An afternoon nap facilitates analogical transfer in creative problem solving
Summary
In analogical problem solving, the solution to a previously experienced problem (source) is used to solve a new but structurally similar problem (target). Yet, analogical transfer is seldom successful, as structural commonalities between source and target problems can be difficult to recognise. Theoretically, memory consolidation processes during REM sleep may help to identify and strengthen connections between weakly related memories, improving the ability to use analogical transfer. In the current experiment, participants attempted to solve source problems, were told the solutions, and then attempted to solve new but structurally similar target problems. After a 2-h break including a nap (n = 28) or wakefulness (n = 30), participants attempted to solve target problems they were unable to solve before the break. Measures of source problem memory and perceived similarity between source and target problems were also obtained. The nap group solved a greater proportion of target problems after the break than the wake group, despite no group differences in solution rates before the break or source problem memory. The nap group also perceived greater similarity between source and target problems after the break than the wake group, and the time spent in REM sleep predicted the proportion of post-break target problems solved. These results indicate that sleep improves the ability to solve target problems that could not be initially solved and suggest that REM sleep improves the use of analogical transfer by highlighting commonalities between source and target problems that were unnoticed before a nap.
1 INTRODUCTION
Early in the 20th century, a heated scientific debate ensued concerning how neurons communicate. Late one night, Otto Loewi suddenly awoke from sleep with a novel idea and headed straight to his laboratory. His ensuing experiment revealed that communication between neurons is chemical; a discovery that won him the Nobel Prize. This is one of several examples in which a creative solution to a tough problem was generated during sleep (Mazzarello, 2000). Beyond this anecdotal evidence, research indicates that sleep may indeed facilitate some forms of creative problem solving by initiating changes in how the brain represents knowledge (Beijamini et al., 2014; Monaghan et al., 2015). Given that creative problem solving can be measured in many ways (e.g. anagrams, analogies, problems with hidden rules, etc.) and that distinct neurophysiological processes occur across different sleep stages, the problem types that benefit from sleep and the mechanisms that support this enhancement are still debated (e.g. Brodt et al., 2018; Lewis et al., 2018).
During sleep, memory consolidation processes stabilise and strengthen newly acquired knowledge for long-term storage, improving memory for this knowledge after sleep (Rasch & Born, 2013). Additionally, qualitative changes to memories can also occur that may be beneficial for problem solving (Stickgold & Walker, 2013). Sleep can facilitate the integration of novel information with existing knowledge schemas (Dumay & Gaskell, 2007; Tamminen et al., 2010) and enable the abstraction of basic principles common to multiple episodes. For example, sleep has been shown to facilitate inferential reasoning (Ellenbogen et al., 2007) and to increase false memories for never-studied words (Payne et al., 2009). Sleep can also enable the extraction of statistical regularities across tone sequences (Durrant et al., 2011) and the discovery of hidden rules to efficiently complete arduous tasks (Wagner et al., 2004).
Memory integration and abstraction rely on processing during stages 2 and 3 of non-rapid eye movement (non-REM) sleep and are initiated by the hippocampally mediated reactivation of recent episodes (Klinzing et al., 2019). In turn, fast sleep spindles, 0.5–3 s bursts of 12–16 Hz activity generated by thalamo–cortical neurons, enable interactions between the hippocampal and cortical areas involved in representing memories, and this exchange is coordinated by the slow-oscillatory activity (0.5–1.5 Hz) characteristic of Stage 3, slow-wave sleep (SWS). This hippocampal-cortical crosstalk decreases hippocampal dependence while creating a richer cortical representation of the memory (Staresina et al., 2015). Supporting this view, non-REM fast sleep spindles, slow-oscillatory activity, and the time spent in SWS are frequently implicated in knowledge integration (Lewis & Durrant, 2011; Tamminen et al., 2010, 2013) and abstraction that occurs across a sleep period (Djonlagic et al., 2009; Durrant et al., 2011, 2016; Payne et al., 2009; Verleger et al., 2013).
