Sleep disorders in a naturalistic cohort of Dutch psychiatric outpatients: prevalence rates and associations with psychopathology symptom severity and well-being
Summary
Sleep problems are very common in individuals with a mental disorder. Given the abundant evidence indicating the negative impact of disturbed sleep on mental health outcome, insight into the prevalence of all types of sleep disorders in specific mental disorders and neurodevelopmental conditions is of practical importance. Therefore, we estimated the prevalence of six types of sleep disorders with the Holland Sleep Disorders Questionnaire in an overall mental health sample (n = 1082) and separately for different mental and neurodevelopmental conditions. Furthermore, associations between specific sleep disorders, psychopathology and well-being were studied. The impact of the total number of sleep disorders on these associations was examined. Overall, 46.2% of all participants scored above the cut-off for having a sleep disorder. Specifically, 26.8% scored on insomnia, 12.1% on sleep breathing disorders, 9.7% on hypersomnia, 13.7% on circadian rhythm sleep–wake disorders, 11.2% on parasomnia, and 17.9% on sleep-related movement disorders. Most sleep disorders were associated with greater severity of psychopathology and lower well-being. These associations got stronger with an increasing number of sleep disorders. Our study revealed higher suspected prevalence of most sleep disorders in a mental disorder sample compared to the general population. Moreover, the presence of sleep disorder(s) was strongly associated with symptom severity and reduced well-being. These findings extend the notion that early detection and treatment of sleep disorders in mental health populations is essential for psychiatric outcome.
1 INTRODUCTION
Sleep is paramount for daily functioning and both physical and mental health (Baglioni et al., 2016; Palagini et al., 2022; Pavlova & Latreille, 2019). Unfortunately, however, various sleep disorders are rather prevalent in the general population. A study in a large nationally representative sample of Dutch individuals demonstrated that 8.2% screened positive for insomnia, 5.3% for circadian rhythm sleep–wake disorders (CRSWD), 6.1% for parasomnia, 5.9% for hypersomnia, 7.1% for sleep-related breathing disorders (SBD), and 12.5% for sleep-related movement disorders (SRMD) (Kerkhof, 2017).
There is substantial evidence that disturbed sleep is even more common in those with a mental disorder (Morin & Ware, 1996). To date, most studies on disordered sleep within the context of mental disorders specifically focused on insomnia disorder. This reportedly affects up to 40% of patients with a depressive, bipolar, or post-traumatic stress disorder (PTSD; Ohayon & Shapiro, 2000; Steinan et al., 2016; Stewart et al., 2006), and 30% of those with an anxiety disorder (Johnson et al., 2006). Only few studies also assessed the prevalence of other sleep disorders. Those that did provide preliminary evidence that hypersomnia is present in about one third of individuals with bipolar disorder (Steinan et al., 2016), and that delayed sleep–wake phase disorder is frequent (30%) in adults with attention-deficit hyperactivity disorder (ADHD; Spera et al., 2020). Unfortunately, these studies often had a small sample size, limiting the generalisability of the findings. A relatively large-scale study (n = 400) in individuals with mental disorders found an overall prevalence of 40.8% for any sleep disorder, 12.5% for narcolepsy, 14.8% for restless legs syndrome (RLS)/periodic limb movement disorder (PLMD), 4.5% for CRSWD, and 14.5% for SBD. However, the validity of these results may be limited due to the unknown psychometric properties of the screening instrument for sleep disorders employed (Hombali et al., 2019).
Insight in how often particular sleep disorders co-occur with particular mental health conditions is especially relevant as disturbed sleep may contribute to mental health problems. Various sleep disorders have been linked to an increased risk of developing mental conditions like depressive, anxiety, post-traumatic stress, and alcohol-abuse disorders (e.g., Freeman et al., 2020; Hertenstein et al., 2019; Kallweit et al., 2016; Lancel et al., 2021). Furthermore, several sleep disorders are associated with more persistent and severe mental health symptoms. For instance, insomnia is associated with increased duration and severity of depression as well as increased relapse rates for depression (Franzen & Buysse, 2022; Hertenstein et al., 2019; Ohayon & Roth, 2003), and individuals with ADHD and comorbid delayed sleep–wake phase disorder experience worse ADHD symptoms then those without this sleep disorder (Spera et al., 2020). Also, sleep disorders are generally associated with suicidal behaviour (B. Krakow et al., 2011; Malik et al., 2014; Wang et al., 2019). In line with this, there is growing evidence indicating that mental disorders and sleep disturbances are increasingly considered as intertwined (co-dependent) conditions that reciprocally worsen each other's symptomatology, resulting in adverse consequences for overall functioning and quality of life (e.g., Abad & Guilleminault, 2022; Sarfan et al., 2022a). Moreover, adequate treatment of various sleep disorders has been shown to positively affect psychopathologies such as depression, PTSD and anxiety disorders (Belleville et al., 2011; Blom et al., 2015, 2017; Maher et al., 2021; Riemann, 2009).
