Actigraphy measurement of physical activity and energy expenditure in narcolepsy type 1, narcolepsy type 2 and idiopathic hypersomnia: A Sensewear Armband study
Abstract
The cause of obesity in narcolepsy type 1 (NT1) patients is not fully understood. The present study investigated if a reduced physical activity could explain weight gain in NT1. Seventy-nine patients were included in this retrospective study and divided into an NT1 group (n = 56) and a non-NT1 group (n = 23), including NT2 and idiopathic hypersomnia (IH). Accelerometry-derived measures of physical activity, total energy expenditure and skin temperature were collected from patients during seven consecutive days without medication. In addition, results from multiple sleep latency tests and the Epworth Sleepiness Scale questionnaire, body weight, height and CSF orexin/hypocretin were acquired. Three measurements of physical activity, including metabolic equivalent of task (MET), the average time of physical activity and step count, were compared without differences between groups. Neither could we find a significant difference in total energy expenditure or skin temperature. Thus, by analysing accelerometric data, we could not find any differences in the amount of physical activity or total energy expenditure explaining overweight in NT1.
1 INTRODUCTION
The neurological sleep disorder narcolepsy is characterized by fragmented sleep, excessive daytime sleepiness (EDS), cataplexy, sleep paralysis and hallucinations associated with sleep (Kornum et al., 2017). Narcolepsy type 1 (NT1) presents with cataplexy and low levels of orexin/hypocretin (hereafter orexin) in cerebrospinal fluid (CSF). In narcolepsy type 2 (NT2) and in idiopathic hypersomnia (IH), cataplexy is absent and orexin levels are typically normal.
The onset of symptoms in NT1 is frequently accompanied by excessive weight gain. The body mass index (BMI) of patients with narcolepsy is higher than that of healthy controls, specifically patients with NT1 (Heier, Jansson, & Gautvik, 2011; Poli et al., 2009). Weight gain increases the risk of developing secondary conditions such as cardiovascular disease, hyperlipidaemia, diabetes mellitus type 2 and obstructive sleep apnea syndrome. The cause of weight gain in NT1 is currently unknown, but in animal models of NT1, loss of orexinergic neurons results in obesity, which has been explained by a reduced physical activity (PA) (Tsunematsu et al., 2008). A decrease in energy expenditure could also be the result of a reduced basal metabolic rate.
There is also a possibility that the reduction in PA is a result of hypersomnolence rather than reduced motivated behaviour. Therefore, we wanted to compare PA levels in NT1 with the primary hypersomnolence disorders NT2 and IH, both of which present with similar symptoms of somnolence but without weight gain.
2 MATERIALS AND METHODS
2.1 Subjects
Results were collected from medical records of patients with NT1, NT2 and IH at the Neurology Department at Sahlgrenska University Hospital in Gothenburg, Sweden, regarding EDS and descriptions of cataplexy, together with Epworth Sleepiness Scale (ESS) scores, polysomnography (PSG), mean sleep latency test (MSLT), actigraphy data and laboratory tests. ICSD-3 diagnostic criteria were used for diagnosis verification of those patients who had actigraphy recordings between November 2009 and October 2018. All patients who were reviewed reported subjective sleepiness and ESS ≥10 points. Seventy-nine patients with a diagnose of NT1 (n = 56), NT2 (n = 7) or IH (n = 16) were included in the study.
2.2 Body weight and height
The first measurements of weight and height following onset of symptoms were used, except if the onset had occurred before the patient’s 18th birthday. In that case, the first measurement after his or her 18th birthday was used instead. Overweight was defined as BMI ≥25.
2.3 Measurements of physical activity
Physical activity (PA) measurements were recorded for approximately 1 week with the Sensewear® armband accelerometer model MF-SW (Bodymedia), as part of the diagnostic evaluation in patients with a suspected primary neurological disorder of hypersomnolence. Psychostimulant medications were discontinued 7 days beforehand. Recorded data were retrieved using the software Sensewear® Professional 7.0. All measurements, including those of PA, were calculated using proprietary algorithms and presented as distinct values for each minute during the day and night of the recording period. Data from periods when the accelerometer was not worn were discarded from analysis. The PA measurements obtained from actigraphy recordings were step count, average MET and duration of the following MET-based grading of physical activity. Additional data acquired from actigraphy recordings were total energy expenditure (TEE), skin temperature measurements, sleep duration and sleep efficiency. Although the Sensewear Armband has not been validated for use in patients with narcolepsy, it is validated for measurement of energy expenditure during light to moderate-intensity free-living activities (Calabro, Lee, Saint-Maurice, Yoo, & Welk, 2014).
2.4 Laboratory tests
All CSF samples used in this study were analysed at the Clinical Neurochemistry Laboratory, Sahlgrenska University hospital, Mölndal, Sweden, using an in-house radio immune assay (RIA) based on a rabbit orexin-A antiserum (Sigma O9756), as described previously in detail (Portelius et al., 2014).
