Efficacy of probiotic, prebiotic, synbiotic and postbiotic supplementation on gastrointestinal health in cats: systematic review and meta-analysis
Abstract
Objectives
The clinical efficacy of the use of probiotics, prebiotics, synbiotics and postbiotics (biotics) in cats is unknown, despite their use in daily practice. The objectives of the study is to evaluate the effectiveness of biotic supplementation in treating and preventing gastroenteropathies, and in reducing gastrointestinal signs associated with antibiotics in cats.
Materials and Methods
A systematic review was conducted by searching four databases for publications before August 2, 2024, following a pre-registered protocol. Eligible publications were trials involving healthy cats or those with gastroenteropathies, supplemented with biotics (and an inactive control), studying outcomes such as faecal consistency, faecal microbiota or vomiting. Risk of bias and quality of reports were assessed. Effects were synthesised by meta-analyses and vote counting based on direction of effect. Certainty of evidence was rated using GRADE approach.
Results
Twenty reports were included, presenting unclear or low risk of bias. The evidence did not permit a high-confidence evaluation of the effectiveness of biotics, although five of the seven probiotic trials showed beneficial effects on faecal consistency. Synbiotics presented no clinically relevant effect in reducing antibiotics-associated vomiting, with very low certainty, in a meta-analysis including 32 adult cats. Probiotics significantly reduce the Bacillota/Actinomycetota ratio, with low certainty, in a meta-analysis involving 34 healthy young-adult cats. Following vote counting, probiotics improved immune profile in young cats, and increased butyric acid concentration in healthy cats.
Clinical Significance
Current data highlight the need for further research, especially focused on at-risk groups and sick cats, before advocating the use of biotic supplementation.
INTRODUCTION
Nowadays, pets occupy a relevant place in people's lives, and owners are more concerned about their quality of life. Gastroenteropathies are common in dogs and cats: the associated signs (diarrhoea, vomiting, weight loss, etc.) are a frequent reason for veterinary consultation. Studies investigating the prevalence of enteropathies in England reported 17.8% among dogs (O'Neill et al., 2014a) and 10% among cats (O'Neill et al., 2014b). However, in a study (Jones et al., 2014), many patients taken to the vet presented a history of illness over the previous days or a complicated diarrhoea. Therefore, the actual number could be higher, as many conditions are self-limiting. Treatment is usually empirical, including nutritional management, with or without antibiotics and sometimes probiotics (Francillon et al., 2023; Jones et al., 2014; Singleton et al., 2019).
The gut microbiota is a set of microorganisms in a host's gastrointestinal system, whose composition and metabolism, such as short-chain fatty acids (SCFAs) production, have well-known effects on health. The microbiota improves functions including gastrointestinal development and permeability, immunomodulation, protection against pathogens, nutrient production, etc., and its influence transcends beyond the digestive system (Binda et al., 2018; Hiippala et al., 2018; Honneffer et al., 2014; Mondo et al., 2019; Sebastián-Domingo & Sánchez-Sánchez, 2018; Suchodolski, 2020; Zeng et al., 2019; Ziese & Suchodolski, 2021). Among the primary SCFAs, butyrate stands out as the main energy source for colonocytes. It exhibits pro-absorptive, anti-secretory and anti-inflammatory intestinal effects, along with potential extraintestinal benefits (Canani et al., 2011). In humans, 90% of gut microbiota is composed by Bacillota (Firmicutes) and Bacteroidota (Bacteroidetes) phyla, and modifications in their ratio (F/B) are associated with many diseases (An et al., 2023; Stojanov et al., 2020). There is currently controversy about the predominant phyla in cats, especially regarding the relevance of Actinomycetota (Actinobacteria), although Bacillota seems to be predominant (Desai et al., 2009; Farhana Binti, 2015; Ritchie et al., 2010; Suchodolski, 2011; Zakošek Pipan, 2023). There is limited information about the role played by the ratio between the most predominant phyla in cats. It is known that the microbiome is altered in pathological states (Honneffer et al., 2014; Mondo et al., 2019; Ziese & Suchodolski, 2021). For all these reasons, in the last years, there has been an increased interest in therapies aimed at its modulation, comprising diverse pathologies (Li, Miao, et al., 2024b; Li, Xiao, et al., 2024a; Luo et al., 2024; Montserrat-Malagarriga et al., 2024).