Clearly then, knowledge on the prevalence of co-occurring sleep disorders may not only give information on the factors that contribute to the refractoriness of mental disorders, but may also aid (timely) identification of sleep disorders in individuals with mental disorders, allowing for early targeted interventions. Therefore, we aimed to establish the prevalence of the six groups of sleep disorders described in the International Classification of Sleep Disorders (ICSD) across a large group of individuals with various types of mental disorders and neurodevelopmental conditions (hereafter referred to as ‘mental health conditions’). Given that sleep disorders are associated with worse mental health-related outcomes (e.g., Franzen & Buysse, 2022; Hertenstein et al., 2019; Spera et al., 2020), we also investigated the association between the presence of suspected sleep disorders and measures of mental health outcome (symptom severity and well-being). The prevalence of sleep disorders was expected to be particularly high in those with PTSD (see Colvonen et al., 2018), especially as insomnia and nightmares are considered symptoms of PTSD (Freeman et al., 2020; Germain, 2013). Furthermore, PTSD and SBD might be bidirectionally related (Krakow et al., 2015). Therefore, we also investigated the association between suspected sleep disorders and PTSD-specific symptoms in patients with PTSD. Finally, as medication prescribed in the context of mental health conditions may have sleep disrupting effects (Neubauer et al., 2018), such as development and/or exacerbation of RLS (Rottach et al., 2008), or onset of insomnia (Alberti et al., 2015), usage of pharmacological agents with known effects on sleep was also assessed.
2 METHODS
2.1 Procedure and population
A total of 1090 individuals receiving care at GGZ Drenthe Mental Health Institute between October 2019 and January 2022 gave informed consent and took part in the Routine Outcome Monitoring that included all questionnaires employed in the present study. Data collection took place within the MOnitoring psychoPHARmacology (MOPHAR) study, which was approved by the local medical ethics committee (RTOP Leeuwarden, study #298). MOPHAR includes patients aged ≥18 years who visited an outpatient department of the Drenthe Mental Health Institute. Specifics of MOPHAR are discussed in a previously published article (Simoons et al., 2019). Only individuals with a fully completed Holland Sleep Disorders Questionnaire (HSDQ) were considered in this study, resulting in a final sample of 1082 participants.
2.2 Measures and materials
2.2.1 Demographic and diagnostic information
Demographic information, mental health diagnoses (Diagnostical and Statistical Manual of Mental Disorders, [DSM], fourth and fifth edition, American Psychiatric Association, 2013) and use of medication were obtained from participants' electronic medical files. Medication was classified according to the Anatomical Therapeutic Chemical (ATC) system (World Health Organisation Collaborating Centre for Drug Statistics Methodology, 2022). The prevalence of the following medication groups within the hypnotics and sedatives ATC-category were established: benzodiazepines (N05CD), Z-drugs (N05CF) and (prescribed) melatonin agonists (N05CH). Furthermore, two off-label pharmacological agents commonly prescribed for sleep, quetiapine (N05AH04; up to 100 mg once daily) and mirtazapine (N06AX11; up to 15 mg once daily) were included (Kamphuis et al., 2015). For sleep-related quetiapine, eight observations were considered missing due to unclear dosage information, while no observations were considered missing for mirtazapine.
2.2.2 Sleep disorders
The HSDQ is a 32-item instrument that is composed of ICSD-2-based (American Academy of Sleep Medicine, 2005) clusters of sleep complaints/symptom descriptions that are specific to six main sleep disorders (Kerkhof et al., 2013). The HSDQ has overall good reliability and accurately discriminates between the six groups of sleep disorders (Kerkhof et al., 2013). Patients report for each item, on a 5-point Likert scale, the degree to which a statement was applicable to them in the past 3 months from 1 ‘never’ to 5 ‘almost always’. Mean scores for each sleep disorder subscale are evaluated against a previously established cut-off value by Kerkhof et al. (2013) to determine the potential presence of a particular sleep disorder and create a binary ‘present’ versus ‘not present’ variable for each group of sleep disorders. The cut-off scores are as follows: 3.68 for insomnia, 2.87 for SBD, 2.90 for hypersomnia, 3.41 for CRSWD (including delayed sleep–wake phase disorder), 2.42 for parasomnia (including symptoms of sleep walking, nightmares, rapid eye movement behaviour disorder, confusional arousals, and night terrors) and 2.70 for SRMD (including RLS and PLMD). Internal consistency for the subscales in our sample was mostly acceptable to excellent with the exception of SBD (insomnia α = 0.91, SBD α = 0.58, hypersomnia α = 0.74, CRSWD α = 0.83, parasomnia α = 0.76, SRMD α = 0.78). The HSDQ subscales were used to establish point prevalence of suspected sleep disorders. Furthermore, for each participant the total number of suspected sleep disorders was computed.
2.2.3 General psychopathology
The Outcomes Questionnaire 45 (OQ-45) is a 45-item instrument measuring severity of psychopathology on a 5-point Likert scale. Respondents indicate how applicable each of the items was in the past week, with options ranging from 0 ‘never’ to 4 ‘almost always’. An earlier study found good internal consistency (α = 0.93) and test–retest reliability (r = 0.79) in clinical samples (de Jong et al., 2007). Internal consistency in our sample was comparably good (α = 0.94). The sleep focused item (item 41) was removed from the total score, and the resultant score was used in regression analyses. The maximum OQ-45 total score in our study was 176.
Additionally, to assess the clinical relevance the OQ-45 cut-off value was computed based on the original OQ-45 cut-off value corrected for the removed sleep item by dividing the total score by the remaining number (44) of items. The percentage of participants scoring above the cut-off was reported.