Memory integration and abstraction resulting from non-REM sleep undoubtedly aid in some forms of problem solving by promoting comprehension of the overall problem structure. However, REM sleep may be necessary to facilitate problem solving when it requires an active re-combination of problem elements, as occurs during creative problem solving. During a creative problem-solving attempt, when an impasse is reached, it is assumed that the mental problem representation is decomposed and reorganised in a novel fashion to generate the correct solution (Schooler & Melcher, 1995). This may involve creating and/or strengthening associations between previously unrelated or distantly related episodes, relaxing needless constraints, or ignoring irrelevant knowledge (Knoblich et al., 1999; McCaffrey, 2012). Research has demonstrated that an incubation period, i.e. time away from a problem, can facilitate unconscious problem restructuring, leading to solution generation (Sio & Ormerod, 2009).
Theoretically, REM should further facilitate unconscious problem restructuring. According to the broader information overlap to extract framework (BiOtA; Lewis et al., 2018), during REM, reduced cortical-hippocampal synchrony favours the reactivation of cortical representations of new memories with low hippocampal dependence (presumably, the cortical representations were established and/or strengthened during the preceding non-REM episode). Simultaneously, ponto-geniculo-occipital (PGO) waves present during REM reactivate other, random, cortically mediated memories, allowing for new associations between both types of reactivated memories to form. Furthermore, the formation of these new connections is facilitated by the influx of postsynaptic calcium concomitant with high cortical cholinergic activity during REM, which allows the synaptic changes necessary to establish these new associations to occur through long-term potentiation.
Another model, network exploration to understand possibilities (NEXTUP; Zadra & Stickgold, 2021), suggests that during REM, new knowledge generation is facilitated through the discovery and exploration of weak associations during dreaming that are typically ignored during wakefulness, and that this process is facilitated by the suppressed release of norepinephrine and serotonin during REM. Whereas high levels of norepinephrine centre attention and reduce the activation of tangential information to maintain the current focus, a lack of norepinephrine allows for the exploration and activation of more distant associations than occurs during non-REM or wakefulness. Furthermore, the lack of serotonin biases the brain to place more value on weak associations than it would otherwise. This increased importance of weak associations in turn allows for the potential generation of new insights that are considered meaningful. Thus, both BiOtA and NEXTUP predict that REM should facilitate creative problem solving by strengthening associations between unrelated or weakly related memories. Consistent with these theories, participants were able to solve more anagrams when awoken from REM than when awoken from non-REM sleep (Walker et al., 2002). Additionally, during the Remote Associates Test (RAT), wherein participants are presented with three unrelated words (e.g. out, dog, cat) and are asked to generate a fourth word that can be linked with each of the other three (e.g. house), REM facilitated performance to a greater extent than an incubation period (Cai et al., 2009). Furthermore, REM, but not other stages, facilitated semantic priming of weakly related words (Stickgold et al., 1999), and sleep facilitated the ability to solve difficult, but not easy, RAT problems (Sio et al., 2013).
Nonetheless, not all research implicates sleep in creative problem solving. For instance, the ability to solve compound remote associates (CRA) problems was not influenced by sleep (Landmann et al., 2016). CRA problems are like RAT problems but require the solution word (e.g. stone) to create a meaningful compound word with the three words presented (e.g. age, mile, sand; Bowden & Jung-Beeman, 2003). Few studies have examined how sleep may impact creative problem solving with other paradigms, with mixed results. Sleep did not increase solution generation for magic tricks or for problems that are typically solved after a burst of insight (Schönauer et al., 2018). Likewise, Brodt et al. (2018) found no effects of sleep on solving change detection, riddle, or anagram puzzles. However, there was also no increase in solution rates in change detection or anagram puzzles after a sleep-free incubation period either, suggesting that neither sleep nor an incubation period facilitates problem restructuring in these puzzle types. Yet another study demonstrated that sleep improved the ability to solve logical reasoning problems (Beijamini et al., 2014), although baseline problem-solving ability was not assessed and problem-solving rates were related to SWS, not REM.