2.5 Statistical analysis
Data for statistical comparisons were performed with a computerized statistical package (SPSS version 25). The two-tailed Mann–Whitney U-test was used for simple group comparisons of continuous variables and Pearson's chi-squared test was used for dichotomous variables. Logistic regression analysis was used for statistical comparison between two variables. Data are presented as means and standard deviations (SDs) and the level of significance was set at 5% (p < .05).
2.6 Ethics
The study was approved by the Regional Board of Medical Ethics at the University of Gothenburg (#549-18).
3 RESULTS
3.1 Comparison of NT1 and non-NT1 patient groups
The results from the comparison between groups are presented in Table 1. Patients with NT1 had a higher male to female ratio, were younger, and had a higher BMI, a shorter sleep duration, lower sleep efficiency and a shorter sleep latency.
| NT1 (n = 56) | Non-NT1 (n = 23) | p-value | |
|---|---|---|---|
| Gender (male), n (%) | 27 (48.2) | 4 (17.4) | .011 |
| Age (years), mean ± SD (n) | 27.6 ± 9.1 (56) | 32.6 ± 10.4 (23) | .005 |
| BMI, mean ± SD (n) | 24.7 ± 3.6 (54) | 22.4 ± 4.1 (20) | .021 |
| Overweight (BMI ≥ 25), n (%) | 22/54 (40.7) | 5/20 (25.0) | .215 |
| CSF orexin-A <1/3 of level in normal population | 43/43 | 0/20 | <.001 |
| ESS score, mean ± SD (n) | 17.1 ± 2.8 (27) | 15.9 ± 3.0 (17) | .181 |
| Sleep duration/day, mean ± SD (n) | 7 hr, 3 min ± 0.066 hr (55) | 7 hr, 17 min ± 0.044 hr (23) | .016 |
| Sleep efficiency (%), mean ± SD (n) | 69.2 ± 10.5 (56) | 81.7 ± 5.8 (23) | <.001 |
| Mean sleep latency (min), mean ± SD (n) | 4.3 ± 2.2 (49) | 5.8 ± 1.4 (23) | <.001 |
| Sleep-onset REM period, mean ± SD (n) | 2.47 ± 1.4 (49) | 0.78 ± 1.1 (23) | <.001 |
Note
- All statistical comparisons between the groups were made using the two-tailed Mann–Whitney U-test, except for the fraction of men and those overweight, where Pearson's chi-squared test was used.
- Abbreviations: BMI, body mass index; CSF, cerebrospinal fluid; ESS, Epworth Sleepiness Scale; REM, rapid eye movement.
3.2 Measurements of physical activity from accelerometric data
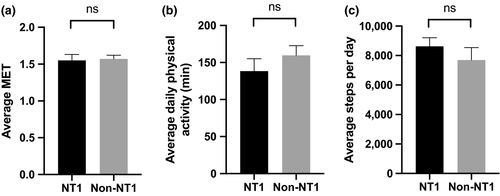
Fifty-six NT1 patients and 23 non-NT1 patients had their PA measured with the same type of accelerometric equipment for 161.5 ± 13.2 consecutive hours, with an on-body time of 96.2 ± 7.2%, corresponding to approximately 1 week (i.e., 164 hr). Metabolic equivalent of task (MET), the average time of PA (defined as minutes per day recorded with MET ≥3) and step count are measured to grade intensity levels of physical activity (Ewald, McEvoy, & Attia, 2010; Jette, Sidney, & Blumchen, 1990). However, none of these measurements were significantly different between groups (MET: 1.55 ± 0.59, 1.57 ± 0.22, p = .28; average time of PA: 138.43 ± 80.1 min, 159.6 ± 98.7, p = .59; step count: 8,629.9 ± 4,351.8, 7,693.8 ± 4,040.5, p = .33 in NT1 and non-NT1, respectively; Figure 1a-c). Also, no significant difference was found in inactivity (MET < 3: 85.7 ± 9.1%, 81.6 ± 10.3%, p = .06), moderate activity (MET 3–6: 13.4 ± 8.3%, 17.8 ± 9.8%, p = .11) or vigorous to very vigorous activity (MET ≥ 6: 0.4 ± 1.7%, 0.2 ± 0.5%, p = .48) in the time awake between groups.
As not all NT1 patients gain weight after onset of symptoms (Wang et al., 2016), we wanted to perform a closer examination of only those who had gained weight, but because we have no data on weight gain we instead used BMI ≥25 as a surrogate delineation. Thus, we tested for differences in PA measurements specifically between NT1 patients without (n = 32) and with (n = 22) overweight. Average MET was significantly higher in normal-weight (BMI <25) as compared to overweight subjects (1.59 ± 0.21 and 1.48 ± 0.19; p = .04), but not time of daily PA (143.1 ± 71.5 and 121.1 ± 59.8 min; p = .33) or step count (9,075.9 ± 3,371.8 and 7,840.1 ± 3,193.7; p = .14). In non-NT1 without (n = 15) or with (n = 5) overweight, there was no difference in MET (1.67 ± 0.18 and 1.52 ± 0.18; p = .15) or time of daily PA (183.4 ± 77.8 and 159.6 ± 54.3 min; p = .55), but step count was higher in overweight non-NT1 subjects (7,438.9 ± 2,519.7 and 10,459.2 ± 2,034.2; p = .02).