2.2.4 Post-traumatic stress disorder symptoms
The PTSD Checklist for DSM-5 (PCL-5) was used as a measure of PTSD-symptom severity. This questionnaire presents 20 statements that participants rate on a 5-point Likert scale from 0 ‘I agree not at all’ to 4 ‘I agree extremely’. Total PCL-5 score ranges from 0 to 80, with higher scores indicating more severe PTSD symptoms (Morrison et al., 2021). The internal consistency (α = 0.96) was excellent and test–retest reliability (r = 0.84) was good in a sample of veterans (Bovin et al., 2016). Internal consistency in our sample was good as well (α = 0.94). The sleep-related statements (items 2 and 20) were omitted from the total score, leaving a maximum score of 72. The total PCL-5 score was used in a regression analysis.
2.2.5 Well-being
The Individual Recovery Outcome Counter (I.ROC) is a 12-item instrument measuring recovery and well-being on a 6-point Likert scale that ranges from 0 ‘never’ to 6 ‘always’. Earlier research found good internal consistency (α = 0.86) and concurrent validity for the I.ROC (Ion et al., 2013; Monger et al., 2013). The internal consistency of the I.ROC was good in our sample (α = 0.91) and the I.ROC was recently validated in a mental health population (Sportel et al., 2023), where good internal consistency (α = 0.88) and test–retest reliability (r = 0.85) were reported. The total I.ROC score, with a maximum of 72, was used as an overall measure of well-being in the regression analysis.
3 STATISTICAL ANALYSES
The point prevalences of suspected sleep disorders overall and per category of mental health condition were assessed using cross-tables. Sex differences in specific sleep disorders were investigated with chi-square tests. Additionally, overall medication use was depicted with a cross-table.
To investigate the association between suspected sleep disorders and mental health symptoms and well-being, three multiple regressions were performed with dichotomous variables indicating the presence of sleep disorders as independent variables and psychiatric outcome measures as dependent variables. Because both age and sex are known to affect sleep (Ford & Cooper-Patrick, 2001; Hombali et al., 2019), these variables were added as covariates. Regressions with OQ-45 and I.ROC total scores as dependent variables were conducted on the total sample, while regressions with PCL-5 were performed in the subset of individuals with a PTSD diagnosis. Interactions between sex and the six sleep disorders on outcome variables were analysed. For all regressions, adjusted R2 was reported as measure of explained variance for the overall model.
To examine whether the number of sleep disorders is related to total OQ-45 and I.ROC scores, multifactorial analyses of variance (ANOVAs) were calculated. In each of these models age and sex were included as covariates and dichotomous variables indicating the presence or absence of one of four mental disorders (depression, anxiety disorder, bipolar disorder, and PTSD) were added to the model. For all significant effects, the η2 was reported as measure of effect size, with η2 ≤ 0.01 denoting a small effect, η2 ≥ 0.06 a medium effect, and η2 ≥ 0.14 a large effect (Lakens, 2013). Within and between comparisons for each model were investigated with Tukey's Honest Significant Difference (HSD) test, where all significant effects were added.
In all statistical analyses, relevant assumptions are met unless specified otherwise. Analyses were performed with the R statistical language (R core team, 2022), using the ‘base’ package for regressions, ANOVAs, and Tukey post hoc tests (‘TukeyHSD’ function).
4 RESULTS
4.1 Demographics
More than half of the participants were female (59.0%). The mean age (SD, range) of the sample was 43.1 (14.5, 18–84) years. Most received post-secondary school education (61.0%), had a paid job (50.8%), and received ambulatory treatment (97.5%).
4.2 Distribution of mental health conditions and sleep disorders
The distribution of mental health conditions is presented in Table 1. Most participants were diagnosed with one mental health condition (n = 500 [46.2%]), 17.7% had two, and 4.9% three or more. A total of 24.9% of participants had not (yet) been clinically diagnosed with a mental health condition, and another 6.3% were diagnosed with mental health condition not listed in Table 1.
| Psychiatric or neurodevelopmental condition | N (%) |
|---|---|
| Depression | 238 (22.0) |
| Bipolar | 201 (18.6) |
| Anxiety disorders | 140 (12.9) |
| PTSD | 118 (10.9) |
| ADHD | 84 (7.8) |
| ASD | 49 (4.5) |
| SUD | 30 (2.8) |
| Personality disorders | |
| Cluster A | 6 (0.6) |
| Cluster B | 51 (4.7) |
| Cluster C | 82 (7.6) |
| Unspecified | 49 (4.5) |
- Abbreviations: ADHD, attention-deficit hyperactivity disorder; ASD, autism spectrum disorder; PTSD, post-traumatic stress disorder; SUD, substance use disorder. Table is not cumulative.
The top rows of Table 2 depict the distribution of sleep disorders in the total sample. Almost half of the patients, 46.2%, scored above cut-off for at least one sleep disorder. More specifically, 21.8% scored positive for one sleep disorder, 10.8% for two, 8.6% for three, and 5.0% for four or more. The most prevalent suspected sleep disorder was insomnia, followed by SRMD. The most frequently occurring combinations were insomnia with CRSWD (122 [11.3%]), insomnia with SRMD (100 [9.2%]), and insomnia with parasomnia (73 [6.8%]). The only significant sex difference concerned SBD, with males scoring more often above cut-off on the SBD scale than females (χ2 = 7.28[1], p < 0.01).