In another form of creative problem solving, analogical transfer, knowledge from a previously encountered problem (source) is applied to a novel (target) problem to solve it. REM may be especially important for analogical transfer, as not only does it depend on memory, as an individual must first recall a source problem, but problem representations must then be restructured to identify commonalities between source and target problems (Gentner & Colhoun, 2010). Analogical transfer is difficult when source and target problems share structural similarities but have different surface features, leading to low problem-solving success rates (Gick & Holyoak, 1980). However, REM could enable the discovery of distant associations between source and target problems with different surface features, resulting in successful analogical transfer after sleep that includes REM.
Monaghan et al. (2015) demonstrated that, after participants attempted to solve six source problems and received their solutions, those who slept during a subsequent 12-h break solved more target problems after the break than participants who remained awake. The authors suggested that during sleep, source-problem representations were integrated into existing schemas that shared similarities with the source problems, establishing broader representations for these schemas in long-term memory. Consequently, participants who slept during the break were able to more readily access these schemas when they encountered the target problems, leading to better analogical transfer for participants who slept compared with those who remained awake. However, objective memory for the source problems was not assessed, leaving open the possibility that the results could be attributable to stronger memories for the source problems after sleep rather than source-problem restructuring. Also, measures of sleep physiology were not obtained. Relationships between sleep physiology and problem-solving success could help to adjudicate between memory strengthening and memory restructuring interpretations. It is also unknown how sleep might impact the ability to solve target problems that could not be initially solved when encountered after an analogous source problem but prior to sleep. Theoretically, REM should enable the detection of structural similarities between source and target problems that were not apparent prior to sleep, but this has never been tested (Lewis et al., 2018; Zadra & Stickgold, 2021).
The goal of the current experiment was to determine if sleep could benefit analogical problem solving by strengthening commonalities between source and target problems that were unnoticed before a period of sleep. After attempting to solve source problems and seeing their solutions, participants attempted to solve analogous target problems prior to a 2-h break filled with wakefulness or sleep. After the break, participants attempted to solve the target problems they were not able to solve prior to the break. In this way, the benefit of sleep for target problems that could not be solved initially could be evaluated. An objective memory test for the source problems was also administered to determine whether sleep improved memory for the source problems, and electroencephalography (EEG) was recorded during sleep to isolate specific aspects of sleep physiology that may enhance analogical problem solving.
2 METHODS
This study was approved by the Texas State University institutional review board.
2.1 Participants
Participants aged 18–29 years were recruited from Texas State University and received either cash or a course credit for participation and were randomly assigned to either the nap (n = 28) or wake group (n = 30). Six additional participants were excluded from the nap group for failure to achieve stable sleep (>10 min). An a priori power analysis indicated this sample size is sufficient to achieve 93% power, α = 0.05, with a moderate effect size (d = 0.4). This sample size is also comparable to similar studies (Beijamini et al., 2014; Brodt et al., 2018; Schönauer et al., 2018).
2.2 Materials
Problems included eight pairs of analogical problems from previous experiments (Duncker, 1945; Gick & Holyoak, 1980; Metcalfe & Wiebe, 1987; Needham & Begg, 1991; Scheerer, 1963). Each problem within a pair could be solved using the same underlying principle but had different surface features. One problem of each pair was randomly designated the source and the other the target. Participants were not privy to the relationship between the source and target problems (Figure 1).
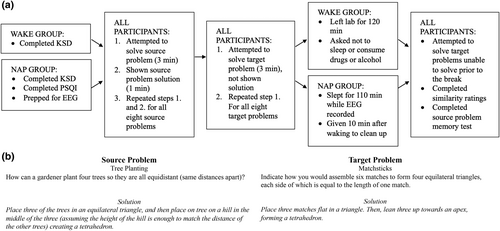
Sleep during the previous month was assessed in nap participants with the Pittsburgh Sleep Quality Index (PSQI; Buysse et al., 1989) to ensure that their sleep habits were comparable to typical college students. PSQI answers are assigned points and combined to yield a global PSQI score (≥5 indicates poor sleep quality). Sleep quality and quantity from the night preceding the experiment was assessed for all participants with the Karolinska Sleep Diary (KSD) to ensure that sleep the night before the experiment did not differ between groups (Åkerstedt et al., 1994). The Stanford Sleepiness Scale (SSS) assessed the perceived sleepiness level after the break by asking participants to rate their current sleepiness level on a 1–7 scale (Hoddes et al., 1973).
