The effect of longitudinal sleep monitoring on clinician agreement in obstructive sleep apnea diagnosis: The ELSA study
Summary
There is strong evidence for clinically relevant night-to-night variability of respiratory events in patients with suspected obstructive sleep apnea. Sleep experts retrospectively evaluated diagnostic data in 56 patients with suspected obstructive sleep apnea. Experts were blinded to the fact that they were diagnosing the same case twice, once based on a short report of a single in-laboratory respiratory polygraphy and once with the additional information of 14 nights of pulse oximetry at home. All experts (n = 22) were highly qualified, 13 experts (59.1%) treated > 100 patients with suspected obstructive sleep apnea per year. In 12 patients, the apnea–hypopnea index in the respiratory polygraphy was < 5 per hr, but the mean oxygen desaturation index of 14 nights of pulse oximetry was ≥ 5 per hr. The additional information of 14 nights of pulse oximetry helped to diagnose obstructive sleep apnea with a 70% consensus in two of those patients (16.7% [95% confidence interval: 4.7/44.8]). In eight patients, experts could not agree to a 70% consensus regarding continuous positive airway pressure therapy recommendation after respiratory polygraphy. The additional information of multiple-night testing led to a consensus in three of those cases (37.5% [95% confidence interval: 14/69]). Change of obstructive sleep apnea diagnosis and continuous positive airway pressure recommendation was significantly negatively associated with the number of treated obstructive sleep apnea patients > 100 per year compared with 0–29 patients per year (Coef. [95% confidence interval] −0.63 [−1.22/−0.04] and −0.61 [−1.07/−0.15], respectively). Experts found already a high level of consensus regarding obstructive sleep apnea diagnosis, severity and continuous positive airway pressure recommendation after a single respiratory polygraphy. However, longitudinal sleep monitoring could help increase consensus in selected patients with diagnostic uncertainty.
1 INTRODUCTION
Obstructive sleep apnea (OSA) is the most common type of sleep-related breathing disorder with an increasing prevalence due to the rising prevalence of world-wide pandemic obesity (Heinzer et al., 2015).
The diagnostic algorithm of OSA is based on a comprehensive sleep evaluation including an overnight sleep recording. Polysomnography (PSG), respiratory polygraphy (RP) or home sleep apnea testing devices determine the apnea–hypopnea index (AHI), which provides the number of apneas and hypopneas per hour of sleep. It is a crucial sleep study parameter to establish the diagnosis of OSA (AHI ≥ 5 per hr) and its severity (AHI ≥ 5 per hr, ≥ 15 per hr, ≥ 30 per hr = mild, moderate, severe OSA, respectively). As recommended by the American Association of Sleep Medicine (AASM) guidelines from 2017, a single-night sleep study is considered the gold-standard to diagnose OSA in uncomplicated patients with suspected OSA (Kapur et al., 2017).
Diagnosing a disease with a single test requires high accuracy of the test method, as well as a stable disease for reducing false-negative and false-positive test results. Recent study results challenge the single-night study protocol due to high intra-individual night-to-night variability of respiratory events leading to misdiagnosed and misclassified patients with suspected OSA (Punjabi et al., 2020; Roeder et al., 2020). Undiagnosed and untreated OSA is associated with daytime sleepiness, decreased quality of life, increased risk for accidents, hypertension, and possibly cardiovascular events, particularly stroke (Eckert & Malhotra, 2008; Jenkinson et al., 1999; Kohler et al., 2011; Loke et al., 2012; Marin et al., 2005; Newman et al., 2000).
Even though several studies pointed out that single-night testing might not draw an accurate picture of the disease burden, there is low evidence on the diagnostic benefit in the clinical setting of longitudinal sleep monitoring. Our group conducted a prospective study, performing up to 14 consecutive sleep studies in over 100 patients with suspected OSA to investigate the accuracy of repeated sleep studies (Roeder et al., 2021). This study revealed that already one additional sleep study increased sensitivity, thereby lowering the number of patients with missed OSA remarkably (Roeder et al., 2021). Because the majority of studies investigating night-to-night variability of respiratory events in OSA are solely based on AHI cut-offs, it is unknown if and how longitudinal sleep monitoring would influence the diagnostic process in a real-life setting.