In order to examine the direct involvement of orexin on physical activity, only those subjects with orexin-A measured in CSF were selected for analysis. Thus, 43 out of 54 (79%) subjects in the NT1 group and 13 out of 20 (65%) in the non-NT1 group were analysed, but with no differences between groups (MET 1.59 ± 0.20 and 1.61 ± 0.13 in NT1 and non-NT1, respectively, p = .37; daily PA: 158.8 ± 72.0 and 159.2 ± 56.8 min, p = .71; and step count: 9,107.6 ± 3,465.8 and 7,483.4 ± 2,640.8, p = .20).
3.3 Measurements of total energy expenditure and skin temperature
Total energy expenditure (TEE) is a measurement of energy expenditure consisting of resting EE (REE, normally 60%–75%), active EE (AEE, 15%–30%) and the thermic effect of food (10%) (Fonseca et al., 2018). Accelerometric data include calculations of total energy expenditure (TEE), which was 9,952 ± 4,557 J per day in NT1 and 9,962 ± 3,086 J in non-NT1, with no significant difference in average TEE between groups (p = .36). Skin temperature of the proximal part of the left upper arm was not significantly different when awake (NT1: 33.3 ± 0.5°C; non-NT1: 33.5 ± 0.6°C, p = .64).
4 DISCUSSION
Our main finding was that NT1 patients did not have a lower PA as compared to patients with NT2 and IH, as measured with accelerometry. Thus, neither metabolic equivalent of task (MET), the time of daily physical activity nor step count was lower in the NT1 group overall. However, obese (BMI >25) subjects had a lower MET as compared to normal-weight subjects in the NT1 group.
In animal models of narcolepsy (Hara, Yanagisawa, & Sakurai, 2005; Tabuchi et al., 2014), weight gain develops in tandem with hypoactivity, leading to the possible explanation that weight gain is the result of reduced energy expenditure, possibly due to a reduction in motivated behaviour. However, sleepiness could also be a possible factor that explains the reduced PA. Our study took advantage of a comparison between two patient groups with primary hypersomnolence who had significantly different mean BMI. Our results of equal PA in both groups suggest that excessive daytime sleepiness, rather than a reduction in motivated behaviour, could explain the reduction in PA observed in animal studies, but it does not result in overweight per se.
This study mainly examined PA as the component of energy expenditure termed active energy expenditure (AEE) in NT1 and non-NT1. In addition to AEE, the other component of energy expenditure is resting energy expenditure (REE), which includes basal energy expenditure and diet-induced thermogenesis. Total energy expenditure was not lower in the NT1 group, indicating that REE is equivalent in both groups. However, as TEE is dependent on the body mass (Fonseca et al., 2018), it is not ideal to compare two groups with observed differences in BMI.
We could also find no differences in skin temperature that would support the notion that weight gain in NT1 is caused by a reduced thermogenesis. In one study where daytime measurements of proximal skin temperature in patients with narcolepsy were performed, a minor difference in skin temperature compared to healthy controls was found upon repeated measurements (Fronczek, Overeem, Lammers, van Dijk, & Van Someren, 2006). Whether this reflects a difference in thermogenesis in narcolepsy patients could however be questioned. Core temperature in NT1 has been measured in several studies, most of which have not found any alterations of mean temperature during the day and night-time (Pollak & Wagner, 1994). Thus, our results are in accordance with earlier studies, indicating that a lower body temperature does not cause overweight in NT1.
The study has limitations as a consequence of the retrospective design with cross-sectional patient record study, including limited and missing data and the lack of a control group of healthy subjects. The use of an accelerometer instead of direct measurements of PA and energy expenditure could also over- or underestimate the results. Also, both the variables of PA recorded with an accelerometer, as well as subsequent analysis, could be insufficient to detect small changes in PA that may exist. In addition, polysomnography measurements were only performed in a minority of cases and thus information on sleep apnea and periodic limb movement is missing.
In conclusion, this study examined measurements reflecting active and resting energy expenditure in patients with narcolepsy and idiopathic hypersomnia, using the Sensewear® armband accelerometer model MF-SW. We found that obese NT1 subjects had a lower metabolic equivalent of task as compared to normal-weight subjects with NT1. No difference was found in other measurements of energy expenditure between a group of patients with NT1 and a group that included both NT2 and idiopathic hypersomnia. Thus, this wearable accelerometer sensor could not detect any differences in physical activity between groups that could explain overweight in NT1.
ACKNOWLEDGEMENTS
This research was founded by the Ingrid and Fredrik Thuring foundation.
CONFLICT OF INTEREST
No conflicts of interest declared.
AUTHOR CONTRIBUTIONS
PW, AB and AH designed the study. AB collected and analysed data. AB, PW and AH wrote the paper.