2.3 Procedure
After giving informed consent, participants completed the KSD (Figure 1). Nap participants then completed the PSQI and were prepped for EEG recording. Next, all participants viewed eight source problems and their associated titles one at a time. The titles provided brief, unique surface descriptions of each problem (e.g. ‘T-shirt Vendor’). The titles and problems appeared in black 24-pt font centred on a white background for 3 min each while participants attempted to solve the problem and wrote their solution attempt(s) on a sheet of paper. After 3 min, the title and solution to the source problem appeared in red 24-pt font in the centre of the screen for 1 min and the participants were asked to remember the solution. Participants then advanced to the next problem by pressing the spacebar. Participants viewed the problems in one of two possible random orders, counterbalanced across participants. After all source problems and solutions were presented, participants took a 5-min break.
Next, participants attempted to solve the eight target problems. Each problem and its unique descriptive title appeared centred on the screen in black 24-pt font on a white background for 3 min and the participants were asked to write their attempted solutions on a sheet of paper. After 3 min, participants were prompted to advance to the next problem by pressing the space bar. Solutions were not presented. The target problems appeared in one of two random orders, counterbalanced across participants, and did not correspond to the orders in which source problems were presented.
All participants were then given a 2-h break. The wake-group participants left the laboratory for 120 min and were asked not to sleep or consume any drugs or alcohol. The nap-group participants slept in a bed in a quiet, dark room in the laboratory while the EEG was recorded. After 110 min, they were woken up, EEG sensors were removed, and they were given 10 min to clean up. Additionally, during the break, a researcher scored the target problem solutions that the participants generated prior to the break to determine which target problems each participant was not able to solve initially.
Finally, all participants completed the SSS and then made a second attempt to solve the target problems they were unable to solve prior to the break. Each unsolved target problem and its title appeared one at a time in the centre of the computer screen for 3 min in a random order while the participant wrote their attempted solution on a sheet of paper. Participants then rated the similarity between the source and target problems using a procedure adapted from Monaghan et al. (2015). Participants were shown the titles of each source problem one at a time in random order and were asked to rate how similar it was to any of the of target problems on a 7-point scale (1 = not similar, 7 = similar). Finally, objective memory for the source problems was tested by giving participants eight sheets of paper with a source problem and its title printed at the top. Participants attempted to recall the solution to each problem and write it on the page.
2.4 Scoring
Problem solutions were scored as either correct (1) or incorrect (0). Because target problems attempted before the break were scored in a narrow time window, a single rater determined which target problems were solved incorrectly and would be re-presented to participants after the break. However, after each participant was tested, a second rater also scored each source and target problem solution generated by participants. There were no discrepancies between raters on any of the scores. The memory test was scored in the same manner as the problem solutions, such that each answer was considered correct (1) or incorrect (0) and was scored by two independent raters who were in complete agreement. Because the number of target problems that participants attempted to solve after the break varied across participants, accuracy was computed as the proportion of correctly solved problems out of the total number of problems that were attempted. Memory for source problem solutions was computed by summing the number of solutions correctly remembered and perceived similarity between source and target problems was computed by averaging the similarity ratings given for each of the eight source problems.
2.5 Electroencephalography
EEG was collected from seven electrodes placed at standard 10–20 system sites (O1, O2, C3, C4, F3, F4, and Fpz) embedded in an elastic cap, referenced to the mastoid average. Additionally, electrooculography was measured with electrodes placed next to each eye, and electromyography was measured with three electrodes on the chin, to aid in sleep staging. The sampling rate was 200 Hz with 0.27 Hz high-pass and 70 Hz low-pass filters. Sleep data were scored according to standard criteria (Iber et al., 2007). Artefacts were rejected based on visual inspection and slow-oscillatory power (0.5–1.5 Hz) was computed by applying a fast Fourier transform with a Hanning function to data from frontal recording sites (F3, F4, Fpz) using 4-s intervals with 50% overlap, averaged across 30-s epochs. Fast spindle density during non-REM sleep was computed using an automated algorithm in PRANA (PhiTools) with the following parameters: frequency range: 12–16 Hz, duration: 0.5–3.0 s, amplitude fluctuations >2.5 SD above the mean amplitude. Fast spindle density was averaged across central recording sites (C3, C4), as central fast spindles have been associated with qualitative memory changes during sleep (Antony et al., 2018; Cowan et al., 2020).