The microbiome (microbiota and their “theatre of activity”) can be modified with probiotics, prebiotics, synbiotics and postbiotics supplementation (Bajagai et al., 2016; Schmitz, 2021; Yadav et al., 2022), hereinafter referred to as “biotics”. The International Scientific Association for Probiotics and Prebiotics (ISAPP) defines probiotics as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” (Hill et al., 2014). Prebiotics are “a substrate that is selectively utilized by host microorganisms conferring a health benefit” (Gibson et al., 2017). The most widely recognised examples include fructooligosaccharides (FOS), such as inulin, and galactooligosaccharides (GOS). Synbiotics are “a mixture comprising live microorganisms and substrate(s) selectively utilized by host microorganisms that confers a health benefit on the host” (Swanson et al., 2020). Postbiotics are a “preparation of inanimate microorganisms and/or their components that confers a health benefit on the host” (Salminen et al., 2021). All these definitions imply that sufficient evidence of their efficacy in the host is required.
There is ambiguous evidence regarding the effectiveness of biotics in treating or preventing gastroenteropathies in pets, despite its daily use (Francillon et al., 2023). A systematic review in dogs, evaluating the efficacy of these compounds, found no clinically relevant effect in shortening the duration of diarrhoea, with low to very low certainty (Scahill et al., 2024). Information on cats is limited.
The aim of this systematic review and meta-analysis was to evaluate the effects of biotic supplementation on gastrointestinal health in cats, specifically its effectiveness in treating and preventing gastroenteropathies, and in reducing gastrointestinal signs associated with administration of antibiotics.
MATERIALS AND METHODS
The detailed methods were described in the protocol, registered previously on Systematic Reviews for Animals and Food (SYREAF): https://syreaf.org/protocols. The methodological quality was ensured by following the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) 2020 statement (Page et al., 2021). No automation tool was used.
PICO questions (population, intervention, comparator, outcome)
- PICO 1: Is biotic supplementation, as a single or adjuvant treatment, effective in treating cat gastroenteropathies compared to no supplementation or placebo?
- PICO 2: Is biotic supplementation effective in preventing gastroenteropathies of healthy cats compared to no supplementation or placebo?
- PICO 3: Is biotic supplementation effective in reducing gastrointestinal signs associated with antibiotic administration in cats?
Each one was categorised into four subgroups, according to the intervention used: (a) probiotics, (b) prebiotics, (c) synbiotics and (d) postbiotics.
Search strategy and eligibility criteria
Four databases were searched, through two search platforms: PubMed for MEDLINE, since 1966; and EBSCOhost for CAB-Abstracts since 1973, Food Science and Technology Abstracts (FTSA) since 1969, and Global Health since 1973, until April 22, 2024. The search was updated on August 2, 2024. The complete search strategy is presented in Appendix S1. There were no language, year or type of publication restriction. The completeness of this search was ensured assessing its ability to retrieve all key studies identified through an initial pilot search (Belà et al., 2024; Fusi et al., 2019; Gookin et al., 2022; Han et al., 2024; Jewell et al., 2022; Lee et al., 2022; Li et al., 2023; Rodrigues et al., 2020; Stokes et al., 2017; Torres-Henderson et al., 2017; Wang et al., 2023; Whittemore et al., 2018, 2019). References of included studies and relevant reviews were also screened. Unpublished data were not sought.
Results were exported to Zotero 6.0.36, and duplicates were merged. They were selected independently in two phases (title/abstract and full text) by two reviewers, and a third resolving discrepancies, according to the eligibility criteria stablished in the protocol. Studies were reviewed if they met the following criteria: controlled randomised or non-randomised trials; in vivo, with healthy cats or cats with gastroenteropathies; supplemented with biotics (per ISAPP definitions, and only considering FOS and GOS as prebiotics), as a single or adjuvant treatment; compared with no supplementation or placebo (active comparators were not accepted); reporting at least one main or secondary outcome. Before-after studies were excluded because disorders of interest (such as episodes of vomiting and diarrhoea) are often self-limiting and time-bound. Cats with gastroenteropathies were grouped in PICO 1; healthy cats in PICO 2; and those receiving antibiotics, in PICO 3.