The aim of this study was to answer the question if and how the additional information of longitudinal sleep monitoring would influence the decision of sleep experts regarding OSA diagnosis, OSA severity, and indication for continuous positive airway pressure (CPAP) therapy.
2 METHODS
2.1 Study design and recruitment of patients with suspected OSA
This study is based on sleep study results deriving from the study “The Accuracy of Repeated Sleep Studies in OSA: A Longitudinal Observational Study With 14 Nights of Oxygen Saturation Monitoring” published in 2020 (Roeder et al., 2021). For this monocentric, prospective observational study, all patients referred to the Department of Pulmonology of the University Hospital Zurich due to suspected OSA were consecutively screened for eligibility between February and October 2019. One-hundred and thirty patients were enrolled. Eligible patients were aged older than 18 years with clinically suspected OSA confirmed by a sleep physician. Inclusion and exclusion criteria can be overviewed in the original publication (Roeder et al., 2021). Eligible patients underwent 1 night of in-laboratory RP (Philips Respironics Alice 6 PSG system) and up to 14 nights of pulse oximetry (PO) at home. RP measurements comprised airflow, thoracic and abdominal respiratory inductance plethysmography, finger PO, three-channel electrocardiogram, snoring sensor, accelerometer and audio-visual recordings by a video camera. The AHI is expressed as apneas plus hypopneas per hours of monitoring time. Lights-off and lights-on were each set by the sleep laboratory technicians based on video monitoring. PO was performed using a wrist-worn finger pulse oximeter (Pulsox-300i; Konica-Minolta-Sensing, Osaka, Japan). The protocol was approved by the local ethic committee (BASEC-Nr. 2018–02305) and patients provided written informed consent.
2.2 Case presentation and recruitment of experts
Only those data of patients with fully completed in-laboratory RP and 14 consecutive nights with PO were used for case presentation. Experts were informed that all patients had high pre-test probability for OSA measured by the NoSAS score of ≥ 11 points (Marti-Soler et al., 2016) and that all patients suffered significant daytime sleepiness. The experts were blinded to the fact that they diagnosed the same case twice: once solely based on in-laboratory RP and once with the additional information of 14 nights PO data. The experts had to decide about OSA diagnosis (yes/no), OSA severity (mild, moderate, severe) and CPAP indication (yes/no) once with the in-laboratory AHI and oxygen desaturation index (ODI; ≥ 3%), and once with the additional information of the highest ODI (≥ 3%) and the mean ODI (≥ 3%) of 14 nights of nocturnal, at-home PO. The exact words of introduction and an example of case presentation can be seen in Appendix S1.
Sleep experts were found through screening the National Medical Professional Registry in Switzerland (https://www.medregom.admin.ch/) and additional internet search. The search filters “specialist in pulmonology” and “certificate of proficiency in sleep medicine (Swiss Society for Sleep Research, Sleep Medicine and Chronobiology)” were applied. Inclusion criteria were current employment as well as 3 years of post-qualification experience in the field of sleep medicine and pulmonology, including regular decision-making about diagnosis and treatment of patients with suspected OSA. One-hundred and sixteen sleep experts were contacted by email invitation with a direct link to the survey. Twenty-two sleep experts completed the survey in full. All experts confirmed the use of the generated data for analysis and publication, and were offered a compensation of 50 CHF for study participation.
2.3 Outcomes
The main outcome of this study was the change of the experts’ opinion regarding OSA diagnosis (yes/no), OSA severity (mild, moderate, severe) and indication of CPAP (yes/no) using the additional information of longitudinal sleep monitoring. As the secondary outcome, we investigated possible predictors of the change of the experts’ opinion defined as any change in OSA diagnosis, OSA severity or CPAP recommendation. We established a threshold of 70% to consider consensus as suggested by the Delphi consensus paper of Vogel et al. (2019).