| N | At least one sleep disorder, n (%) | Insomnia disorder, n (%) | SBD, n (%) | Hypersomnia, n (%) | CRSWD, n (%) | Parasomnia, n (%) | SRMD, n (%) | |
|---|---|---|---|---|---|---|---|---|
| Total sample | 1082 | 500 (46.2) | 290 (26.8) | 131 (12.1) | 105 (9.7) | 148 (13.7) | 121 (11.2) | 194 (17.9) |
| Females | 638 | 301 (47.1) | 170 (26.6) | 63 (9.9) | 89 (11.0) | 84 (13.2) | 77 (12.1) | 116 (18.2) |
| Males | 444 | 199 (44.8) | 120 (27.0) | 68 (15.3) | 35 (7.9) | 64 (14.4) | 44 (9.9) | 78 (17.6) |
| Depression | 238 | 119 (50.0) | 76 (31.9) | 28 (11.8) | 31 (13.0) | 43 (18.1) | 31 (13.0) | 47 (19.7) |
| Bipolar | 201 | 64 (31.8) | 26 (12.9) | 27 (13.4) | 9 (4.5) | 8 (4.0) | 9 (4.5) | 20 (10.0) |
| Anxiety disorders | 140 | 64 (45.7) | 42 (30.0) | 11 (7.9) | 14 (10.0) | 20 (14.3) | 13 (9.3) | 28 (20.0) |
| PTSD | 118 | 79 (67.0) | 44 (37.3) | 21 (17.8) | 11 (9.3) | 24 (20.3) | 35 (29.7) | 32 (27.1) |
| ADHD | 84 | 47 (56.0) | 26 (31.0) | 19 (22.6) | 11 (13.1) | 14 (16.7) | 9 (10.7) | 19 (22.6) |
| ASD | 49 | 28 (57.1 | 17 (34.7) | 7 (14.3) | 7 (14.3) | 13 (26.5) | 7 (14.3) | 8 (16.3) |
| SUD | 30 | 20 (66.7) | 10 (33.3) | 10 (33.3) | 3 (10.0) | 8 (26.7) | 7 (23.3) | 7 (23.3) |
| Personality disorders | ||||||||
| Cluster A | 6 | 3 (50.0) | 2 (33.3) | 1 (16.7) | 3 (50.0) | 0 (0) | 1 (16.7) | 2 (33.3) |
| Cluster B | 51 | 34 (66.7) | 22 (43.1) | 15 (29.4) | 8 (15.7) | 12 (23.5) | 11 (21.6) | 18 (35.3) |
| Cluster C | 82 | 47 (57.3) | 27 (32.9) | 7 (8.5) | 8 (9.8) | 14 (17.1) | 13 (15.9) | 17 (20.7) |
| Unspecified | 49 | 28 (57.1) | 22 (44.9) | 6 (12.2) | 7 (14.3) | 9 (18.4) | 4 (8.2) | 10 (20.4) |
- Note: Table has non-cumulative frequencies, percentages are calculated row-wise.
- Abbreviations: ADHD, attention-deficit hyperactivity disorder; ASD, autism spectrum disorder; CRSWD, circadian rhythm sleep–wake disorder; HSDQ, Holland Sleep Disorders Questionnaire; PTSD, post-traumatic stress disorder; SBD, sleep breathing disorder; SRMD, sleep-related movement disorder; SUD, substance use disorder.
4.3 Prevalence of sleep disorders in specific mental health conditions
As depicted in Table 2, suspected insomnia disorder seems especially prevalent in those with personality disorders and PTSD. Suspected SBD seemed most prevalent within substance-use disorder (SUD) and ADHD. Suspected hypersomnia appeared not very common, and there were no large differences between the various mental health conditions. The highest prevalence of suspected CSWRD was observed in autism spectrum disorder (ASD), SUD, cluster B personality disorder, and PTSD. Suspected parasomnias were most frequently found in patients with PTSD, followed by SUD, and cluster B personality disorders. Suspected SRMD appeared particularly common in cluster B personality disorder, PTSD, SUD, and ADHD.
4.4 Use of sleep-related medication
Medication data was available for 1072 patients (0.9% missing). The prevalence of hypnotic medication was 7.3% (n = 78) for benzodiazepines, 3.6% (n = 39) for Z-drugs, and 0.4% (n = four) for melatonin agonists. The prevalence of off-label prescriptions of quetiapine and mirtazapine was 5.9% (n = 63) and 1.4% (n = 15), respectively.
4.5 Associations between sleep disorders, symptoms of psychopathology and well-being
For the OQ-45 total score, the regression model was significant (Table 3). Significant positive associations were found between OQ-45 scores and insomnia, hypersomnia, CRSWD, parasomnia and SRMD, while significant negative associations were found between age and OQ-45 scores. This implies that the presence of these sleep disorders is related to more severe symptoms of psychopathology, while with increasing age symptoms of psychopathology are less severe. No significant interaction effects were observed. Based on the adjusted R2, this model explained 28% of the variation in OQ-45 total scores.
