The efficacy of weighted blankets for sleep in children with attention-deficit/hyperactivity disorder—A randomized controlled crossover trial
Summary
Weighted blankets are a non-pharmacological intervention for treating sleep and anxiety problems in children with attention-deficit/hyperactivity disorder. However, research on the efficacy of weighted blankets is sparse. The aim of this randomized controlled trial with a crossover design (4 + 4 weeks) was to evaluate the efficacy of weighted blankets on sleep among children with attention-deficit/hyperactivity disorder and sleeping problems. Children diagnosed with uncomplicated Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition attention-deficit/hyperactivity disorder with verified sleep problems were randomized to start with either a weighted blanket or a lighter control blanket. Data collection was performed at weeks 0, 4 and 8 using actigraphy, questionnaires and a daily sleep diary. T-tests were used to evaluate efficacy. The study included 94 children with attention-deficit/hyperactivity disorder (mean age 9.0 [sd 2.2] years; 54 [57.4%] boys). Weighted blankets had a significant effect on total sleep time (mean diff. 7.72 min, p = 0.027, Cohen's d = 0.24), sleep efficiency (mean diff. 0.82%, p = 0.038, Cohen's d = 0.23) and wake after sleep onset (mean diff. −2.79 min, p = 0.015, Cohen's d = −0.27), but not on sleep-onset latency (p = 0.432). According to our exploratory subgroup analyses, weighted blankets may be especially beneficial for improving total sleep time in children aged 11–14 years (Cohen's d = 0.53, p = 0.009) and in children with the inattentive attention-deficit/hyperactivity disorder subtype (Cohen's d = 0.58, p = 0.016). Our results suggest that weighted blankets may improve children's sleep and could be used as an alternative to pharmacological sleep interventions.
1 INTRODUCTION
Attention-deficit/hyperactivity disorder (ADHD) affects 6% of children, with diverse adverse outcomes for both child and family (Faraone et al., 2021; Wernersson et al., 2020). The prevalence of sleep problems among children with ADHD is 25%–50% (Sung et al., 2008), and it is suggested that there is a common neurological aetiology between ADHD and sleep problems (Hvolby, 2015). ADHD symptomology and sleep problems may vary due to differences in background characteristics such as age (Kelly & El-Sheikh, 2014; Sadeh et al., 2009), gender (Becker et al., 2018) and ADHD subtype (Bondopadhyay et al., 2021; Hvolby, 2015). Sleep problems in children with ADHD may cause sedation, increased symptoms of ADHD and behaviour, and compromise academic performance (Ruiz-Herrera et al., 2021; Turnbull et al., 2013).
Non-pharmacological interventions are recommended as first-line treatment for children with sleep problems (Ogundele & Yemula, 2022). However, behavioural interventions and other non-pharmacological interventions are rare and less commonly applied than pharmacological interventions (Bliddal et al., 2022; Vriend & Corkum, 2011) for sleep problems in children with ADHD (Bondopadhyay et al., 2021; Larsson et al., 2023).
Weighted blankets (WBs) have been used as a non-pharmacological intervention for treatment of sleep and anxiety problems (Eron et al., 2020). ADHD is the most common diagnosis among children receiving WBs in healthcare (Cederlund et al., 2023). Despite weak evidence of effectiveness, the rationale for using WBs in practice stems from theories on deep pressure and sensory integration, where the weight is hypothesized to reduce the physiological level of arousal, anxiety and stress (Mullen et al., 2008). In addition, there is recently published evidence of WBs augmenting levels of melatonin (Meth et al., 2022), which might improve sleep.
However, there is limited evidence on the effects of WBs in general (Bondopadhyay et al., 2021; France et al., 2018). To our best knowledge, only three randomized controlled trials (RCT) are available on the effects of WBs on sleep, showing that WBs reduced subjective sleep disruption in adults with psychiatric disorders (Ekholm et al., 2020), increase melatonin levels in healthy adults (Meth et al., 2022), but no objective impact on sleep among children with autism (Gringras et al., 2014). WBs improved sleep-onset latency (SOL) compared with their regular blanket in two open studies in children with ADHD (Hvolby, 2020; Hvolby & Bilenberg, 2011); however, the possible benefits of WBs for children with ADHD have as yet not been shown in an RCT.
The primary aim of this study was to evaluate the efficacy of WBs on sleep, among children with ADHD and sleep problems. A secondary aim was to explore and compare possible moderators such as age, gender and ADHD subtype. We hypothesized that sleep measured by actigraphy would improve with WBs compared with lighter control blankets (CBs).
2 MATERIALS AND METHODS
2.1 Study design
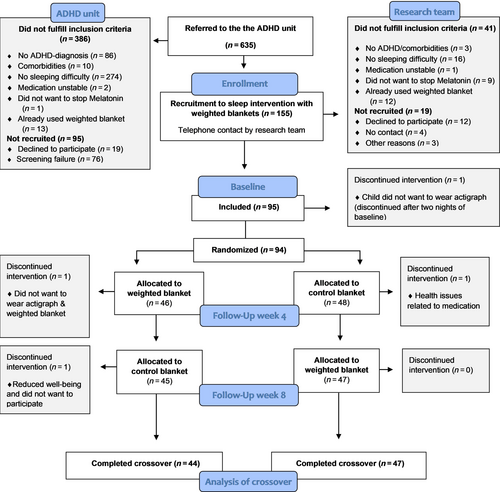
The study was a randomized controlled crossover trial of a sleep intervention using WBs versus lighter CBs (Larsson et al., 2022), reported in accordance with the CONSORT 2010 statement for crossover trials (Dwan et al., 2019). The crossover trial was conducted during 4 + 4 weeks in 2019–2022. Children were initially randomized to either a WB or a CB (Figure 1). The children randomized to start with the WB received it in the first period of 4 weeks and the CB in the second period of 4 weeks and vice versa. Randomization was sealed in envelopes and completed 1:1 in blocks of 10 to ensure a balance over time (Suresh, 2011). A lighter fibre blanket was chosen to be used as a CB for the comparison instead of usual care. Parents and children were informed about the study design with two different kinds of fibre blankets: one lighter and one heavier fibre blanket. No further information was given about the weight of the blankets, although when crossing over to the other blanket, the difference in weight became apparent.
2.2 Recruitment and participants
The study was conducted in collaboration with the ADHD unit at a child and adolescent mental health service (CAMHS) in the south of Sweden.