Data collection
Data from included studies were extracted by one reviewer and checked by a second one. The articles that required it were translated with DeepL Translator (DeepL GmbH, Cologne, Germany). All authors were contacted for missing information (e.g., values used for the calculation of the estimate, dispersion measures, excluded data, or incomplete trial descriptions). The necessary data presented in the figures were extracted using WebPlotDigitizer 5.0 (Rohatgi, 2024).
Data were sought, regarding study identification (author and year), funding sources, population characteristics (breed, sex, age and health status), study groups and sample size, intervention (product and dosage), comparator, duration and outcomes measured. Unless otherwise specified, cats were assumed to be healthy.
- Faecal consistency: measured by faecal consistency scores (FS), as change, final or mean scores; by qualitative description (pathological or normal); or by days until first normal stool.
- Faecal microbiota: there was no restriction on units of measurement, but molecular methodologies such as 16S ribosomal ribonucleic acid (rRNA) gene sequencing, were preferable. Relative abundances of relevant taxonomic categories were selected from among those most prevalent throughout the studies, regardless of their statistical significance.
- Severity of chronic enteropathy: measured by Feline Chronic Enteropathy Activity Index (FCEAI), as change or final scores.
- Incidence of vomiting after starting supplementation.
Secondary outcomes were body condition/weight, appetite, serum cytokines and faecal metabolites.
All time points of measurement were considered. When multiple forms of data presentation were available, those that enabled comparison between reports were selected, regardless of their statistical significance. For each measurement, the time point, method and sample size were recorded, assuming the measurement included all cats unless stated otherwise; these assumptions were clearly indicated.
Risk of bias and methodological quality assessment
SYRCLE's Risk of Bias tool (Hooijmans et al., 2014) and ROBINS-I (Risk of Bias in Non-Randomised Studies of Interventions) (Sterne et al., 2016) were used to establish the risk of bias of randomised and non-randomised trials, by two reviewers per study. The authors were contacted when the published information was insufficient to make a judgement. An attempt was made to obtain the protocol of the trials to establish risk of selective reporting bias: otherwise, it was established by comparison of the results with the methods section, and by identification of missing critical variables (Hooijmans et al., 2014).
CONSORT (Consolidated Standards for Reporting Trials) 2010, their extensions, and TREND (Transparent Reporting of Evaluations with Nonrandomized Desing) statements were used to assess the quality of reports of randomised and non-randomised trials (Des Jarlais et al., 2004; Dwan et al., 2019; Juszczak et al., 2019; Schulz et al., 2011).
Summary of results
To estimate the pooled effect size measure across studies, a meta-analysis was performed with ReviewManager (RevMan) 5.4 (The Cochrane Collaboration, 2020) using the inverse variance method, and representing it by forest plots. A random-effects model was selected due to the expected diversity among the included studies and real differences in the intervention effects. This was conducted when studies showed similarity in design, intervention (type of biotic and duration of supplementation), population (health status, age) and measurement of variables (method and time of measurement). Continuous outcomes were combined using mean difference (MD) with a 95% confidence interval (95% CI). Data were processed for statistical combination when necessary (e.g., calculation of standard deviation, SD, or imputation from similar studies). Any decision to exclude studies in a synthesis was justified. A p-value of less than 0.05 was considered statistically significant.
Heterogeneity among the included studies was evaluated using the I2 statistic values, and explored through subgroup analyses, given a sufficient number of trials (Deeks et al., 2023). A sensitivity analysis was conducted to assess the robustness of the measured effect by omitting, one study at a time, those substantially different in design, population, intervention or outcome measurement, or at high risk of bias. The risk of publication bias would be studied if five or more studies were included in a meta-analysis, through funnel plot asymmetry. The certainty of the evidence from statistical syntheses was assessed using the GRADE approach (Grading Recommendations Assessment, Development and Evaluation), which considers risk of bias (at the outcome level), inconsistency (heterogeneity), indirectness, and imprecision (Guyatt et al., 2008). Imprecision was assessed by clinical threshold crossing and review information size (RIS) calculation (Schünemann et al., 2022). For variables where clinical thresholds could not be established (e.g., faecal microbiota), imprecision was evaluated based on the relative width of the 95% CI and sample size.
