody is as effective as heparin in preventing clotting in an ECMO circuit in rabbits, but produces less bleeding.98 Moreover, FXII depletion reduced thrombin generation induced by components of mechanical heart valves to background levels without increasing the risk for bleeding.99 Thus, although being evaluated only in animal models of ECMO, FXII-directed therapies are promising and may be safer than heparin for prevention of clotting while on ECMO support.100
5 SUMMARY
HIT is a serious, antibody-mediated complication of heparin therapy. It confers significant risks of thrombosis and devastating outcomes with limb- and life-threatening consequences. Although HIT is a recognizable and treatable complication, because of its relative infrequency, patients are at increased risk for delayed diagnosis and significant morbidity. It is apparent that HIT is an intensely procoagulant disorder and in the setting of patients on ECMO it is associated with a high thrombotic morbidity and mortality, thus a high index of suspicion is mandatory based on clinical signs of HIT. It is crucial to intervene early with alternative anticoagulants when HIT is suspected as this step may improve outcome in these patients. It has to be mentioned that the presence of heparin/PF4 antibodies, rather than platelets activating assays, is not diagnostic for HIT. Further clinical research in alternative anticoagulants is critical in both pediatric and adult patients so that therapy can be optimized without placing the ECMO patients at undue risk.
AUTHOR CONTRIBUTION
Dr Pollak conceived of the presented idea, planned and performed the literature search, reviewed the results and wrote the manuscript.
CONFLICTS OF INTEREST
None were declared. The author certifies that he has no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or nonfinancial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.