Children diagnosed with uncomplicated Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) ADHD spectrum disorder; Inattentive, Hyperactive and Combined subtype, without significant comorbidities (that would be a primary concern for pharmacological or psychosocial interventions), were triaged to the ADHD unit. Children triaged to the ADHD unit (about half of children receiving an ADHD diagnosis at the CAMHS) have fewer comorbidities than children triaged to usual care at CAMHS. The admission and diagnostic procedures for the ADHD unit have been described in more detail elsewhere (Wernersson et al., 2020). The brief child and family phone interview (BCFPI) was used to triage children to the ADHD unit, and children with significant comorbidities or severe psychosocial stress were transferred to usual care at CAMHS. Thus, some comorbidities of minor concern were diagnosed at the ADHD unit, but these patients remained and received treatment at the ADHD unit. Very few cases triaged to the ADHD unit were diagnosed with a non-ADHD primary disorder. These cases were subsequently referred to usual care at CAMHS.
The inclusion criteria for the sleep intervention were an ADHD diagnosis, sleep problems, stable medication, lack of or discontinuation of melatonin medication, and no prior use of WBs. The children were recruited to the sleep intervention after initial contact at the ADHD unit, and underwent a screening for sleep problems by either a psychiatrist or resident supervised by a psychiatrist who screened for sleep issues and informed the eligible children and parents about the study. Sleep problems were verified by three selected questions from the Children's Sleep Habits Questionnaire (CSHQ; Owens et al., 2000), concerning sleep initiation (> 20 min, 3–7 days per week), sleep maintenance (waking up several times per night, 3–7 days per week), and sleep duration (sleep too little, 3–7 days per week). The parents had to report their child's sleep problem as a problem in order to be eligible.
A total of 637 children were triaged for assessment to the ADHD unit during the recruitment period. A total of 386 children were excluded, of whom 86 did not receive an ADHD diagnosis and 274 did not have a sleep difficulty (Figure 1). A further 95 children were not recruited due to declining participation or screening failure (Figure 1).
The eligible children recruited at the ADHD unit (n = 155) and their parents were invited to participate in the study by the research team, and received written and verbal information about the study. Instructions about the study procedure, actigraphy, daily sleep diary and questionnaires were given by phone. Information confirming stable medication, that melatonin had been discontinued, and no prior use of WBs was obtained during this phone call. This resulted in a further 60 children being determined as ineligible or not willing to participate (Figure 1). A final total of 95 children and one of their parents (the participating parent agreed to answer all the parent questionnaires) were enrolled for baseline measurements and further randomized to either WB or CB.
2.3 Interventions—the WB and the CB
Fibre WBs from Novista of Sweden (Novista.se) were used in this study (150 × 210 cm, a standard size for children and adults in Sweden). Two occupational therapists independently chose the blanket's weight ranging from 6 to 10 kg according to the child's ADHD subtype (Inattentive or Hyperactive/Combined), degree of sleeping problems, weight, height, age and sex of the child (mean weight of WB/child body weight: 1.9/10 kg). The CB had a weight of 2 kg. The blankets had the same design and identical carrying bags. The weight was the only aspect that distinguished them.
2.4 Ethics
The parents and children were informed verbally and in writing about the study during the assessment at the ADHD unit, and provided written informed consent.
The research team contacted the parents by phone shortly after, and received more thorough information about the study procedures. Some children could start the intervention immediately after this phone call, but others had to wait until medication stabilization and a revisit to the ADHD unit. The research team then updated screening information about sleep problems, and eligible children started the intervention soon after.
The thorough information given to families during their initial visit to the ADHD unit and later by the researchers enabled the families to make informed consent. This thorough information was considered crucial to ensure adherence to the trial, and that both the child and the participating parent were willing to participate. Ethical approval was obtained from the Swedish Ethical Review Authority, Sweden (2019-02158/2019-03-18), and the study followed the principles outlined in the Declaration of Helsinki (World Medical Association, 2013).
2.5 Data collection
Measurements took place in the child's home environment. Data were collected during three measurement weeks; at baseline, and during the 4th and 8th weeks of the study (see flowchart in Figure 1). Data were collected through actigraphy, daily sleep diary text messages, child questionnaires, and parent questionnaires. Data collection was during autumn, winter and spring (7 September–4 June), only during regular schooldays and weekends, and not during holidays. No data collection was carried out during the summer break.
2.6 Measures
Primary outcomes and the focus in this study of crossover evaluation of efficacy of WBs were on objective sleep as measured by actigraphy. The four objective actigraph measures were: SOL; wake after sleep onset (WASO); total sleep time (TST); and sleep efficiency (SE).
Secondary outcomes were subjectively measured parent- and child-reported sleep. Sleep diary data were also collected in support of the interpretation of actigraphic sleep measurements.
Demographic variables and background characteristics were collected through parent questionnaires. ADHD diagnosis was determined according to DSM-5 by an intern or psychiatrist at CAMHS. More information on outcome measures is found in the study protocol (Larsson et al., 2022).
2.6.1 Objectively measured sleep–actigraphy
Sleep was measured objectively with actigraphy during baseline, week 4 and week 8. The children wore the actigraph (Motionware 1.2.47 Camntech) during each measurement week (7 nights). The measurements were performed during the last week of each period (4 weeks during each period) to minimize the risk of bias due to carry-over effects. Parents were encouraged to contact the research team if any problems occurred during the week, such as unusual events, technical issues, or concerns that were noted in a log.
Actigraph data were analysed together with information from the daily sleep diary to support the interpretation of data (Meltzer et al., 2012). The daily sleep diary is a digital diary where parents answer a daily text message each morning during 1 week of measurement. Additional information on the six questions in the daily sleep diary text message is found in the study protocol (Larsson et al., 2022).
Actigraph data were analysed for each night separately, with a mean value for the whole week for SOL, WASO, TST and SE, and defined as presented in Table 1. Three nights were considered a minimum for the actigraph sleep analysis (Littner et al., 2003).
| Actigraphic measures | Definition |
|---|---|
| Sleep-onset latency (SOL) | The duration of time from turning the light off to falling asleep (min) |
| Wake after sleep onset (WASO) | The total amount of wakefulness occurring after defined sleep onset until wake up (min) |
| Total sleep time (TST) | The total amount of sleep time from sleep onset until wake up (min) |
| Sleep efficiency (SE) | The ratio of total sleep time (TST) to time in bed (lights off until wake up) (multiplied by 100 to yield a percentage) (%) |
The first set of analyses was performed together by two researchers (ML, KA) to find a consensus in the interpretation and scoring of sleep data. The actigraph provides data on time asleep and awake according to the algorithm by Kushida (Kushida et al., 2001). The sensitivity setting was set to medium, and the epoch length was set to 30 s.
2.6.2 Subjectively measured sleep
Sleep was evaluated subjectively with parent- and child-reported outcomes.
Children's sleep habits questionnaire—parent-reported
The CSHQ has 33 items divided into eight subscales concerning sleep behaviour and sleep problems (Owens et al., 2000). Parents rate each item over the last week on a three-point scale from rarely (1), sometimes (2), and usually (3). Overall sleep problems are summarized in a total score from 33 to 99. The clinical cut-off is 41. A higher score indicates greater sleep problems.