| Model | b | β | SE | t | p | F | Adj R2 | p model |
|---|---|---|---|---|---|---|---|---|
| Whole sample N = 940, OQ-45 | ||||||||
| HSDQ scale scores (df = 931) | 46.10 | 0.28 | <0.001 | |||||
| Intercept | 75.70 | 2.74 | 27.66 | <0.001 | ||||
| Insomnia | 18.10 | 0.31 | 1.92 | 9.40 | <0.001 | |||
| SBD | 3.36 | 0.04 | 2.35 | 1.43 | 0.154 | |||
| Hypersomnia | 7.77 | 0.09 | 2.63 | 2.95 | 0.003 | |||
| CRSWD | 6.37 | 0.09 | 2.44 | 2.61 | 0.009 | |||
| Parasomnia | 8.43 | 0.10 | 2.47 | 3.41 | <0.001 | |||
| SRMD | 7.03 | 0.10 | 2.02 | 3.49 | <0.001 | |||
| Sex (F) | 0.48 | 0.01 | 1.48 | 0.33 | 0.745 | |||
| Age | −0.29 | −0.15 | 0.06 | −5.30 | <0.001 | |||
| Whole sample N = 1015, I.ROCa | ||||||||
| HSDQ scale scores (df = 1006) | 35.63 | 0.21 | <0.001 | |||||
| Intercept | 41.55 | 1.16 | 35.88 | <0.001 | ||||
| Insomnia | −7.43 | −0.29 | 0.85 | −8.79 | <0.001 | |||
| SBD | −1.92 | −0.005 | 1.03 | −1.86 | 0.064 | |||
| Hypersomnia | −4.53 | −0.12 | 1.11 | −4.09 | <0.001 | |||
| CRSWD | −2.71 | −0.08 | 1.08 | −2.51 | 0.012 | |||
| Parasomnia | −2.66 | −0.07 | 1.09 | −2.44 | 0.015 | |||
| SRMD | −1.38 | −0.05 | 0.90 | −1.54 | 0.124 | |||
| Sex (F) | 0.02 | −0.001 | 0.65 | 0.03 | 0.980 | |||
| Age | 0.10 | 0.12 | 0.02 | 4.22 | <0.001 | |||
| PTSD N = 103, PCL-5 | ||||||||
| HSDQ scale scores (df = 94) | 4.69 | 0.22 | <0.001 | |||||
| Intercept | 31.57 | 4.67 | 6.76 | <0.001 | ||||
| Insomnia | 3.07 | 0.11 | 2.93 | 1.05 | 0.297 | |||
| SBD | −0.44 | −0.01 | 3.18 | −0.14 | 0.890 | |||
| Hypersomnia | 4.10 | 0.08 | 4.40 | 0.93 | 0.353 | |||
| CRSWD | 4.56 | 0.14 | 3.60 | 1.27 | 0.208 | |||
| Parasomnia | 10.17 | 0.36 | 2.87 | 3.54 | <0.001 | |||
| SRMD | 3.52 | 0.12 | 2.64 | 1.33 | 0.187 | |||
| Sex (F) | 1.06 | 0.04 | 2.53 | 0.42 | 0.676 | |||
| Age | −0.02 | −0.02 | 0.10 | −0.23 | 0.818 | |||
- Note: significant effects are depicted in bold.
- Abbreviations: Adj, adjusted; b, unstandardised regression weights; β, standardised regression weights; CRSWD, circadian rhythm sleep–wake disorder; HSDQ, Holland Sleep Disorders Questionnaire; I.ROC, Individual Recovery Outcome Counter; OQ-45, Outcomes Questionnaire 45; PCL-5, PTSD Checklist for DSM-5; PTSD, post-traumatic stress disorder; SBD, sleep breathing disorder; SE, standard error; SRMD, sleep-related movement disorders.
- a Some heteroscedasticity was noticeable in this model: the variability of the residuals increased over the range of the fitted values. Therefore, the assumption of equal variance across fitted values was violated.
The regression analysis for the total I.ROC score was significant as well (Table 3). Significant negative associations were observed between I.ROC total scores and insomnia, hypersomnia, CRSWD, and parasomnia, and a significant positive association was found for age. Thus, the presence of these sleep disorders is associated with lower mental well-being, while age is related to higher mental well-being. Again, no interaction effects between them were found. Based on the adjusted R2, this model explained 21% of the variation in I.ROC total scores.
In participants with PTSD the regression model for the PCL-5 total scores was significant (Table 3), explaining 22% of the variation. A positive association was found between PCL-5 scores and parasomnia, meaning that patients with PTSD with a positive score on parasomnia had more severe PTSD symptoms than those with a score below parasomnia cut-off.
4.6 Association between the number of sleep disorders and psychopathology
Overall, the number of sleep disorders was positively associated with OQ-45 total scores (F [4,913] = 76.87, p < 0.001, η2 = 0.25). There were significant differences in OQ-45 total scores between having no suspected sleep disorder and all other categories, between having one and three or at least four, and between two and three suspected sleep disorders (Figure 1), whereby having more suspected sleep disorders was indicative of higher psychopathology scores.