3 RESULTS
All analyses were completed in SPSS v27 (IBM Corp).
3.1 Problem solving
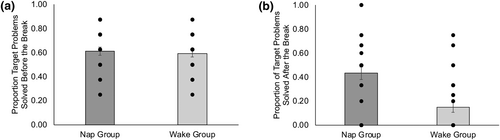
The proportion of source problems solved by nap (M = 0.16, SD = 0.21) and wake (M = 0.18, SD = 0.11) participants did not differ, F(1, 56) = 0.36, p = 0.55, indicating no difference in baseline problem-solving ability between groups. There was also no difference in the proportion of target problems solved before the break between nap (M = 0.61, SD = 0.18) and wake (M = 0.59, SD = 0.15) participants, F(1, 56) = 0.21, p > 0.6. However, after the break, sleep participants (M = 0.43, SD = 0.29) solved a greater proportion of target problems than wake participants (M = 0.15, SD = 0.22), F(1, 56) = 17.8, p < 0.001, ηp2 = 0.24 (Figure 2).
3.2 Memory
One nap participant was omitted from this analysis due to data loss from computer error. There was no difference in the number of source problem solutions recalled between nap (M = 6.85, SD = 1.12) and wake (M = 6.80, SD = 1.19) participants, F (1, 55) = 0.03, p > 0.85. Therefore, better memory for source problem solutions cannot account for the higher proportion of target problems solved after the break in nap participants compared with wake participants.
3.3 Similarity
Fifteen participants were excluded from the similarity analysis (12 nap, 3 wake) due to data loss from computer error. Nonetheless, average similarity scores were higher for nap (M = 6.05, SD = 0.71) than for wake participants (M = 5.40, SD = 1.22), t(41) = 2.24, p = 0.03, d = 0.62.
3.4 Sleep and arousal
Consistent with typical PSQI scores in college students (Dietch et al., 2016), the mean global PSQI score for nap participants was 5.00 (SD = 2.21). The SSS scores did not differ significantly between nap (M = 2.61, SD = 0.92) and wake (M = 3.13, SD = 1.19) participants, although a trend for greater sleepiness in wake participants was present, t(56) = 1.89, p = 0.06. To rule out the possibility that group differences in problem solving after the break could be attributed to differences in sleepiness after the break, a one-way ANCOVA was conducted with SSS scores as a covariate. The results confirmed the previous pattern of results, showing that nap participants solved a greater proportion of problems after the break than wake participants, F(1, 55) = 16.8, p < 0.001, ηp2 = 0.23, indicating that this difference is not due to group differences in sleepiness after the break. There were also no group differences on any of the KSD questions (p > 0.06) with one exception. On average, it took wake participants longer to fall asleep (M = 9.4 min, SD = 15.7) than nap participants (M = 3.1 min, SD = 4.70) the night before the experiment, t(56) = 2.07, p = 0.04, d = 0.54.
On average, nap participants slept for 76.3 min (SD = 8.2) total during the break. The average time spent in stage 1 and stage 2 was 12.5 min (SD = 6.7) and 29.6 min (SD = 14.1), respectively. Averaged across all participants, the time spent in SWS was 28.0 min (SD = 16.8) and the time spent in REM was 6.1 min (SD = 8.5). However, not all participants achieved SWS and/or REM. For those who achieved SWS (n = 26), the average time spent in SWS was 30.1 min (SD = 15.4) and for those who achieved REM (n = 13), the average time spent in REM was 13.1 min (SD = 7.9). The mean non-REM slow-oscillatory power averaged across frontal electrodes was 657.8 μV2 (SD = 414.8) and the mean non-REM fast spindle density averaged across central electrodes was 2.7 spindles/min (SD = 2.1).