When it was not possible to perform a meta-analysis, alternative and acceptable synthesis methods were used, based on the available data (McKenzie & Brennan, 2023). If p-values and effect directions were presented, they were combined. If only the effect direction was available, vote counting based on it was performed, using a harvest plot. For this, each study was visually weighted based on the established risk of bias quality, at outcome level: a score of 3 was “low risk” (every item at low risk), 2 was “unclear risk” (at least one item at unclear risk) and 3 was “high risk” (at least one item at high risk). For cases where this was not feasible, a narrative synthesis structured around the PICOs was performed.
Deviations from the protocol
The amendments of the protocol together with justifications, before study selection completion, are available on OSF (https://osf.io/eu5jr/).
Discrepancies in the risk of bias assessment were resolved through discussion. Methodological quality was assessed by one reviewer, except for items 20 to 22 (CONSORT) and 22 to 24 (TREND), which were assessed by two reviewers, as other items were deemed objective. There were no additional deviations.
RESULTS
Characteristics of included studies
The search strategy identified 825 studies after duplicates were removed, of which 20 reports, from 19 trials, met the eligibility criteria (Fig 1). References of included studies and a relevant systematic review (Jensen & Bjørnvad, 2019) were screened, and two clinical trials were retrieved (Hesta et al., 2001; Zhang et al., 2023). Reasons for exclusion of potentially eligible studies are presented in Appendix S2.
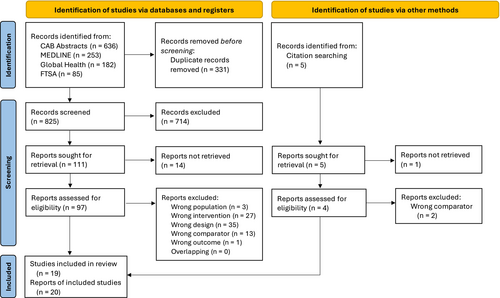
Included studies and their characteristics are presented in Tables 1 to 3. The publications ranged from 2001 to 2024. The trials were performed in the United States (Abecia et al., 2010; Barry et al., 2014; Bybee et al., 2011; Gookin et al., 2022; Stokes et al., 2017; Torres-Henderson et al., 2017; Watson et al., 2019; Whittemore et al., 2018, 2019), China (Han et al., 2024; Li et al., 2023; Wang et al., 2023; Wang et al., 2024; Zhang et al., 2023), Belgium (Hesta et al., 2001, 2005; Verbrugghe et al., 2009) and Brazil (Aquino et al., 2012, 2013; Rodrigues et al., 2020). The majority of them were included in PICO 2, and some trials answered more than one question. Probiotics were the most frequent supplementation (10 from 19 trials). Most of the trials were conducted under controlled laboratory conditions, except for one study conducted in a shelter (Bybee et al., 2011), and another in foster houses (Gookin et al., 2022). Excluding these two trials, which involved a much larger sample, the average population was 14 ± 7 cats. The studies mostly included cats from both sexes and all ages, from kittens (≤6 months) (Gookin et al., 2022; Wang et al., 2024; Watson et al., 2019; Zhang et al., 2023) to senior cats (≥9 years) (Barry et al., 2014), although the majority used adult cats.