Insomnia Severity Index (ISI)—child-reported
The ISI has a total of seven items concerning night-time and daytime consequences of insomnia (Bastien, 2001; Kanstrup et al., 2014). Children rate each item over the last week on a four-point scale from none (0), mild (1), moderate (2), severe (3), and very severe (4). A higher score indicates a higher level of insomnia severity. The total score range is 0–28, and the clinical cut-off in adolescents is 9 (Chung et al., 2011).
2.6.3 Satisfaction with the WB evaluated with child-reported data
Children's quality of sleep and satisfaction with the blanket during each period is scored by the child on a visual analogue scale of 0–100 clarified with sad/happy faces. The crossover design allows the child to state their satisfaction with the WB and the lighter CB. Questions are “How well have you slept with the blanket that you have tried out the last month?” and “How pleased are you with the blanket that you have tried out the last month?” A higher score indicates a greater preference.
2.7 Sample size
A power analysis based on estimated changes in SOL, one of the primary outcome variables, was performed (Larsson et al., 2022). This analysis indicated that 58 children (29 in each group) would be sufficient if accepting a 30% change in SOL, from a mean of 35 min (SD 15), to detect 80% power. To allow for a 40% drop-out, 90–100 children were determined as an appropriate sample size.
2.8 Statistical analysis
2.8.1 Crossover analysis
The correct analysis of crossover trials consists of three t-tests, which evaluate the treatment effect, the period effect, and the treatment–period interaction effect (Altman, 1990).
The period effect is tested with a two-sample t-test by comparing the difference between the periods (period 1 – period 2) in the two randomized groups. Period 1 encompasses the first 4 weeks with the first blanket, and period 2 the second 4 weeks with the next blanket. Due to randomization, period 1–period 2 is WB–CB for children randomized to start with the WB, and vice versa.
The treatment–period interaction is tested with a two-sample t-test comparing the average response of the two blankets ([period 1 + period 2]/2) between the two randomized groups (Altman, 1990). If the treatment effect during the first period is carried over to the next period, the average response will increase. Measurements were taken on the last week of each period to minimize treatment–period interaction.
The treatment effect is tested with a one-sample t-test comparing the within-subject differences between the WB and the CB (Altman, 1990). Mean differences of WB versus CB found not meeting the criteria for normal distribution according to Shapiro–Wilk W-test for normal data were also analysed with Wilcoxon signed rank test.
2.8.2 Pre–post analysis
Pre–post analysis was conducted to further evaluate the found period effects. Sleep was evaluated over time (baseline–week 4 & week 4–week 8) for each randomized group by one-sample t-tests. Significant differences were set at a p-value of 0.05 (two-sided). Figures were used to illustrate pre–post changes for outcomes with significant period effects in crossover analysis.
2.8.3 Exploratory subgroup analyses by age group, gender and ADHD subtype
Exploratory crossover subgroup analyses were conducted for primary outcomes comparing the within-subject differences between the WB and the CB by age group (6–10/11–14 years), gender (boys/girls), and subtype (Inattentive/Hyperactive or Combined). The age groups were chosen according to changes in emotional regulation and sleep during onset of puberty (Kelly & El-Sheikh, 2014; Lucien et al., 2021; Sadeh et al., 2009). These analyses were not specified a priori and are considered exploratory.
2.8.4 Sensitivity analysis
Sensitivity analyses were conducted on primary outcomes to rule out any potential impact of the chosen method of analysis on the results for all crossover treatment comparisons (t-test or Wilcoxon signed rank test). Missing data, removal of outliers, and removal of non-adherent participants were also considered as part of sensitivity analyses (Thabane et al., 2013). Data from participants were analysed in their assigned blanket group, irrespective of adherence to assigned treatment, as “intended-to-treat” (Ranganathan et al., 2016). Missing data were not imputed if considered not to be significantly different from the remaining sample regarding sex, age, stimulant medication, or parent education.
Children included were stable on ADHD medication before inclusion, and were encouraged not to initiate other sleep adjustments during the study period or adjust their medications. Without our knowledge, seven children did not discontinue their melatonin treatment during the study period due to the severity of their sleeping problems. This was revealed after crossover. These children were excluded as part of a sensitivity analysis. Because no change was found in results or level of significance when these children were excluded, a decision was made to include these children in analyses. All analyses were conducted using STATA 16.
3 RESULTS
3.1 Sample characteristics
A total of 94 children participated in the sleep intervention with WBs (Figure 1). Baseline sample characteristics are summarized in Table 2. The mean age at baseline (n = 94) was 9.0 years (2.2 sd; range 6–14 years), and 54 (57.4%) were boys. Of the ADHD subtypes, four children had hyperactive subtype, 25 had inattentive subtype, and 65 children had combined subtype. The children's mean baseline values were: SOL, 35 min; WASO, 42 min; TST, 489 min; and SE, 87%. Complete baseline and period measurements by randomized blanket are found in Appendix A; Table A1.
| Characteristics | WB–CB (n = 46) | CB–WB (n = 48) |
|---|---|---|
| Gender male, n (%) | 28 (60.9) | 26 (54.2) |
| Age (years), mean (SD) | 9.5 (2.4) | 9.4 (2.1) |
| Child weight (kg), mean (SD) | 39.8 (13.1) | 37.7 (10.9) |
| Child height (cm), mean (SD) | 141.3 (13.4) | 139.9 (11.6) |
| ADHD subtype, n (%) | ||
| Hyperactive | 1 (2.2) | 2 (4.2) |
| Inattentive | 12 (26.1) | 13 (27.1) |
| Combined | 33 (71.7) | 33 (68.8) |
| Global assessment of functioning, mean (SD) | ||
| CGAS | 51.0 (3.5) | 51.9 (4.7) |
| ADHD medication at baseline, n (%) | ||
| Stimulant | 20 (43.5) | 25 (52.1) |
| No ADHD medications | 26 (56.5) | 23 (47.9) |
| Melatonin during baseline, n (%) | ||
| Melatonin | 2 (4.3) | 5 (5.3) |
| No sleep medication | 44 (95.7) | 43 (89.6) |
| Comorbidities, n (%) | ||
| Autism spectrum disorder | 1 (2.2) | 1 (2.1) |
| Generalized anxiety disorder | – | 1 (2.1) |
| Oppositional defiant disorder | 4 (8.7) | 4 (8.3) |
| Motor or vocal tic disorder | 2 (4.3) | – |
| Language disorder | – | 2 (4.2) |
| No comorbidities | 43 (93.5) | 44 (91.7) |
| Gender of participating parent | ||
| Male, n (%) | 8 (17.4) | 2 (4.2) |
| Age of participating parent, mean (SD) | 39.5 (5.7) | 38.8 (5.6) |
| Highest education of parent, n (%) | ||
| Compulsory school | 1 (2.2) | 5 (10.4) |
| Upper secondary school | 18 (39.1) | 17 (35.4) |
| University | 27 (58.7) | 26 (54.2) |
| Single parent, n (%) | 9 (19.6) | 9 (18.8) |
| Alternates between two households, n (%) | 11 (23.9) | 10 (20.8) |
- Note: Characteristics for children randomized to start with a WB (WB–CB; n = 46) or to start with a CB (CB–WB; n = 48).