A linear regression was conducted to examine whether any aspects of sleep physiology predicted the proportion of target problems solved after the break. The time spent in REM, the time spent in non-REM (stages 2 and 3), non-REM fast spindle density, and non-REM slow-oscillatory power were predictor variables. Additionally, the interaction between the time spent in non-REM and the time spent in REM was included as a predictor, as the influence of REM on problem solving may depend on how much time was spent in non-REM. The model was not significant, F(5, 22) = 2.35, p = 0.07, but the time spent in REM predicted the proportion of target problems solved after the break (p = 0.004). No other predictor variables achieved significance (Table 1). When these other variables were excluded from the model and only the time spent in REM was entered as a predictor, the model was significant F(1, 26) = 5.15, p = 0.032, R2 = 0.165 (Figure 3). In contrast, when the time spent in non-REM was entered as the only predictor, the model was not significant, F(1, 26) = 0.037, p > 0.8.
| Predictor variable | ß | SE | p | 95% CI |
|---|---|---|---|---|
| REM (min) | 0.637 | 0.007 | 0.004 | [0.008, 0.036] |
| Non-REM (min) | 0.166 | 0.004 | 0.443 | [−0.005, 0.011] |
| Non-REM slow-oscillatory power (μV2) | −0.047 | <0.001 | 0.820 | [0.000, 0.000] |
| Non-REM fast spindle density (spindles/min) | −0.360 | 0.026 | 0.063 | [−0.103, 0.003] |
| REM × Non-REM interaction | 0.277 | <0.001 | 0.162 | [0.000, 0.001] |
- Abbreviations: 95% CI, 95% confidence intervals; REM, rapid eye movement sleep; SE, standard error; ß, beta coefficient; SWS, slow-wave sleep.
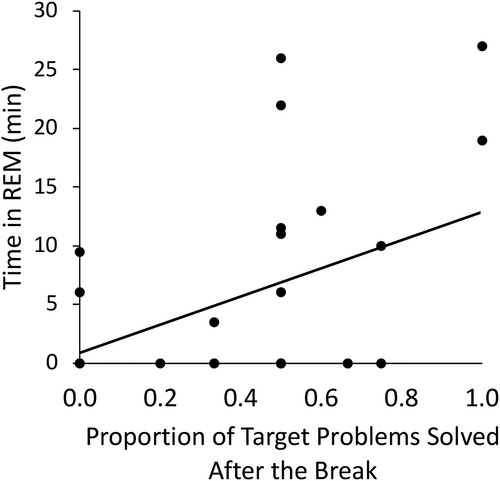
To further explore the potential relationship between REM and problem-solving ability, a final analysis compared the proportion of problems solved after the break for nap participants who achieved REM (n = 13) and nap participants who did not achieve REM (n = 15). Although the proportion of problems solved was numerically greater for nap participants who achieved REM (M = 0.48, SD = 0.33) compared with those who did not (M = 0.40, SD = 0.25), this difference did not approach significance, p > 0.4, likely due to the small group sizes.
4 DISCUSSION
The current results are the first to demonstrate that sleep improves the ability to use analogical transfer to solve target problems that could not be solved prior to sleep. Participants who slept between target problem-solving attempts solved a greater proportion of target problems after the break than participants who remained awake, despite no group differences in solution rates before the break, sleep quality, or quantity from the prior night, sleepiness level, or the ability to solve source problems. This finding is not attributable to better memory for the source problems for those who slept, as memory for source problem solutions did not differ between groups. Additionally, perceived similarity between source and target problems after the break was greater for nap than for wake participants. Finally, the proportion of target problems solved after the break was positively associated with the time spent in REM in nap participants. Collectively, these findings suggest that REM promotes the discovery of connections between source and target problems, increasing the ability to creatively solve problems that could not be solved prior to sleep.