| Author and year | Population N, health status, breed, sex and age | Design | Intervention and comparator | Measured outcomes | Subgroup | Funding sources |
|---|---|---|---|---|---|---|
| Abecia et al., 2010 |
N = 8 IBD
|
Cross-over
|
n = 8/group
|
② | (b) | Hill's Pet Nutrition; Basque Government: Education, Universities and Research Department |
| Watson et al., 2019 |
N = 8 (Group A) Induced aEPEC (108 CFU/cat)
|
Parallel
|
n = 4/subgroup
|
① ② ⑤ ⑥ | (a) | North Carolina State University, Comparative Medicine Institute, and the College of Veterinary Medicine; National Institutes of Health; North Carolina State University. |
| Zhang et al., 2023 |
N = 20 Mild diarrhoea
|
Multi-arm parallel
|
n = 10/group
|
① ② ⑤ ⑥ ⑦ ⑧ | (a) | No external funding |
- Ad Adaptation phase to diet (without intervention), aEPEC Atypical enteropathogenic Escherichia coli, BP Bacteriological peptone, CFU Colony-forming units, Co Control group, d Day(s), E. Enterococcus (genus), Ex Experimental phase (intervention or comparator), GOS Galactooligosaccharides, In Intervention group, IBD Inflammatory bowel disease, N Total sample size, n Sample size per group or test, N.I. Arm not included in the systematic review, n.s. Not supplemented, S. Saccharomyces (genus)
- Measured outcomes: ① faecal consistency; ② faecal microbiota; ⑤ body condition or weight; ⑥ appetite; ⑦ serum cytokine levels; ⑧ faecal metabolites. Subgroup: (a) probiotics; (b) prebiotics
| Author and year | Population N, breed, sex and age | Design | Intervention and comparator | Measured outcomes | Subgroup | Funding sources |
|---|---|---|---|---|---|---|
| Abecia et al., 2010 |
N = 10
|
Cross-over
|
n = 10/group
|
② | (b) | Hill's Pet Nutrition; Basque Government: Education, Universities and Research Department |
| Aquino et al., 2012 |
N = 20
|
Multi-arm parallel
|
n = 5/group
|
② ⑤ ⑥ ⑧ | (d) | N.D. |
| Aquino et al., 2013 |
N = 20
|
Multi-arm parallel
|
n = 5/group
|
② | (d) | Alltech Brazil |
| Barry et al., 2014 |
N = 18
|
Multi-arm parallel
|
n = 6/group
|
① ② ⑤ ⑥ ⑧ | (b) | N.D. |
| Bybee et al., 2011 |
N = 222
|
Non-randomised
|
|
① | (a) | Nestlé Purina PetCare |
| Gookin et al., 2022 |
N = 220
|
Parallel Variable duration (up to ≥907 g of weight): Median: In: 17d; Co: 22d |
|
① ② ⑤ | (a) | Winn Feline Foundation; PetSmart Charities®; North Carolina State University; Center for Gastrointestinal Biology and Disease; UNC Nutrition Obesity Research Center |
| Han et al., 2024 |
N = 12
|
Parallel
|
n = 6/group
|
② ⑧ | (a) | Central Public-Interest Scientific Institution Basal Research Fund; Natural Science Fnd. of Jiang Xi, China |
| Hesta et al., 2001 | N = 4
|
Cross-over and multi-arm Experiment 1:
|
n = 4/group
|
① ⑥ | (b) | N.D. |
| Hesta et al., 2005 |
N = 4
|
Cross-over
|
n = 4/group Moderately protein-restricted diet
|
① ⑥ | (b) | N.D. |
| Li et al., 2023 |
N = 12
|
Parallel
|
n = 6/group
|
② ⑧ | (a) | No external funding |
| Rodrigues et al., 2020 |
N = 18
|
Multi-arm parallel
|
n = 6/group
|
① ② ⑥ | (a) | N.D. |
| Verbrugghe et al., 2009 |
N = 8
|
Cross-over
|
n = 8/group Diet aimed at producing insulin resistance.
|
⑤ ⑥ | (b) | Institute for Promotion of Innovation through Science and Technology in Flanders; Beneo-Orafti, Beneo-group (Tienen, Belgium) |
| Wang et al., 2023 |
N = 20
|
Parallel
|
n = 10/group
|
① ② ⑦ ⑧ | (a) | National Natural Science Fnd. of China; Natural Science Fnd. of Zhejiang province; National High-Tech R&D Program Project of China |
| Wang et al., 2024 | N = 10
|
Parallel
|
n = 5/group
|
① ② ⑤ ⑥ ⑦ | (a) | Fnd. of Key Technology Research Project of Henan Province |
- Ad Adaptation phase to diet (without intervention), B. Bacillus (genus), bw Body weight, CFU Colony-forming units, Co Control group, d Day(s), DM Dry matter of diet, E Enterococcus (genus), Ex Experimental phase (intervention or comparator), Fnd Foundation, FOS Fructooligosaccharides, GOS Galactooligosaccharides, In Intervention group, L. Lactobacillus (genus), MER Metabolizable energy requirements, N Total sample size, n Sample size per group or test, N.D. Undeclared, n.s. Not supplemented, S. Saccharomyces (genus), YCW Yeast cell wall extract (Saccharomyces cerevisiae)
- Measured outcomes: ① faecal consistency; ② faecal microbiota; ⑤ body condition or weight; ⑥ appetite; ⑦ serum cytokine levels; ⑧ faecal metabolites; Subgroup: (a) probiotics; (b) prebiotics; (d) postbiotics
- † Intervention alternation between rooms
| Author and year | Population N, health status, breed, sex and age | Design | Intervention and comparator | Measured outcomes | Subgroup | Funding sources |
|---|---|---|---|---|---|---|
| Stokes et al., 2017 Whittemore et al., 2019 |
N = 16 Healthy
|
Cross-over
|
n = 16/group Clindamycin (75 mg/d PO) during Ex.