- Abbreviation: ADHD, attention-deficit/hyperactivity disorder; CB, control blanket; CGAS, children's global assessment scale; WB, weighted blanket.
3.2 Randomization, treatment acceptability and adherence
Forty-six children were randomized to start the first period with the WB, and 48 children were randomized to start the first period with the CB. Three children dropped out during the 4 + 4 week crossover periods, leaving 91 children (44 + 47) available for analyses (Figure 1). Two of the three children dropped out due to unwillingness to wear actigraph and/or blankets. One of the three children withdrew due to health issues related to a change of medication, decreased well-being, and perceived ineffectiveness of the CB. Self-reported side-effects of the WB included panic (n = 1), anxiety (n = 1) and pain (n = 2). A drop-out rate of n = 3 and almost complete adherence during the trial resulted in crossover analysis of actigraph data (n = 85), daily sleep diary data (n = 89), child questionnaire data (n = 88), and parent questionnaire data (n = 87; Appendix B; Table B1). The average time the children wore the actigraph during measurement weeks was: for WB, 6.33 days (SD 0.97, range 4–7 days); and for CB, 6.47 days (SD 0.93, range 3–7 days). Children with missing data were no different from the remaining sample regarding gender, age, stimulant medication, or parent education.
Children's adherence (6–7 days with blanket per week) to WB was 86.2%–90.8% and to CB was 85.1%–92.0% (Table 3).
| Number of days/week | WB adherence (4 weeks), n = 87 | CB adherence (4 weeks), n = 87 |
|---|---|---|
| Week 1 | ||
| 6–7 days, n (%) | 79 (90.8) | 76 (87.4) |
| 4–5 days, n (%) | 6 (6.9) | 6 (6.9) |
| 0–3 days, n (%) | 2 (2.3) | 5 (6.3) |
| Week 2 | ||
| 6–7 days, n (%) | 76 (87.4) | 80 (92.0) |
| 4–5 days, n (%) | 7 (8.0) | 4 (4.6) |
| 0–3 days, n (%) | 3 (3.4) | 3 (3.4) |
| Week 3 | ||
| 6–7 days, n (%) | 75 (86.2) | 74 (85.1) |
| 4–5 days, n (%) | 5 (5.7) | 10 (11.5) |
| 0–3 days, n (%) | 7 (8.0) | 3 (3.4) |
| Week 4 | ||
| 6–7 days, n (%) | 78 (89.7) | 78 (89.7) |
| 4–5 days, n (%) | 3 (3.4) | 6 (6.9) |
| 0–3 days, n (%) | 6 (6.9) | 3 (3.4) |
- Note: Number of days with blanket use during nighttime for period with WB and period with CB according to parent-reported questionnaires. Children used each blanket (WB or CB) during 4 + 4 weeks.
- Abbreviation: CB, control blanket; WB, weighted blanket.
3.3 Treatment effect of WBs
3.3.1 Objectively measured sleep
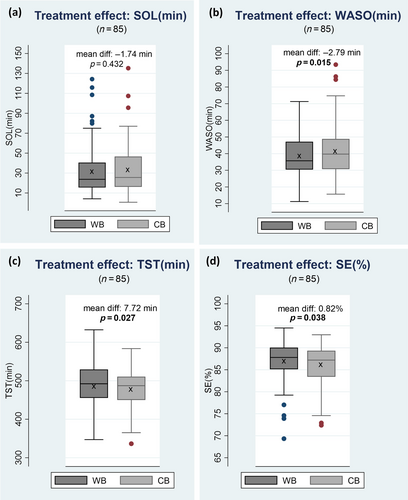
Comparing the WB with the CB in crossover analyses, a significant treatment effect at p < 0.05 emerged for three of the four primary outcome measures WASO, TST and SE, but not for SOL (Table 4). The sensitivity analyses of data showing non-normality distribution (i.e. SE and CSHQ) showed that the treatment effects were unchanged when analysed with Wilcoxon signed rank test (Appendix C; Table C1).Children had significantly lower WASO (WASO mean diff. [SD]:−2.79 [10.38]), longer TST (TST mean diff. [SD]: 7.72 [31.69]) and improved SE (SE mean diff. [SD]:−0.82 [3.60]) when sleeping with the WB as compared with when sleeping with the CB (Figure 2a–d).
| Outcomes | n | WB–CB mean diff. (SD) | CB–WB mean diff. (SD) | WB versus CB mean diff. (SD) | Treat. effect t/p-value | Treat. effect Cohens d | Period effect t/p-value | Treat. × period effect t/p-value |
|---|---|---|---|---|---|---|---|---|
| Objectively measured sleep | ||||||||
| SOL, min | 85 | −3.11 (16.11) | 0.46 (23.64) | −1.74 (20.29) | −0.79/0.432 | −0.086 | −0.60/0.550 | −1.68/0.097 |
| WASO, min | 85 | −0.92 (11.01) | 4.53 (9.55) | −2.79 (10.38) | −2.48/0.015 | −0.269 | 1.62/0.109 | 0.38/0.706 |
| TST, min | 85 | 16.63 (26.59) | 0.59 (34.03) | 7.72 (31.69) | 2.25/0.027 | 0.244 | 2.41/0.018 | 0.12/0.906 |
| SE, % | 85 | 0.97 (2.81) | −0.69 (4.23) | 0.82 (3.60) | 2.11/0.038 | 0.229 | 0.36/0.721 | 1.29/0.199 |
| Subjectively measured sleep | ||||||||
| Sleep problems, CSHQ total scorea | 81 | −0.08 (4.40) | 1.99 (4.00) | −1.05 (4.29) | −2.20/0.031 | −0.245 | 2.04/0.045 | −1.76/0.082 |
| Insomnia severity, ISI total score | 88 | −1.72 (4.90) | −0.24 (3.57) | −0.71 (4.36) | −1.54/0.127 | −0.164 | 2.16/0.034 | 0.26/0.796 |
- Note: Significant p-values (p < 0.05) in bold. Wilcoxon signed rank of WB versus CB was conducted on treatment outcomes showing non-normality distributed data. p-Values from Wilcoxon signed rank; SE: p = 0.015; CSHQ: p = 0.010. Mean differences in objectively and subjectively measured sleep with WBs compared with CBs. Treatment effect, period effect and treatmentxperiod effect.
- Abbreviation: CSHQ, Children's Sleep Habits Questionnaire; ISI, Insomnia Severity Index; SE, sleep efficiency; SOL, sleep-onset latency; TST, total sleep time; WASO, wake after sleep onset.