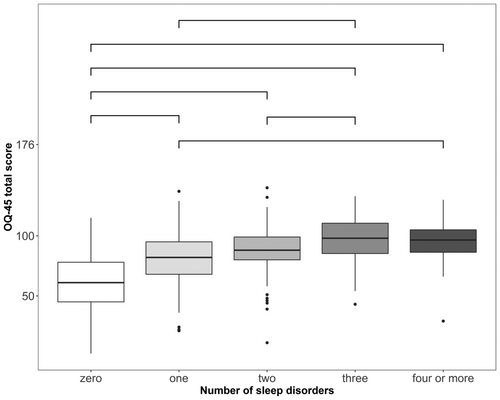
Overall, 76.5% of patients scored above the adjusted cut-off value of the OQ-45. A higher proportion of patients with one or more suspected sleep disorders appeared to have clinically relevant psychopathology as indicated by their OQ-45 scores (Table 4).
| Number of sleep disorders | OQ-45 total score above cut-off, n (%) |
|---|---|
| None | 319 (63.0) |
| One | 180 (87.8) |
| Two | 94 (93.1) |
| Three | 77 (98.7) |
| Four or more | 49 (98.0) |
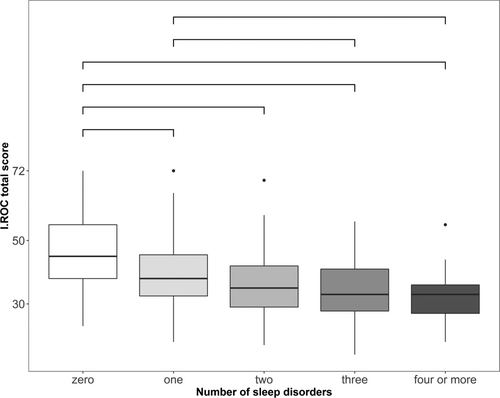
A higher number of suspected sleep disorders was associated with lower I.ROC total scores (F [4,988] = 54.25, p < 0.001, η2 = 0.18). There were significant differences in I.ROC scores between no sleep disorder and all other categories, and between one and three or four suspected sleep disorders (Figure 2). In all comparisons a more suspected sleep disorders was associated with lower well-being.
5 DISCUSSION
In this study, we established the co-occurrence of suspected sleep disorders and specific mental health conditions, and the associations sleep disorders have with psychopathology and well-being. To this end, we included a large outpatient sample that was diverse, and relatively complex in terms of both mental health conditions and sleep disorders. Sleep disorders were common in our sample: the screening questionnaire indicated that almost half of the participants met HSDQ criteria for a sleep disorder. This is about twice as frequent as established previously in the general Dutch population (Kerkhof, 2017). Specifically, all types of sleep disorders detected by the HSDQ occur substantially more often among individuals with a mental health condition. Earlier studies (Dolsen et al., 2014; Khurshid, 2018) already identified insomnia as frequently occurring in psychiatric disorders. The present study supports this notion and extends it to most other sleep disorders.
With respect to the occurrence of insomnia, the most extensively studied sleep disorder, our results are relatively comparable to or even more pronounced than earlier findings in participants with anxiety, ADHD, ASD, depression, PTSD, cluster B personality disorders, and SUD (Fadeuilhe et al., 2021; Hohn et al., 2019; Johnson et al., 2006; Ohayon & Shapiro, 2000; Provencher et al., 2020; Stewart et al., 2006; Van Veen et al., 2017). Potential explanations for the high occurrence of insomnia in depression include shared neurochemical imbalances, hypothalamus–pituitary–adrenal-axis dysregulation, abnormal activity in emotion regulatory brain areas and shared environmental influences (Freeman et al., 2020). For PTSD/anxiety disorders, shared neurobiological and/or genetic factors and overlapping characteristics such as hyperarousal have been implied as well (Freeman et al., 2020). In our study, the prevalence of suspected SBD was moderate compared to previously reported prevalence rates in a clinical mental health sample (Behr et al., 2018). We did observe a relatively high prevalence of suspected SBD in participants with SUD and those with ADHD. Alcohol use in particular is known to contribute to SBD (Chakravorty et al., 2018). Although our SUD group was too small to analyse subgroups, most reported problems related to alcohol, which may underlie the high prevalence of suspected SBD in this group. Hypersomnia, which represents a group of rare sleep disorders (Billiard & Sonka, 2016), also occurred less frequently than the other sleep disorders in our sample. However, compared to the general population its prevalence was higher, which may be partly due to the use of sedative pharmacotherapeutic agents in our population (Abad & Guilleminault, 2022). We observed the highest prevalence of suspected CRSWD, especially delayed sleep–wake phase symptoms, in patients with ASD, which is in accordance with the literature (Baker & Richdale, 2017; Carmassi et al., 2019). CRSWD also occurred frequently in those with cluster B personality disorders, PTSD, and depression. Remarkably, CRSWD was less common in patients with ADHD and bipolar disorder in this study than might be expected from previous reports (Bijlenga et al., 2019; Takaesu et al., 2016). This may in part be due to differences in factors like diagnostic criteria or sample characteristics, such as DSM instead of ICSD sleep diagnoses or patients with bipolar disorder with current mania or depression versus euthymic patients. Parasomnia was frequently suspected in patients with PTSD and cluster B personality disorders. Particularly nightmares (HSDQ item 20) were frequently reported in these groups. Prevalence of suspected SRMD was high in patients with affective disorder and type B personality disorders, which could be related to the RLS stimulating effects of many antidepressants, and atypical antipsychotics (Patatanian & Claborn, 2018; (Rottach et al., 2008; Wichniak et al., 2017). Additionally, in agreement with literature (Snitselaar et al., 2016), SRMD was commonly found in those with ADHD. One may speculate whether either the effects of stimulants prescribed for ADHD or overlap in aetiology between RLS and ADHD such as dopaminergic dysregulation could be partially responsible for this trend (Wajszilber et al., 2018). We found relatively low prevalence estimates for sleep disorders in individuals with bipolar disorder (Steinan et al., 2016). An explanation could be that many of those with a bipolar disorder were euthymic at the time they completed the HSDQ. In patients with bipolar disorder in remission, longer duration of euthymia is associated with a higher sleep quality (Samalin et al., 2016), thus one may expect that the prevalence of sleep disorders is lower in stable euthymic bipolar patients. Potentially a similar phenomenon is present in our sample.