|
① ② ④ ⑤ ⑥ ⑧ | (c) | Nutramax Laboratories Veterinary Sciences, Inc. Lancaster, SC |
| Torres-Henderson et al., 2017 |
N = 34 Healthy
|
Parallel
|
n = 17/group Amoxicillin-clavulanic acid (62.5 mg/12 h PO) during Ex. I
|
① ② ④ ⑥ | (a) | Center for Companion Animal Studies in the Department of Clinical Sciences, Colorado State University |
| Watson et al., 2019 |
N = 8 (Group B) Induced aEPEC (108 CFU/cat)
|
Parallel
|
n = 4/subgroup Amoxicillin-clavulanic acid (12.5 mg/kg/12 h PO) + pradofloxacin (7.5 mg/kg/24 h PO), ATB phase
|
① ② ⑤ ⑥ | (a) | North Carolina State University, Comparative Medicine Institute, and the College of Veterinary Medicine; National Institutes of Health; North Carolina State University. |
| Whittemore et al., 2018 |
N = 16 Healthy
|
Parallel
|
n = 8/group Clindamycin (150 mg/d PO) during Ex.
|
① ② ④ ⑤ ⑥ ⑧ | (c) | University of Tennessee, College of Veterinary Medicine; Nutramax Laboratories Veterinary Sciences, Inc., Lancaster, SC. |
- Ad Adaptation phase to diet (without intervention), aEPEC Atypical enteropathogenic Escherichia coli, ATB Antibiotic phase (without intervention), B. Bifidobacterium (genus), BP Bacteriological peptone, CFU Colony-forming units, Co Control group, d Day(s), E Enterococcus (genus), Ex Experimental phase (intervention or comparator), FOS Fructooligosaccharides, In Intervention group, L. Lactobacillus (genus), N Total sample size, n Sample size per group or test, PO Per os (oral administration)
- Measured outcomes: ① faecal consistency; ② faecal microbiota; ④ vomiting; ⑤ body condition or weight; ⑥ appetite; ⑧ faecal metabolites; Subgroup: (a) probiotics; (c) synbiotics
All but one publication (Verbrugghe et al., 2009) reported at least one primary outcome, mostly faecal consistency or microbiota. No studies evaluated the FCEAI. Contact with the authors allowed to obtain responses to assess the risk of bias, and unreported data; however, response rate was low (five out of 20).
Risk of bias and quality of included studies
Risk of bias was generally low or unclear (Fig 2). It was low for all domains for the non-randomised trial (Bybee et al., 2011). Justifications for the judgements are described in Appendix S3.