- a Partially missing data in some of the included parent items. For CSHQ, data is partially missing for six children, generating a total of 81 children for crossover analysis.
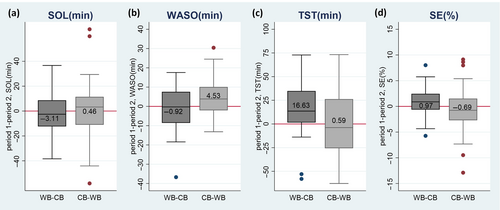
No treatment by period effects were present for any of the primary outcomes, although a period effect was present for TST (t = 2.41, p = 0.018), i.e. a larger change in TST for children using the WB in the first period (TST mean diff. [SD]: 16.63 [26.59]; Figure 3c; Table 4). TST was thus further evaluated in pre-post comparisons (Figure 4).
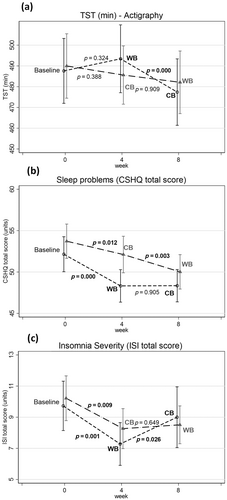
Pre–post comparisons showed a significantly decreased TST (comparing week 4–week 8) for children using the WB in the first period (week 4) (t = −4.00, p = 0.000). There was no significant change in TST (comparing week 4–week 8) for children using the WB in the second period (week 8; t = −0.11, p = 0.909; Figure 4; Appendix D; Table D1).
3.3.2 Subjectively measured sleep
Comparing the WB with the CB in crossover analyses, a significant treatment effect was present for parent-reported sleep problems CSHQ but not for child-reported sleep severity ISI (Table 3). No treatment by period effects were present for any of the parent- or child-reported sleep measures, although a period effect was present for both CSHQ and ISI (Table 3).
The evaluation of sleep problems (CSHQ) and sleep severity (ISI) in pre–post comparisons showed significantly decreased sleep problems comparing baseline with first period (week 4) for both CSHQ and ISI (Figure 4b,c; Appendix D; Table D1). This decrease was present for both the WB and the CB. For CSHQ and ISI, the decrease for children using the WB in the first period (week 4) was significantly changed comparing baseline with first period (CSHQ mean diff. [SD]: −3.68[4.85], t = −4.80, p = 0.000; ISI mean diff. [SD]: −2.2[4.2], t = 3.41, p = 0.001). The clinical cut-off for CSHQ is 41 and for ISI is 9. Children's sleep problems decreased from baseline to first period with WB to: CSHQ 48.4 [6.5] and ISI 7.27 [4.6].
For children using the WB in the second period, the decrease was significant comparing both baseline with first period (CSHQ mean diff. [SD]: −1.70[4.33], t = −2.63, p = 0.012) and comparing first period with second period (CSHQ mean diff. [SD]: −1.99[4.01], t = −3.18, p = 0.003; Figure 4b).
3.4 Child satisfaction with WBs
Children rated their quality of sleep with the WB as improved compared with the CB (mean diff. [SD]: 18.60[33.70], t = 5.18, p < 0.000). Their satisfaction with the WB was also higher (mean diff. [SD]: 26.28[41.27], t = 5.97, p < 0.000) as compared with the CB. There was no treatment by period effect, although a significant (p < 0.05) period effect was found for both quality of sleep and blanket satisfaction. Children using the WB in the first period (week 4) were more satisfied with the WB (mean satisfaction rate [SD]: 85.88[20.10]) compared with children using the WB in the second period (week 8; mean satisfaction rate [SD]: 77.27[26.95]). There was no difference in children's ratings concerning the CB (first period mean satisfaction rate [SD]: 55.00[34.60]; second period mean satisfaction rate [SD]: 55.39[33.71]).
3.5 Exploratory subgroup analyses by age group, gender and ADHD subtype
3.5.1 Baseline sleep measurement by age group, gender and ADHD subtype
Demographics and baseline characteristics by age group, gender and ADHD subtype are found in Table 5. Additional information on children's different background characteristics by subgroup can be found in Appendix E; Table E1. Children aged 11–14 years had lower baseline measurements in TST as compared with children aged 6–10 years (p < 0.001). Children with Inattentive subtype had lower baseline TST than children with Hyperactive/Combined subtype (p < 0.01). Children aged 6–10 years had longer baseline WASO than children aged 11–14 years (p < 0.05).
| Objectively measured sleep | Age group | Gender | ADHD subtype | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 6–10 years (n = 64) | 11–14 years (n = 28) | Boys (n = 53) | Girls (n = 39) | Hy/C (n = 67) | In (n = 25) | ||||
| Mean (SD) | Mean (SD) | p-Value | Mean (SD) | Mean (SD) | p-Value | Mean (SD) | Mean (SD) | p-Value | |
| SOL, min | 33.0 (27.9) | 39.9 (30.1) | 0.292 | 36.5 (25.0) | 33.2 (33.0) | 0.579 | 32.6 (24.8) | 41.8 (36.6) | 0.173 |
| WASO, min | 44.3 (16.2) | 35.9 (16.8) | 0.025 | 43.3 (15.1) | 39.7 (18.8) | 0.306 | 43.1 (18.0) | 38.1 (12.6) | 0.208 |
| TST, min | 504.7 (47.2) | 452.5 (42.3) | 0.000 | 495.1 (45.8) | 480.3 (58.0) | 0.175 | 498.3 (47.2) | 463.4 (55.1) | 0.003 |
| SE, % | 86.8 (4.5) | 85.9 (5.3) | 0.428 | 86.2 (4.2) | 87.0 (5.5) | 0.449 | 86.9 (4.4) | 85.5 (5.7) | 0.233 |
- Note: Mean difference in baseline data evaluated with two-sample t-test, p < 0.05. Significant group differences in bold.
- Abbreviation: ADHD, attention-deficit/hyperactivity disorder; C, Combined subtype; Hy, Hyperactive subtype; In, Inattentive subtype; SE, sleep efficiency; SOL, sleep-onset latency; TST, total sleep time; WASO, wake after sleep onset.