2.4 Statistical analysis
Descriptive statistics of baseline patient and experts' characteristics are presented as mean and standard deviation (SD) or median and interquartile range for continuous measurements, and as number and percentage of total for categorical measurements. Ninety-five percent confidence intervals (CIs) for proportions were calculated using the Wilson interval. Multivariable logistic regression analysis and univariable negative binomial regression analysis (represented as incidence rate ratio [IRR]) were used to assess associations between opinion changes (dependent variable) and possible predictors (experience of the expert, change in AHI/ODI). All estimates were reported with 95% CI. Analyses were conducted with Stata 16.1 (StataCorp, Texas, USA).
3 RESULTS
3.1 Patient characteristics
Between February and October 2019, 130 eligible patients with suspected OSA were enrolled consecutively (Figure 1). Eighteen patients were excluded, mostly due to low protocol adherence (e.g. sending back the PO device with no or less than two data nights) or logistic problems with the PO device delivery. Fifty-four patients were excluded for not completing 14 nights of PO or missing in-laboratory RP. In the end, 56 patients were included in the final analysis. The subjects were predominantly male (73.2%) and overweight (median [quartiles] body mass index of 28.2 [24.9/33.0] kg m−2). The mean (SD) in-laboratory AHI and ODI were 12.8 (12.2) per hr and 11.6 (12.9) per hr, respectively. The mean (SD) ODI of 14 nights of at-home PO was 12.9 (9.0) per hr, the mean (SD) of the highest PO was 20.6 (14.3) per hr.
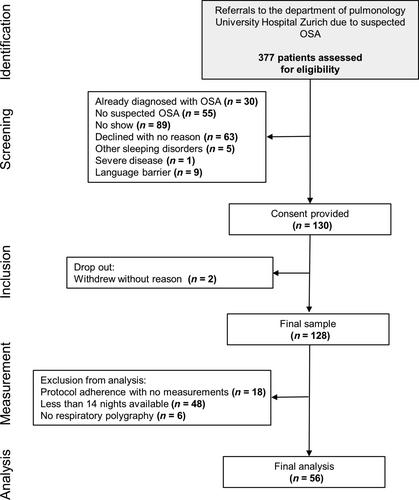
3.2 Experts' characteristics
All experts (n = 22) worked in the field of pulmonology and sleep medicine, and had more than 3 years of experience. Sixteen (72.7%) experts claimed > 10 years of experience in the field of sleep medicine. Twenty experts of 22 (90.9%) had a national diploma in sleep medicine. While three experts (13.6%) diagnosed and treated 0–29 patients per year, six experts (27.3%) saw 60–100 patients and 13 experts (59.1%) > 100 patients with suspected OSA per year.
3.3 Change of OSA diagnosis
In 32 of 56 patients (57.1% [95% CI: 44.1/69.2]) with suspected OSA, at least one expert changed OSA diagnosis status from no OSA to OSA or vice versa using the additional information of the longitudinal sleep monitoring. After the RP, experts reached a consensus level of 70% in 53 of 56 cases (94.6% [95% CI: 85.4/98.1]). In three cases, no consensus was reached using only the RP results. With the additional information of the longitudinal sleep monitoring, consensus was reached in only one further case (1.8% [95% CI: 0.3/9.4]). In contrast, the longitudinal sleep monitoring led to the loss of consensus in four patients (7.1% [95% CI: 2.8/16.8]). In two patients (3.6% [95% CI: 0.9/1.2]), experts denied OSA in consensus after RP and changed the OSA diagnosis to YES in consensus using the additional information of the longitudinal sleep monitoring. In 12 patients, the in-laboratory AHI in the RP was < 5 per hr, but the mean ODI of 14 nights of PO was ≥ 5 per hr (Table 1). The additional information of 14 nights of PO helped to find a 70% consensus in two of those patients (16.7% [95% CI: 4.7/44.8]). The experts also used the longitudinal sleep monitoring to change their CPAP recommendations in those patients, but reached a consensus only in those two patients (16.7% [95% CI: 4.7/44.8]). Only one patient showed a mean ODI < 5 per hr but an in-laboratory AHI suggesting mild OSA. In this case, the results of the multi-night testing did not help to reach a 70% consensus regarding a new OSA diagnosis status (Table 2).