Aside from the high prevalence of suspected sleep disorders, associations between specific sleep disorders and measures of psychopathology and well-being became apparent. Positive relationships with overall psychopathology were found for all suspected sleep disorders except SBD. The regression analysis revealed that all these sleep disorders, (partially) independent from each other, significantly contribute to general symptom severity. This supports the notion that particularly in mental health services the screening for sleep disorders should encompass the full range of ICSD sleep disorders. Moreover, in patients with PTSD, parasomnia (mostly nightmares) was associated with increased daytime PTSD symptoms. Furthermore, insomnia, hypersomnia, CRSWD, and parasomnia were related to lower levels of well-being.
When multiple suspected sleep disorders were present overall psychopathology increased and well-being decreased further. It thus seems that the negative effects of sleep disorders potentially cumulate when they co-occur with each other. Furthermore, clinically relevant differences in overall psychopathology were observed with an increasing number of comorbid sleep disorders. This is especially relevant because around a quarter of patients in our sample screened positive for multiple suspected sleep disorders, mostly insomnia in combination with another sleep disturbance. This is in agreement with previously described associations between insomnia, nightmare disorder and/or RLS (Ohayon et al., 1997; Phillips et al., 2006) and consistent with the notion that insomnia often develops in the course of other sleep disorders. For example, people with nightmares may develop insomnia symptoms due to fear of sleep (Werner et al., 2021).
A growing number of studies that focused on treatment of particularly insomnia suggest a causal relationship between disturbed sleep and symptoms of psychopathology (Belleville et al., 2011; Hertenstein et al., 2022; Maher et al., 2021; Scott et al., 2021). Although causal relationships cannot be inferred from our correlational study, all our findings corroborate and extend the notion that disordered sleep (both due to insomnia and other sleep disorders) and mental disorders are intertwined.
The absence of associations between SBD and psychopathology/well-being is striking. In line with this, a recent meta-analysis found no association between obstructive sleep apnea and depressive symptoms in cross-sectional studies (Edwards et al., 2020). One explanation for the absence of a relationship between suspected SBD and outcome measures in the present study is that the employed sleep screener does not include common daytime consequences, such as excessive daytime sleepiness, which are nowadays deemed very relevant for accurate diagnosis. Therefore, the HSDQ SBD scale may not have optimal discriminative properties. Individuals scoring erroneously below the SBD threshold due to mainly experiencing daytime SBD symptoms may obscure associations between SBD and psychopathology and well-being. Although our study did not find direct associations between suspected SBD and psychiatric outcome, diagnosis and treatment of SBD remains important in psychiatric populations due to the negative impact of SBD per se (Akashiba et al., 2002).
Overall use of hypnotic medication likely prescribed for sleep was comparable to that in the general population, both in United States-based (Chong et al., 2013) and Dutch samples (National Drug Monitor, 2023). This finding is striking as the prevalence of insomnia was high in our sample. It may be explained by reluctance of psychiatrists to prescribe medication to aid sleep. Alternatively, they may often not be aware of a potential sleep disturbance or consider disturbed sleep as a mere symptom of the mental health condition. Apart from benzodiazepines, the off-label drug quetiapine was used with the highest frequency in this sample. Furthermore, actual use of off-label medication might be even higher as we only considered two specific pharmacological agents in this category. Thus, apparently, off-label prescription of sedative drugs for sleep disorders has become common practice. Melatonin agonists were rarely prescribed (only four patients). However, melatonin is also available over the counter in the Netherlands, so the actual use of melatonin in this population might be higher.
A few limitations of our study warrant discussion. We used a screening instrument, which, while well validated in a general population, has not yet been validated for use in populations with a mental disorder. Furthermore, clinical sleep diagnoses were not available, thus our prevalence rates are estimates of suspected sleep disorders based on the HSDQ. Particularly the prevalence estimates of sleep disorders that require extensive physiological diagnostic procedures, such as SBD, may not be sufficiently accurate. For instance, a study in a clinical mental health population utilising polysomnography-based diagnosis of SBD found the substantially higher prevalence of 23.7% (Behr et al., 2018), which may, among others, suggest that our suspected prevalence might be an underestimation. In contrast to SBD, the clinical diagnosis of the SRMD RLS is based on self-reported symptoms. A study using such an RLS diagnosis in a sample of individuals with psychiatric disorders found an RLS prevalence of 16.4% (Weber et al., 2022). The prevalence shown in the present study (17.9%) falls well within the confidence interval reported by Weber et al. (2022). This is consistent with earlier findings indicating that a self-report screener like the HSDQ has moderate to excellent diagnostic accuracy for assessing sleep disorders for which diagnoses are (largely) based on subjective reports of symptoms (i.e., insomnia, CRSWD, RLS, and most parasomnia; e.g., Kerkhof et al., 2013). Although using the HSDQ to estimate the prevalence of sleep disorders has the obvious limitation of being less reliable/precise than a clinical assessment, it has the important pro that it can be used to assess sleep disorders in large samples such as the present naturalistic sample of 1082 psychiatric patients. Clearly, assessing each patient for all ICSD sleep disorders based on their diagnostic criteria in a sample of this size would not be feasible in psychiatric practice. An additional advantage of the use of a (quick) self-report screener is that it may result in a limited selection bias (i.e., individuals who do not experience (severe) sleep problems might be less inclined to participate in a study employing more elaborate and time-consuming diagnostic procedures).