3.5.2 Treatment effect of WBs by age group, gender and ADHD subtype
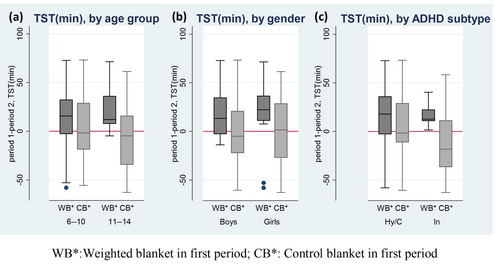
In crossover analysis, a significant treatment effect (Table 6) emerged on TST comparing WB versus CB for children 11–14 years (16.3 min, p = 0.009, Cohen's d = 0.53) and for children with Inattentive subtype (15.6 min, p = 0.016, Cohen's d = 0.58), but no difference by gender in TST (Figure 5a–c). A significant treatment effect was also found in WASO for boys (−3.5 min, p = 0.034, Cohen's d = −0.31) and in SE for children aged 11–14 years (2.1%, p = 0.009, Cohen's d = 0.53), as well as in SE for boys (1.0%, p = 0.042, Cohen's d = 0.30; Table 6).
| Objectively measured sleep | Age | Gender | ADHD subtype | |||
|---|---|---|---|---|---|---|
| 6–10 years (n = 57) | 11–14 years (n = 28) | Boys (n = 49) | Girls (n = 36) | Hy/C (n = 64) | In (n = 21) | |
| Mean diff./p-value | Mean diff./p-value | Mean diff./p-value | Mean diff./p-value | Mean diff./p-value | Mean diff./p-value | |
| SOL, min | 1.1/0.668 | −7.4/0.098 | −2.4/0.375 | −0.8/0.828 | −0.2/0.937 | −6.5/0.322 |
| WASO, min | −2.6/0.083 | −3.2/0.070 | −3.5/0.034 | −1.9/0.241 | −2.4/0.079 | −4.0/0.065 |
| TST, min | 3.5/0.405 | 16.3/0.009 | 8.05/0.069 | 7.5/0.194 | 5.1/0.216 | 15.6/0.016 |
| SE, % | 0.2/0.628 | 2.1/0.009 | 1.0/0.042 | 0.6/0.381 | 0.5/0.212 | 1.9/0.093 |
- Note: Significant p-values (p < 0.05) are in bold. One sample t-test used for crossover evaluation of WB versus CB, p < 0.05. Wilcoxon signed rank of WB versus CB was conducted on treatment outcomes showing non-normality distributed data. P-Values from Wilcoxon signed rank; SOL (11–14): p = 0.035; SE (11–14): p = 0.001; SE (boys): p = 0.037.
- Abbreviation: C, Combined subtype; Hy, Hyperactive subtype; In, Inattentive subtype; SE, sleep efficiency; SOL, sleep-onset latency; TST, total sleep time; WASO, wake after sleep onset.
4 DISCUSSION
To our knowledge, this study is the first RCT evaluating the efficacy of WBs in children with ADHD. Our principal findings showed that WBs significantly improved actigraph-measured WASO, TST and SE, but not SOL as compared with using a lighter CB. Furthermore, parent ratings of children's sleeping problems were also improved with WBs.
Using WBs improved TST, with a mean change of 8 min for the total group of children and 16 min improvement for children with the inattentive subtype and children aged 11–14 years. There is a change in sleep and emotional systems as children develop and begin their transition toward adolescence (Sadeh et al., 2009). Increased sleep duration could be especially beneficial during this transition (Kelly & El-Sheikh, 2014), and especially among individuals with sleep problems. The statistically significant treatment effects in our crossover trial are in magnitude similar to other sleep intervention studies (most commonly parallel trials), for example, in non-pharmacological interventions for children in general (Magee et al., 2022) and for children with ADHD (Hiscock et al., 2015; Larsson et al., 2023). There are no standards for the exact number of minutes of prolonged sleep that are clinically relevant. However, treatment effects from sleep interventions rarely exceed 30 min improvements in TST (Morin, 2003), and 15–30 min improvement is considered a moderate treatment effect in children with ADHD (Larsson et al., 2023). Comparing changes in subjective sleep quality from baseline to post-treatment to a normative sample could be a more valid finding (Morin, 2003). From our pre–post comparisons, from baseline to use of the WB, the analyses showed decreased sleep problems for the total score of CSHQ and ISI to ranges within normative values for children with ADHD. This can be interpreted as clinically significant reductions in total scores and is in line with previous studies, where CSHQ total scores in children aged 5–14 years with ADHD with no/mild sleep severity were found to be within the same range as our post-treatment values (Lycett et al., 2015). Also, children's self-reported sleep severity decreased in our sample to values far below the clinical cut-off level (Chung et al., 2011). However, more research needs to be carried out concerning the effectiveness of WBs in clinical settings.
A main finding in this study is the efficacy of WBs on WASO and SE. Difficulties in settling at night and night-time awakenings are the most commonly described sleep complaint in children with ADHD and other neurodevelopmental disorders (Bruni, 2018), emphasizing the clinical relevance of our results. If children's restlessness and subsequent sleep problems decrease during night-time, the whole family may experience improved functioning and overall well-being. Improved child and family functioning when using WBs was confirmed in interviews with children (Lönn et al., 2023) and parents (Larsson et al., 2021).
In this study, there was no significant effect on SOL, even if the parents experienced improved sleep initiation in a qualitative study including children with ADHD when using WBs (Larsson et al., 2021). Improvement in SOL has also been shown in two open studies of children with ADHD when using ball blankets (Hvolby, 2020; Hvolby & Bilenberg, 2011). However, according to a clinical register study, melatonin is used to promote sleep initiation, and WBs are used as a complement to melatonin, with the intention of improving sleep maintenance (Cederlund et al., 2023).
Thus, various interventions need to be available in clinical practice to target children's different needs and purposes for intervention, as the preferences of families struggling with sleep problems may differ. WBs can provide an important addition to current sleep intervention practices for children with ADHD. Even if non-pharmacological sleep interventions are recommended as first-line treatment for children with sleep problems (Ogundele & Yemula, 2022), they are less commonly applied than pharmacological interventions (Bliddal et al., 2022; Vriend & Corkum, 2011). One explanation for this is that few evidence-based non-pharmacological interventions exist for sleep problems of children with ADHD (Bondopadhyay et al., 2021; Larsson et al., 2023). The findings in our study thus contribute important evidence for WBs as a non-pharmacological sleep intervention.
4.1 Strengths and limitations
The main strength of the present study is the rigorous research design with a randomized controlled comparison of WBs with lighter CBs with the same design. Further strengths are the sample size and trial adherence. The validity is strengthened through the prospectively published study protocol (Larsson et al., 2022), the robust testing of the results (Thabane et al., 2013), and the evaluation of different aspects of sleep both objectively and subjectively. The crossover design has the strength of detecting differences without the issue of confounding due to differences in health states (Dwan et al., 2019). This strengthens the results concerning the causality of the effect of WBs on sleep, but somewhat underestimates the effect sizes. Objective measures of sleep are especially well suited for the crossover design, as they are not likely to be affected by subjective preferences or blinding issues.
However, a limitation could be the imperfect blinding as parents possibly could figure out which was intended to be the active intervention. This could explain the larger standard deviation and decreased efficacy for children randomized to start with the CB.