| PI | In-hospital AHI, n per hr | In-hospital ODI3%, n per hr | PO ODI3%, highest, n per hr | PO ODI3% mean, n per hr | Experts RP OSA «yes», n (%) | Experts RP + PO OSA «yes», n (%) | Experts RP CPAP «yes», n (%) | Experts RP + PO CPAP «yes», n (%) |
|---|---|---|---|---|---|---|---|---|
| 03 | 3.0 | 3.0 | 8.0 | 5.1 | 0 (0) | 3 (13.6) | 0 (0) | 3 (13.6) |
| 08 | 0.4 | 0.3 | 37.9 | 20.0 | 1 (4.5) | 15 (68.2) | 1 (4.5) | 15 (68.2) |
| 10 | 4.9 | 6.1 | 26.5 | 20.0 | 1 (4.5) | 20 (90.1)* | 0 (0) | 18 (81.8)* |
| 12 | 1.6 | 1.3 | 9.6 | 6.2 | 0 (0) | 6 (27.3) | 0 (0) | 4 (18.2) |
| 13 | 2.4 | 1.0 | 8.8 | 6.3 | 0 (0) | 4 (18.2) | 0 (0) | 3 (13.6) |
| 16 | 3.3 | 3.8 | 9.3 | 5.1 | 0 (0) | 4 (18.2) | 0 (0) | 2 (9.1) |
| 24 | 0.9 | 0.1 | 29.1 | 15.7 | 0 (0) | 16 (72.7)* | 0 (0) | 16 (72.7)* |
| 28 | 4.6 | 3.6 | 15.4 | 6.7 | 1 (4.5) | 10 (45.4) | 0 (0) | 4 (18.2) |
| 39 | 3.4 | 2.7 | 10.5 | 7.7 | 0 (0) | 11 (50) | 0 (0) | 7 (31.8) |
| 41 | 1.0 | 1.6 | 17.4 | 7.4 | 0 (0) | 7 (31.8) | 0 (0) | 5 (22.7) |
| 50 | 4.1 | 1.5 | 13.7 | 9.1 | 0 (0) | 11 (50) | 0 (0) | 7 (31.8) |
| 52 | 1.4 | 0.6 | 7.4 | 5.6 | 0 (0) | 3 (13.6) | 0 (0) | 2 (9.1) |
- Note: In-hospital RP, at-home PO data and experts' decisions after RP or RP and PO in patients with in-laboratory AHI < 5 per hr during RP and ODI3% mean ≥ 5 per hr during PO.
- Abbreviation: AHI, apnea–hypopnea index; CPAP, continuous positive airway pressure therapy; n, number; ODI, oxygen desaturation index; OSA, obstructive sleep apnea; PI, patient ID; PO, pulse oximetry; RP, respiratory polygraphy.
- * Reached new consensus of ≥ 70%.
| PI | AHI, n per hr | In- laboratory ODI3%, n per hr | PO ODI3%, highest, n per hr | PO ODI3% mean, n per hr | Experts RP OSA «yes», n (%) | Experts RP + PO OSA «yes», n (%) | Experts RP CPAP «yes», n (%) | Experts RP + PO CPAP «yes», n (%) |
|---|---|---|---|---|---|---|---|---|
| 09 | 6.3 | 2.2 | 9.5 | 4.3 | 14 (63.6) | 14 (63.6) | 5 (22.7) | 5 (22.7) |
- Note: In-hospital RP, at-home PO data and experts' decisions after RP or RP and PO in patients with in-laboratory AHI ≥ 5 per hr during RP and ODI3% mean < 5 per hr during PO.