Previous research suggested that structural changes to source problems during sleep increases their accessibility after sleep, which enhances the ability to solve new target problems that are structurally analogous to source problems encountered prior to sleep (Monaghan et al., 2015). The current results demonstrate that, beyond merely increasing the accessibility of source problems, sleep also promotes the discovery of distant associations between source and target problems that were not apparent during wakefulness, and that this benefit may depend on REM. Supporting this interpretation, nap participants perceived greater similarity between source and target problems at the end of the experiment than did wake participants. However, it should be noted that in the current experiment, all target problems attempted after the break were problems that were attempted before the break. Thus, the benefit of sleep for solving previously encountered target problems relative to "new" target problems (i.e. problems that share structural similarities with previously encountered source problems but were never encountered previously themselves) could not be assessed. Including additional problems in the current paradigm was not practical, given the experiment length and the need to include enough target problems prior to the break to ensure that multiple target problems would be initially unsolved. Future research in which the ability to solve new and previously encountered target problems after sleep would help to determine whether the same or different mechanisms are associated with the ability to solve each problem type.
These results are also broadly consistent with BiOtA (Lewis et al., 2018) and NEXTUP (Zadra & Stickgold, 2021), although BiOtA and NEXTUP make slightly different predictions about how REM contributes to creative problem solving. According to BiOtA, hippocampal–cortical communication during non-REM sleep generates cortical schemas representing individual problems. Subsequently, during REM, spontaneous reactivation of new schemas in concert with the activation of random, unrelated schemas by PGO waves provides opportunities for new connections between schemas to form (between multiple new or between new and previously existing schemas), and high acetylcholine levels facilitate the synaptic changes necessary to make these connections. It is possible that in the current experiment, initially distinct schemas for source and target problems were created during non-REM and then new connections between schemas were forged during REM, leading to the ability to transfer analogically structural information from the source to the target problems after REM. Alternatively, NEXTUP posits that during REM, low norepinephrine levels enable the exploration of weak associations between recently encoded and other memories (other recently encoded memories and/or remote memories) that were ignored during waking, and this exploration is what comprises the REM dream content. Additionally, low serotonin during REM amplifies the importance of these weak connections, further strengthening associations. In the current study, exploration of distant associations during REM may have led to the discovery and strengthening of the structural similarities between source and target problems, resulting in enhanced analogical transfer after REM. Although this experiment was not designed to test between BiOtA and NEXTUP, research that includes measures of REM dreaming may help to refine and/or arbitrate between these theories. It should also be noted that although aspects of non-REM sleep, including duration, slow-oscillatory power, and fast spindle density, did not predict problem solving success, non-REM processing is likely requisite for REM-related enhancements in problem solving given the nature of the sleep cycle, in which non-REM stages occur prior to REM. Although the exact roles of non-REM and REM in memory consolidation are still debated, current theories posit that non-REM and REM have complementary functions, with REM processes building upon processes that took place in non-REM (e.g., Ambrosini & Giuditta, 2001; Diekelmann & Born, 2010).
Another important aspect of the current results is that memory for the source problem solutions did not differ between nap and wake groups. Recalling a comparable source problem is the first step in successfully using analogical transfer to solve a target problem (Gentner & Colhoun, 2010). Therefore, in the current experiment, the ability of nap participants to solve more initially unsolved target problems than wake participants is not attributable to better memory for source problems. Rather, group differences likely stem from processes involved in discovering commonalities between source and target problems. The lack of a memory difference between groups may seem surprising, given the abundance of research indicating that memories are typically remembered better after a period containing sleep relative to a comparable period spent awake (Rasch & Born, 2013). However, memory consolidation processes are not uniformly applied to all recent experiences (Payne & Kensinger, 2018; Stickgold & Walker, 2013). Along with emotionality and future relevance, initial memory strength can also influence whether a memory will be strengthened during sleep, with weaker memories prioritised for consolidation over stronger memories (Denis et al., 2020; Schapiro et al., 2017). In the current experiment, participants in both groups recalled seven of the eight source problem solutions on average. Source problems and their solutions may have been strongly encoded, which could explain why no group differences in source problem memory were observed. It is also possible that non-memory processes contributed to performance on the source memory test. Participants were asked to recall the solutions and write them down on a sheet of paper with the problem typed at the top. Although they were not instructed to do so, if they could not remember the solution, they may have used non-memory processes to generate a solution.