Sleep is a complex phenomenon characterized and influenced by several components. For example, we did not evaluate the efficacy based on primary sleep disorder. Also, participating in a sleep intervention might improve sleep hygiene practices even if the participants were informed not to change anything in their environment (Larsson et al., 2021; Lönn et al., 2023) but, in such cases, this should have affected both groups. This could also explain why some self-reported sleep outcomes were improved for participants using both WBs and CBs. Parent and child experience of children's sleep problems over time differs from objectively measured impact on sleep (Owens et al., 2016), as indicated in our results.
Another limitation may be that children with major comorbidities, like autism, mood and anxiety disorders, were not included in the current study. Comorbid anxiety, associated with increased sleep latency, is common among children with ADHD (Spruyt & Gozal, 2011). Our sample without major comorbidities might underestimate SOL. This could be a limitation concerning generalizability to children with ADHD in general, and possibly underestimating the effects of the intervention as a more clinically complex subgroup of ADHD would also be expected to have more severe sleeping issues. Future studies should evaluate the effectiveness of WBs in a clinical setting, and include children with comorbidities such as anxiety, depression and externalizing syndromes to increase the external validity of the results.
5 CONCLUSION
This crossover RCT study showed that the use of WBs is effective in improving sleep duration, sleep maintenance and decreasing sleep disruption in children with ADHD. The WBs significantly improved actigraph-measured WASO, TST and SE, but not SOL as compared with using a lighter CB. In addition, the results showed that WBs were especially beneficial for children aged 11–14 years and children with the inattentive ADHD subtype. WBs could be used as a non-pharmacological sleep intervention, and be recommended in clinical guidelines as a first-line intervention for sleep difficulties in ADHD.
AUTHOR CONTRIBUTIONS
Petra Svedberg, Håkan Jarbin, Jens Nygren, Katarina Aili and Ingrid Larsson conceptualized and designed the study. Maria Lönn, Petra Svedberg, Håkan Jarbin, Katarina Aili and Ingrid Larsson contributed to data collection. Maria Lönn performed the statistical analyses. Maria Lönn, Petra Svedberg, Jens Nygren, Håkan Jarbin, Katarina Aili and Ingrid Larsson interpreted the data. Maria Lönn drafted the manuscript, and all the authors contributed to review and to editing the manuscript. All authors approved the final version.
ACKNOWLEDGEMENTS
The authors thank all the children and parents who have participated in this study, and the secretaries, nurses, psychiatrists and residents at the ADHD unit who made this study possible. The authors thank Ulf Strömberg at the Department of Research and Development, Region Halland, for his helpful support on statistical analysis of crossover trials. The authors also thank Julia Malmborg Söderström for support in the analysis of raw data from the actigraph sleep measurements. The authors are grateful for the support from Novista of Sweden AB for contributing with the fibre blankets used in this study, and Carmona AB for database creation and maintenance.
FUNDING INFORMATION
This work was supported by external grants from The Knowledge Foundation [number 20200012], Swedish Research Council for Health Working Life and Welfare (Forte) [number 2021-00664]; Majblomman foundation and different grants from Region Halland.
CONFLICT OF INTEREST STATEMENT
Maria Lönn, Katarina Aili, Petra Svedberg, Jens Nygren, Håkan Jarbin and Ingrid Larsson have no conflicts of interest to disclose. The funding bodies and the companies had no role in or influence on the study design, data collection, analysis, interpretation of data, or manuscript preparation.
APPENDIX A
| Group (WB–CB) | Group (CB–WB) | |||||||
|---|---|---|---|---|---|---|---|---|
| n | Baseline mean (SD) | Period 1 WB mean (SD) | Period 2 CB mean (SD) | n | Baseline mean (SD) | Period 1 CB mean (SD) | Period 2 WB mean (SD) | |
| Objectively measured sleep | ||||||||
| SOL, min | 45 | 33.00 (25.41) | 27.54 (18.13) | 30.41 (21.54) | 47 | 37.83 (31.30) | 36.20 (25.30) | 35.61 (27.36) |
| WASO, min | 45 | 42.24 (17.50) | 40.41 (13.01) | 41.39 (13.77) | 47 | 41.74 (17.27) | 42.09 (17.40) | 37.61 (14.51) |
| TST, min | 45 | 486.11 (52.39) | 492.76 (53.69) | 476.12 (53.34) | 47 | 490.40 (53.32) | 482.93 (46.94) | 482.98 (50.83) |
| SE, % | 45 | 87.05 (4.11) | 87.82 (3.36) | 86.87 (3.63) | 47 | 85.89 (5.37) | 85.85 (5.11) | 86.58 (5.14) |
| Subjectively measured sleep | ||||||||
| Sleep problems, CSHQ total score | 46 | 52.14 (6.84) | 48.36 (6.49) | 48.45 (6.37) | 48 | 53.72 (7.09) | 52.01 (7.83) | 50.02 (7.12) |
| Insomnia severity, ISI total score | 46 | 9.61 (5.22) | 7.27 (4.60) | 9.00 (6.53) | 48 | 10.21 (4.82) | 8.27 (4.40) | 8.51 (4.16) |
- Abbreviation: CB, control blanket; CSHQ, Children's Sleep Habits Questionnaire; ISI, Insomnia Severity Index; SE, sleep efficiency; SOL, sleep-onset latency; TST, total sleep time; WASO, wake after sleep onset; WB, weighted blanket.
APPENDIX B
| Participated in crossover with WBs | ||||
|---|---|---|---|---|
| WB–CB n = 46 | CB–WB n = 48 | |||
| Analysed | Reason for missing data | Analysed | Reason for missing data | |
| 0 w (n = 94) | Actigraph data n = 45 | Do not want to wear actigraph n = 1 | Actigraph data n = 47 | Technical error n = 1 |
| Daily sleep diary n = 46 | Daily sleep diary n = 48 | |||
| Child survey data n = 46 | Child survey data n = 48 | |||
| Parent survey data n = 46 | Parent survey data n = 48 | |||
| 4 w (n = 92) | Actigraph data n = 43 | Do not want to wear actigraph n = 1 | Actigraph data n = 45 | Do not want to wear actigraph n = 1 |
| Technical error n = 1 | Only 2 nights with actigraph n = 1 | |||
| Daily sleep diary n = 44 | Only 1 night with sms n = 1 | Daily sleep diary n = 46 | Missing daily sleep diary n = 1 | |
| Child survey data n = 45 | Child survey data n = 47 | |||
| Parent survey data n = 44 | Missing survey n = 1 | Parent survey data n = 47 | ||
| 8 w (n = 91) | Actigraph data n = 42 | Do not want to wear actigraph n = 1 | Actigraph data n = 45 | Do not want to wear actigraph n = 1 |
| Technical error n = 1 | Technical error n = 1 | |||
| Daily sleep diary n = 44 | Daily sleep diary n = 46 | Missing daily sleep diary n = 1 | ||
| Child survey data n = 43 | Missing survey n = 1 | Child survey data n = 45 | Missing survey n = 2 | |
| Parent survey data n = 43 | Missing survey n = 1 | Parent survey data n = 45 | Missing survey n = 2 | |
| Included in crossover analysis | ||||
|---|---|---|---|---|
| Crossover | Analysed | Reason for missing data | Analysed | Reason for missing data |
| n = 85 | Actigraph data n = 41 | Missing data in week 4 or 8 | Actigraph data n = 44 | Missing data in week 4 or 8 |
| n = 88 | Child survey data n = 43 | Missing data in week 4 or 8 | Child survey data n = 45 | Missing data in week 4 or 8 |
| n = 87a | Parent survey data n = 42 | Missing data in week 4 or 8 | Parent survey data n = 45 | Missing data in week 4 or 8 |
- Abbreviation: CB, control blanket; WB, weighted blanket.