- Abbreviation: AHI, apnea–hypopnea index; CPAP, continuous positive airway pressure therapy; n, number; ODI, oxygen desaturation index; OSA, obstructive sleep apnea; PI, patient ID; PO, pulse oximetry; RP, respiratory polygraphy.
In 24 patients (42.9% [95% CI: 30.8/55.9]), multi-night testing did not lead to changed OSA diagnosis through any of the experts. Three patients were diagnosed with no OSA, while 21 patients were diagnosed with OSA.
3.4 Change of indication for CPAP therapy
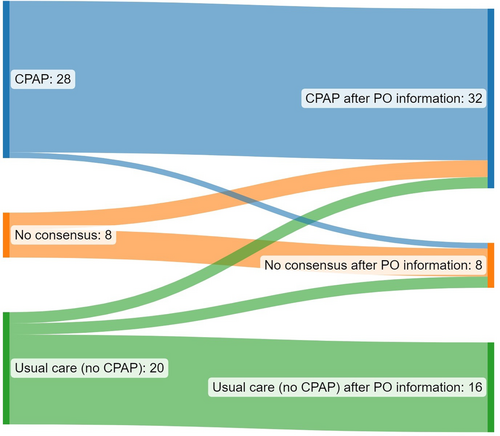
In 37 patients (66.1% [95% CI: 53.0/77.1]), CPAP therapy recommendations were changed by at least one expert giving the additional information of 14 nights PO. In eight patients (14% [95% CI: 7.4/25.7]), experts could not agree to a 70% consensus regarding CPAP therapy recommendation just after the RP. The additional information of multi-night testing led to a consensus in three of those cases (37.5% [95% CI: 14/69]). In addition, consensus change happened from 70% consensus no CPAP to a 70% consensus pro CPAP therapy in two patients (3.6% [95% CI: 0.9/12.1]; Figure 2). In three patients, consensus was lost after multi-night testing (5.4% [95% CI: 1.8/14.6]).
3.5 Change of OSA severity
The severity of OSA was changed in 50 patients (89.2% [95% CI: 78.5/95.0]) at least by one expert using data of the longitudinal sleep monitoring. Either way, experts found no consensus about OSA severity in only five patients (8.9% [95% CI: 3.8/19.3]). Using the information of the longitudinal sleep monitoring, experts were able to reach consensus in two of those cases (40% [95% CI: 15.0/85.0]). Only in one case consensus changed from mild OSA to moderate OSA. On the contrary, longitudinal sleep monitoring was responsible for lost consensus in 10.7% (95% CI: 5.0/21.5) of the patients where there was an initial consensus after evaluating the RP results.
3.6 Effect of patients' baseline and experts' characteristics on diagnostic changes
While the in-laboratory AHI of the RP and the mean ODI of 14 nights of PO were associated with changed OSA diagnosis status (Coef. [95% CI] 0.78 [0.74/0.82] and Coef. [95% CI] 0.96 [0.90/1.03], respectively), there was no significant association between the highest ODI and changed OSA diagnosis (Table 3).
| Odds ratio | 95% CI | p-Value | |
|---|---|---|---|
| In-laboratory AHI | 0.78 | 0.74/0.82 | < 0.001 |
| Highest ODI3% PO | 0.96 | 0.90/1.03 | 0.261 |
| Mean ODI3% PO | 1.16 | 1.04/1.30 | 0.010 |
- Note: Multiple regression analysis of in-hospital RP and at-home PO data.
- Abbreviation: AHI, apnea–hypopnea index; CI, confidence interval; ODI, oxygen desaturation index; PO, pulse oximetry.