It is also noteworthy that improvements in problem solving ability were apparent after a 90-min afternoon nap. This finding is consistent with previous studies showing that 90-min naps are sufficient to improve creative problem solving using other paradigms (Beijamini et al., 2014; Cai et al., 2009). However, not all studies have found beneficial effects of a nap (Brodt et al., 2018; Schönauer et al., 2018). To the extent that creative problem solving depends on the unique neural milieu present during REM, it is possible that the relatively small amount of REM afforded by a nap was sufficient to establish the connections necessary to use analogical transfer in the current experiment. Yet, for other problem types, a brief nap may not provide enough REM to effectively forge the connections necessary to reach a solution. This could account for the null findings observed in some experiments in which other problem types were used.
Nap participants also perceived stronger similarity between source and target problems than wake participants, supporting the interpretation that connections between source and target problems are strengthened during sleep. Theoretically, the higher perceived similarity by nap participants likely resulted from processing that occurred during REM sleep (Lewis et al., 2018; Zadra & Stickgold, 2021). However, due to computer error, data were not obtained from approximately 10% of wake and 40% of nap participants. Although the uneven distribution of participants in each group limits the usefulness of this underpowered and potentially skewed analysis, this possible difference in similarity ratings across groups corroborates the idea that sleep amplifies connections between source and target problems.
One limitation of the current experiment is that wake participants left the laboratory and were only instructed not to sleep or consume drugs or alcohol. The experiment was conducted on a large campus where student parking and housing is not conveniently located near the academic building where the study took place. Thus, it is likely that most wake participants remained on campus during the break. Although this helped to limit the potential experiences had by participants during the break, it is possible that variability in these activities could have influenced problem-solving ability after the break. Another limitation is that because they had an opportunity to rest, nap participants may have been more motivated to solve problems after the break than wake participants, but motivation was not assessed.
5 CONCLUSION
Past experiences provide a wealth of information that can be beneficial for our future behaviours, including the ability to creatively solve problems. Unfortunately, we are not always able to use that information to our best advantage. The current results indicate that when a problem seems unsolvable, the phrase "just sleep on it" may carry some merit, especially if sleep includes REM. This sleep stage may play a key role in putting past experiences to best use by establishing and strengthening associations that are not readily apparent in our waking lives, although additional research will be necessary to establish a causal relationship between REM and analogical problem solving. Nonetheless, a brief nap including REM may be an efficient way to overcome impasses during creative problem solving.
AUTHOR CONTRIBUTIONS
Carmen E. Westerberg: Conceptualization; funding acquisition; writing – original draft; writing – review and editing; investigation; methodology; formal analysis; project administration; resources. Sean E. Fickle: Conceptualization; data curation; methodology; funding acquisition; writing – original draft; investigation; writing – review and editing; formal analysis. Chloe E. Troupe: Formal analysis; data curation; writing – review and editing; investigation. Anna Madden-Rusnak: Investigation; writing – review and editing; data curation; formal analysis. Rebecca G. Deason: Writing – review and editing.
ACKNOWLEDGEMENTS
Funding was provided by a Graduate Thesis Supplement from the Master of Arts in Psychological Research Program at Texas State University awarded to SEF and a Research Enhancement Program grant from Texas State University awarded to CEW. We thank Marissa Lira, Karla Reyes Fierros, and Linda Etufugh for assistance with experimental setup and data collection.
CONFLICT OF INTEREST STATEMENT
This project was approved by the Institutional Review Board at Texas State University. We have complied with the ethical standards of the Declaration of Helsinki and all participants gave consent to participate in the study. We report no conflict of interest for C.E. Westerberg, S.E. Fickle, C.E. Troupe, A. Madden-Rusnak, or R.G. Deason.
Open Research
DATA AVAILABILITY STATEMENT
The data underlying this article are available in Open Science Framework at https://osf.io/ta6ez.