- a Partially missing data in some of the included parent items. For CSHQ, data are partially missing for six children, generating a total of 81 children for crossover analysis.
APPENDIX C
| n | Distribution of dataa | WB 25/50/75 percentile | CB 25/50/75 percentile | Treat. effect z/p-value | Treat. effect t/p-value | |
|---|---|---|---|---|---|---|
| Objectively measured sleep | ||||||
| SOL, min | 85 | Normal | 15.5/23.7/40.5 | 16.2/25.57/46.6 | −0.91/0.366 | −0.79/0.432a |
| WASO, min | 85 | Normal | 30.4/35.7/47.2 | 30.7/39.7/48.8 | −1.93/0.054 | −2.48/0.015a |
| TST, min | 85 | Normal | 455.3/492.1/529.4 | 450/487.2/510.7 | 2.52/0.012 | 2.25/0.027a |
| SE, % | 85 | Non-normal | 85.1/87.8/90.0 | 83.4/87.2/89.3 | 2.44/0.015b | 2.11/0.038 |
| Subjectively measured sleep | ||||||
| Sleep problems, CSHQ total score | 81 | Non-normal | 44.8/49.0/54.0 | 45.0/50.0/55.0 | −2.57/0.010b | −2.20/0.031 |
| Insomnia severity, ISI total score | 88 | Normal | 5.0/7.0/10.5 | 5.0/7.0/12.0 | −1.10/0.276 | −1.54/0.127a |
- Note: Distributional characteristics with treatment effect evaluated with Wilcoxon signed rank test (z-value) and t-test (t-value). Significant p-values (p < 0.05) are in bold.
- a Normal distributed data according to Shapiro–Wilk W-test (p > 0.05).
- b Non-normal distributed data according to Shapiro–Wilk W-test (p < 0.05).
- Abbreviation: CB, control blanket; CSHQ, Children's Sleep Habits Questionnaire; ISI, Insomnia Severity Index; SE, sleep efficiency; SOL, sleep-onset latency; TST, total sleep time; WASO, wake after sleep onset; WB, weighted blanket.
APPENDIX D
| Baseline–WB–CB | Baseline–CB–WB | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Baseline–w4 mean diff. (SD) | t/p-Value | n | w4–w8 mean diff. (SD) | t/p-Value | n | Baseline–w4 mean diff. (SD) | t/p-Value | n | w4–w8 mean diff. (SD) | t/p-Value | |
| Objectively measured sleep | ||||||||||||
| SOL, min | 43 | −4.13 (22.00) | −1.23/0.2252 | 41 | 3.11 (16.11) | 1.24/0.224 | 44 | −1.01 (29.20) | −0.23/0.819 | 44 | −0.46 (23.64) | −0.13/0.899 |
| WASO, min | 43 | −1.75 (11.06) | −1.04/0.3053 | 41 | 0.92 (11.01) | 0.53/0.596 | 44 | 0.50 (9.67) | 0.34/0.736 | 44 | −4.54 (9.55) | −3.15/0.003 |
| TST, min | 43 | 5.14 (33.77) | 1.00/0.3244 | 41 | −16.64 (26.59) | −4.00/0.000 | 44 | −5.12 (37.95) | −0.87/0.388 | 44 | −0.59 (34.03) | −0.11/0.909 |
| SE, % | 43 | 0.91 (3.13) | 1.91/0.0627 | 41 | −0.97 (2.81) | −2.21/0.033 | 44 | −0.06 (4.11) | −0.09/0.928 | 44 | 0.69 (4.23) | 1.08/0.287 |
| Subjectively measured sleep | ||||||||||||
| Sleep problems, CSHQ total score | 40 | −3.68 (4.85) | −4.80/0.000 | 40 | 0.08 (4.41) | 0.12/0.905 | 45 | −1.70 (4.33) | −2.63/0.012 | 41 | −1.99 (4.01) | −3.18/0.003 |
| Insomnia severity, ISI total score | 43 | −2.21 (4.24) | −3.41/0.001 | 43 | 1.72 (4.90) | 2.30/0.026 | 45 | −1.89 (4.62) | −2.74/0.009 | 45 | 0.24 (3.57) | 0.46/0.649 |
- Note: Significant p-values (p < 0.05) in bold.
- Abbreviation: CB, control blanket; CSHQ, Children's Sleep Habits Questionnaire; ISI, Insomnia Severity Index; SE, sleep efficiency; SOL, sleep-onset latency; TST, total sleep time; WASO, wake after sleep onset; WB, weighted blanket.
APPENDIX E
| Age group | Gender | ADHD subtype | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All (n = 94) | 6–10 years (n = 66) | 11–14 years (n = 28) | Boy (n = 54) | Girl (n = 40) | Hy/C (n = 69) | In (n = 25) | ||||
| Mean (SD) | Mean (SD) | Mean (SD) | p-Value | Mean (SD) | Mean (SD) | p-Value | Mean (SD) | Mean (SD) | p-Value | |
| Demographics | ||||||||||
| Age, mean (SD) | 9.4 (2.2) | 8.3 (1.4) | 12.2 (0.9) | – | 8.9 (2.0) | 10.2 (2.3) | 0.004 | 9.0 (2.2) | 10.7 (1.6) | 0.001 |
| Male, n (%) | 54 (57.4) | 43 (65.2) | 11 (39.3) | 0.029 | – | – | - | 44 (63.8) | 10 (40.0) | 0.039 |
| ADHD subtype Hy/C, n (%) | 69 (73.4) | 53 (80.3) | 16 (57.1) | 0.020 | 44 (81.1) | 25 (63.4) | 0.039 | – | – | - |
- Note: Two-sample t-tests used for continuous variables. Pearson chi-square used for categorical variables. Significant p-values (< 0.05) indicating differences between groups are presented in bold.
- Abbreviation: C, Combined subtype; Hy, Hyperactive subtype; In, Inattentive subtype.
Open Research
DATA AVAILABILITY STATEMENT
The data presented in this study are available upon reasonable request from the corresponding author. The data are not publicly available due to ethical restrictions.