There was a negative association between changed OSA diagnosis status and expert experience in terms of number of treated patients with suspected OSA > 100 per year (IRR [95% CI] 0.53 [0.29/0.96]; Table 4). Furthermore, there was a negative association between changed CPAP recommendation and the number of treated patients > 100 per year (IRR [95% CI] 0.54 [0.34/0.86]; Table 4). Changed CPAP recommendation was also negatively associated with years of experience > 10 years (IRR [95% CI] 0.64 [0.44/0.94]). Further regression analysis results can be seen in Table 5.
| IRR | 95% CI | p-Value | |
|---|---|---|---|
| Number of OSA changes (dependent) | |||
| 0–29 patients per year (reference), n = 3 | |||
| 30–59 patients per year, n = 0 | na | ||
| 60–100 patients per year, n = 6 | 0.58 | 0.30/1.13 | 0.110 |
| > 100 patients per year, n = 13 | 0.53 | 0.29/0.96 | 0.037 |
| 60–100 patients per year versus > 100 patients per year | 0.91 | 0.52/1.59 | 0.745 |
| Number of CPAP changes (dependent) | |||
| 0–29 patients per year (reference), n = 3 | |||
| 30–59 patients per year, n = 0 | na | ||
| 60–100 patients per year, n = 6 | 0.59 | 0.35/0.999 | 0.049 |
| > 100 patients per year, n = 13 | 0.54 | 0.34/0.86 | 0.009 |
| 60–100 patients per year versus > 100 patients per year | 0.91 | 0.58/1.43 | 0.690 |
| Number of severity changes (dependent) | |||
| 0–29 patients per year (reference), n = 3 | |||
| 30–59 patients per year, n = 0 | na | ||
| 60–100 patients per year, n = 6 | 0.71 | 0.34/1.51 | 0.376 |
| > 100 patients per year, n = 13 | 0.63 | 0.32/1.24 | 0.185 |
| 60–100 patients per year versus > 100 patients per year | 0.89 | 0.52/1.53 | 0.666 |
- Abbreviation: CI, confidence interval; CPAP, continuous positive airway pressure; IRR, incidence rate ratio; n, number; OSA, obstructive sleep apnea.
| IRR | 95% CI | p-Value | |
|---|---|---|---|
| Number of OSA changes (dependent) | |||
| 3–10 years of experience (reference), N = 6 | |||
| > 10 years of experience, N = 16 | 0.65 | 0.40/1.05 | 0.077 |
| Number of CPAP changes (dependent) | |||
| 3–10 years of experience (reference), N = 6 | |||
| > 10 years of experience, N = 16 | 0.64 | 0.44/0.94 | 0.023 |
| Number of severity changes (dependent) | |||
| 3–10 years of experience (reference), N = 6 | |||
| > 10 years of experience, N = 16 | 0.71 | 0.43/1.20 | 0.202 |
- Abbreviation: CI, confidence interval; CPAP, continuous positive airway pressure; IRR, incidence rate ratio; N, number; OSA, obstructive sleep apnea.
4 DISCUSSION
Numerous studies report high intra-individual night-to-night variability of respiratory events in patients with suspected OSA, thereby challenging the present single-night protocol to diagnose OSA (Aarab et al., 2009; Bittencourt et al., 2001; Gouveris et al., 2010; Stoberl et al., 2017). Night-to-night variability in the number of obstructive apneas or hypopneas is not unique to supine OSA, and appears to be particularly relevant to treatment recommendations for lower AHI categories. Even though multi-night testing has been recognised by sleep physicians and researchers (Roeder & Kohler, 2020), the effect of longitudinal sleep monitoring on the decision process of sleep experts has only been gradually investigated. To our knowledge, this is the first analysis of such an extent, asking 22 sleep experts to evaluate in a blinded fashion 56 patients with suspected OSA who underwent one in-laboratory cardio-RP and a longitudinal sleep monitoring comprising 14 nights of PO.
We have found that sleep experts show already a high level of agreement based on just 1 night's assessment of RP regarding OSA diagnosis status, CPAP treatment recommendation and OSA severity in patients with daytime sleepiness. However, the additional information about the course of respiratory events over 14 nights helped to find consensus in those patients with initial diagnostic uncertainty.
A recent meta-analysis pooling data of 24 studies comprising 3250 patients with suspected OSA showed high intra-individual night-to-night variability of respiratory events. On average, 41% (95% CI: 27/57) of all participants showed changes of respiratory events > 10 per hr from night to night, and 12% (95% CI: 8/19) of patients with moderate OSA would have been missed using a single-night sleep study assessment (Roeder et al., 2020).