However, by using self-report instruments, biases related to response styles, such as an extreme response, which are related to characteristics (anxiety and neuroticism) that might be common in our population, cannot be ruled out and might have introduced some bias in the statistical analyses (Deng et al., 2018; Van Vaerenbergh & Thomas, 2013). Also, sleep disorders often overlap in symptoms, especially insomnia and CRSWD, which share common items in the HSDQ, and thus might have resulted in a high number of participants scoring on multiple sleep disorders and a potential overestimation of insomnia and/or CRSWD. Another important limitation is that in our data collection we were unable to include patients with psychotic disorders. This is a relevant study population, as it is a large patient population with chronic and severe psychopathology who often have disturbed sleep (Reeve et al., 2015). Finally, our cross-sectional study design renders us unable to assess the directionality of the relationship between sleep and mental health conditions. Future research should include a longitudinal design in a similarly diverse mental health sample. In addition, it would be valuable to study whether treatment of mental health condition reduces the occurrence of sleep disorders and vice versa. And if not, whether the refractory sleep problems exert negative effects on mental disorder trajectory and relapse.
Concerning the clinical implications of our study, the strong (and cumulative) associations between suspected sleep disorders and psychopathology and well-being stress the importance of assessing sleep disorders in persons with mental health conditions. The high prevalence of suspected sleep disorders found in this study (and others) supports this as well. Furthermore, a potential lack of identification of sleep disorders in mental health settings was also apparent in our sample, as only 1.29% had a sleep disorder diagnosis recorded in their medical file. This is in stark contrast to the 46.2% who screened positively on the HSDQ. Incorporating screening instruments like the HSDQ and queries concerning the six clusters of sleep disorders in clinical interviews could aid early detection and guide further treatment steps.
Concretely, insomnia can be diagnosed based on self-reported symptoms and treated by cognitive behavioural therapy for insomnia (CBT-I), which has also been found to be effective in individuals with mental disorders (Hertenstein et al., 2022; Wu et al., 2015). There are several CBT-I protocols adapted to specific mental disorders and neurodevelopmental conditions available (Mijnster et al., 2022; Nowakowski et al., 2021). CRSWD can be treated using chronotherapy and light therapy (Auger, 2009; Lack & Wright, 2007). An alternative may be the more broadly oriented sleep intervention Transdiagnostic Sleep and Circadian Intervention (TranS-C; Harvey, 2016; Sarfan et al., 2022b). The parasomnia nightmare disorder can be treated by imagination and rescripting therapy alongside treatment for one's mental disorder (Van Schagen et al., 2015, 2016). For the other sleep disorders—SBD, hypersomnia, SRMD, and some specific parasomnias—referral to a sleep centre is required for both diagnosis and treatment. Finally, it seems relevant to consider that individuals with mental health conditions are often treated pharmacologically, and that many of these drugs exert negative effects on sleep (Neubauer et al., 2018). Therefore, a thorough assessment of prescribed pharmacological agents should be part of sleep assessment in those with mental health conditions.
In conclusion, we found high suspected prevalence rates of sleep disorders in a large and diverse sample of individuals with mental disorders or neurodevelopmental conditions with especially high rates in individuals with PTSD and cluster B personality disorders. Furthermore, presence of suspected sleep disorders was strongly associated with increased psychopathology and decreased well-being. These effects were larger with increasing numbers of suspected sleep disorders. While more (longitudinal) research is necessary, our results support the overt need to diagnose and treat the full range of sleep disorders in those with mental health conditions. This is especially relevant as a substantial part of our sample had more than one type of sleep disorder. Early detection by using a sleep screening questionnaire for diagnostics, and subsequent timely treatment of sleep disorders in patients with psychiatric and/or neurodevelopmental conditions could not only improve sleep, but also psychiatric outcome, and overall well-being.
AUTHOR CONTRIBUTIONS
Teus Mijnster: Formal analysis; investigation; methodology; project administration; software; validation; visualization; writing – original draft; writing – review and editing. Gretha J Boersma: Conceptualization; formal analysis; funding acquisition; investigation; methodology; project administration; resources; supervision; validation; writing – original draft; writing – review and editing. Maaike M van Veen: Conceptualization; funding acquisition; investigation; writing – review and editing. Edith Liemburg: Data curation; investigation; resources; writing – review and editing. Danielle Cath: Data curation; funding acquisition; investigation; resources; writing – review and editing. Gerdina H.M. Pijnenborg: Conceptualization; funding acquisition; validation; writing – review and editing. Peter J De Jong: Conceptualization; funding acquisition; validation; writing – review and editing. Marike Lancel: Conceptualization; formal analysis; funding acquisition; investigation; methodology; project administration; resources; supervision; validation; writing – original draft; writing – review and editing.
ACKNOWLEDGEMENTS
This work is supported by ZonMW, grant number 626310014. The MOPHAR database is funded by the Espria innovation fund. We thank Fiona ter Heege, Esther Meijer, and Manon Schallig for their contribution to the data collection.
CONFLICT OF INTEREST STATEMENT
None of the authors have conflicts of interest to disclose.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