Our data document that the experts showed already a high level of consensus based on one in-laboratory RP regarding OSA diagnosis in more than 94% of all patients. Either way, 21.4% (95% CI: 0.13/0.34) of patients with symptomatic OSA diagnosed by the mean ODI of 14 nights PO and using the diagnostic threshold of five events per hour (AHI or ODI3%, respectively) would have been missed in the in-laboratory RP. This ratio is slightly higher as the reported 12% (95% CI: 9/15) of missed OSA using an AHI threshold of five events per hour in the meta-analysis mentioned above (Roeder et al., 2020). One reason could be the high number of nights available for assessment in this study as it has been suggested that night-to-night variability rises with the number of recorded nights (Roeder et al., 2020). Although the AASM guidelines suggest treating symptomatic patients with an AHI ≥ 5 per hr (Kapur et al., 2017), a 70% consensus to change the OSA diagnosis status and CPAP therapy recommendation was generated only in 16.7% (95% CI: 4.7/44.8) of those patients. The reasons why a remarkable percentage of sleep experts did not change their diagnostic opinions are probably multi-factorial. Our data suggest that experience in the form of number of treated patients with suspected OSA per year and years of clinical work in the field was negatively associated with the willingness to change OSA diagnosis or CPAP recommendation. It seems reasonable to assume that more experienced experts use a single-night sleep study as the gold-standard to diagnose OSA as suggested by recent guidelines (Kapur et al., 2017). As a result, those experts ascribe higher value to the results of a multi-channel recording device as to the results derived from PO. It is unclear whether those experts would also neglect the results of a longitudinal sleep monitoring using repetitive PSG or RPs.
The effect of multi-night testing was more substantial when it came to CPAP therapy recommendation. Experts were indecisive in 14% (95% CI: 7.4/25.7) of all cases. The information of multi-night testing helped experts to find consensus in more than 30% of those cases.
While the longitudinal sleep monitoring helped to reach consensus in some patients with suspected OSA regarding OSA status, OSA severity and CPAP recommendation, our data also describe the tendency of increased diagnostic uncertainty receiving the additional information of a longitudinal sleep monitoring. For example, while multi-night testing helped to reach consensus in 5.4% (95% CI: 0.02/0.17) of all patients regarding OSA diagnosis, it also led to loss of consensus in 7.1% (95% CI: 2.8/16.8) of all evaluated patients. The only other study investigating the influence of night-to-night variability of respiratory events in OSA on sleep experts’ decision was conducted by Ahmadi et al. (2009). The group presented two consecutive PSGs of only five patients with high intra-individual night-to-night variability of respiratory events to 22 international experts (Ahmadi et al., 2009). Only two cases were presented as isolated cases undergoing a single-night PSG. While one patient was already diagnosed with OSA after the first PSG with a 70% consensus, the other patient was diagnosed initially with OSA only by 64%. After receiving the results of the second PSG, 90% of the experts diagnosed OSA in this patient. The authors suggested that 27%–36% of patients with OSA would have been missed by the experts if only the sleep study results of the PSG with the lower AHI would have been presented (Ahmadi et al., 2009). Anyway, these patients harboured a noteworthy selection bias as these five patients were handpicked from a cohort of 193 patients due to their outstanding intra-individual night-to-night variability of AHI in two consecutive PSGs. Our cohort of 56 consecutively screened patients with suspected OSA presents a scenario closer to real life with patients with different degrees of night-to-night variability of respiratory events.
So far, there is no consensus on how to deal with results of repetitive or even contradicting sleep study results. While some authors suggest following a “treat the worst” approach using the highest event rate, others prefer to use the mean of all measured nights to diagnose or exclude OSA (Punjabi et al., 2020; Redline et al., 1998). Using only the highest respiratory event index might overestimate the disease burden. It is questionable if a single, isolated night with increased event rates might already trigger adaption processes (e.g. catecholamine, erythropoietin levels) to an extent that leads to OSA-typical pathophysiological changes such as cardiovascular risk, increased risk for car accidents and excessive daytime sleepiness (Bliwise et al., 1991). Comparative effectiveness trials comparing both diagnostic approaches – “treat the worst” and “mean AHI” – are needed. However, in addition to the AHI, several other sleep study parameters and, above all, symptoms and comorbidities are relevant for treatment recommendations. Our study found that the change in OSA diagnosis status was significantly associated with the mean ODI but not with the highest ODI, suggesting that sleep experts prefer to use the mean over the most extreme measured event rate of a longitudinal sleep monitoring to let their diagnostic decisions be influenced.
5 LIMITATIONS
This study has some limitations. It is indisputable that the diagnostic process of OSA should comprise a comprehensive sleep evaluation including assessment of potential symptoms of OSA, risk factors for OSA, relevant comorbidities, medical history, physical examination and manual review of the adequate type of sleep study. To increase comparability, all cases were presented as symptomatic and with high pre-test probability taking the experts’ opportunity to use a multi-factorial clinical approach to diagnose those patients. Anyway, the main outcome was the effect of a longitudinal sleep monitoring on the diagnostic process focused on the respiratory event rates. A more individual report of those cases would lead to a series of confounders and would have made the survey even more time consuming. For the same reason, the expert clinicians had no access to the full data of RP or OP. There is a risk of over-simplification that might conceal essential diagnostic elements and might lead to incorrect interpretation. Moreover, the small sample size of cases (n = 56) is a limitation given the focus on proportions of patients changing diagnosis or in which a consensus is achieved. Furthermore, the small number of experts limits the statistical reliability of reported associations derived from regression analysis. Nevertheless, this study represents the biggest study population that has been investigated regarding the influence of longitudinal sleep monitoring. Consequently, further studies with larger study populations are needed. The conduction of those studies could be more feasible by reducing the number of study nights, as our group found already a significant increase of accuracy by adding one additional sleep study night (Roeder et al., 2021). This study is not able to answer the question why our experts changed their decisions regarding OSA diagnosis and therapy recommendations. Nevertheless, it seems reasonable to assume that there is a particular level of uncertainty regarding a single-night testing protocol for patients with suspected OSA. In future studies, it would be useful to understand why clinicians changed their diagnoses by directly querying the information that went into their decisions.
6 CONCLUSION
This study revealed a high degree of consensus of sleep experts regarding OSA diagnosis, OSA severity and CPAP recommendation based on a single-night in-laboratory RP in patients with excessive sleepiness. However, longitudinal sleep monitoring can help increase consensus in patients with diagnostic uncertainty especially regarding CPAP therapy recommendations.
AUTHOR CONTRIBUTIONS
Maurice Roeder: Conceptualization; data curation; formal analysis; investigation; methodology; visualization; writing – original draft. Noriane Sievi: Conceptualization; formal analysis; methodology; writing – review and editing. Nina Frei: Formal analysis; investigation; writing – review and editing. Esther Irene Schwarz: Formal analysis; writing – review and editing. Carolin Steinack: Formal analysis; writing – review and editing. Thomas Gaisl: Conceptualization; methodology; writing – review and editing. Malcolm Kohler: Conceptualization; funding acquisition; methodology; supervision; writing – original draft.
ACKNOWLEDGEMENT
Open access funding provided by Universitat Zurich.
FUNDING INFORMATION
This study was supported by a grant from Lunge Zurich. The funders of the study had no role in the study design, data collection, data analysis, data interpretation or writing of this report.
CONFLICT OF INTEREST STATEMENT
Professor Malcolm Kohler reports grants and personal fees from Bayer, personal fees from Novartis, personal fees from Boehringer, personal fees from GSK, personal fees from Astra Zeneca, grants from Roche, personal fees from CSL Behring, and personal fees from Mundipharma, during the conduct of the study. The authors report no other conflicts of interest in this work. Thomas Gaisl reports personal fees from Bayer (outside the submitted work) during the conduct of this study.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.