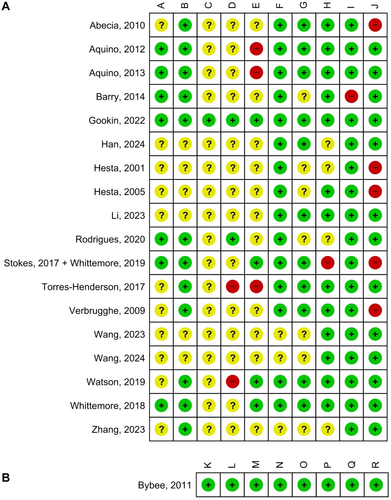
Selection bias could not be assessed in most studies, due to the absence of information about how the random sequence was generated or implanted. After contacting with the authors, two studies performed adequate randomisation (Aquino et al., 2012, 2013). The risk of bias was downgraded in those studies that measured baseline values of outcomes and when population characteristics distribution (sex, age and weight) was similar. Performance bias was increased when each experimental group was housed in a different room, potentially compromising blinding if the effects of the intervention were pronounced. However, there was little information about the housing distribution and blinding among the studies. Detection bias was considered unclear only in those studies that measured serum cytokines (that may be affected by the time of measurement, due to the circadian cycle) and in those that did not describe blinding of FS evaluators (subjective variable) and was generally low in other trials. Attrition bias was generally low, but it was considered high in a trial where elimination from treatment was related to the intervention (Stokes et al., 2017; Whittemore et al., 2019). Reporting bias was judged to be low, but no protocol could be retrieved. The absence of a washout phase in crossover designs (Abecia et al., 2010; Hesta et al., 2001, 2005; Verbrugghe et al., 2009) or an insufficient duration of it (Stokes et al., 2017; Whittemore et al., 2019) was a frequent source of high risk of bias.
Evaluation of the methodological quality showed that 39% ± SD 9 and 12% ± SD 5 of the items were either unpresented or incomplete, respectively (Appendix S3). The absence of reporting on the trial registration or protocol availability was prominent.
Effect of biotic supplementation
Faecal consistency
This was studied in 513 cats of all ages, corresponding to 13 trials, from all three PICO (Barry et al., 2014; Bybee et al., 2011; Gookin et al., 2022; Hesta et al., 2001, 2005; Rodrigues et al., 2020; Stokes et al., 2017; Torres-Henderson et al., 2017; Wang et al., 2023; Wang et al., 2024; Watson et al., 2019; Whittemore et al., 2018; Zhang et al., 2023). FS was predominantly used, although the incidence of cats with at least one episode of diarrhoea and the proportion of pathological faeces were also studied. Details of individual studies are presented in Table 4.
| Study | Measurement (time, sample size and method) | Summary of results | Estimated effect | |
|---|---|---|---|---|
| PICO 1 | ||||
| Watson et al., 2019 |
Daily, all study Group A (n = 4/subgroup†) |
Nestlé Purina FS (1 to 7) (1: hard; 2: normal; 7: watery) | Values by group were not provided | No comparison reported |
| Zhang et al., 2023 |
Days 0, 14 and 28 (n = 10/group†) |
FS (1 to 5) (1: hard; 2 to 2.5: ideal; 3 to 3.5: normal; 5: watery) | Daily mean
|
Days 0 and 14: ns Day 28: MD = −0.9 (P < 0.05) |
| PICO 2 | ||||
| Barry et al., 2014 |
Last 3d of Ex II (n = 5/group) |
FS (1 to 5) (1:hard; 2 to 3: normal; 5:watery) | Values by group were not provided: period mean = 2.5 | ns |
| Bybee et al., 2011 |
Daily, all study (n = Co: 87; In: 130) |
Nestle Purina FS (1 to 7) (≥4: diarrhoea) |
Incidence of cats with diarrhoea. Co: 28/87; In: 34/130 Diarrheal/total stool ratio‡ Co: 60/337; In: 52/475 |
OR = 0.75 (P = 0.336) OR = 0.57 (P = 0.006) |
| Gookin et al., 2022 |
Daily, all study (n = Co: 72; In: 58) |
Veterinary diagnosis | Incidence of cats with diarrhoea. Co: 20/72; In: 6/58 | OR = 0.30 (P = 0.017) |
| Hesta et al., 2001 |
Last 5d of each Ex (n = 4/group) |
FS (1 to 4) (1: watery; 3: normal; 4: hard) | Mean. Co: 2.68; In I: 2.52; In II: 2.22; In III: 1.99 | SEM = 0.044 (P = 0.002) |
| Hesta et al., 2005 |
Last 5d of each Ex (n = 4/group) |
FS (1 to 4) (1: watery; 3: normal; 4: hard) | Mean ± SD. Co: 2.73 ± 0.32; In: 2.68 ± 0.36 | MD = −0.05 ± 0.47 (P > 0.999) |
| Rodrigues et al., 2020 |
Days 16, 18 and 20 (n = 6/group†) |
FS (0 to 5) (0: watery; 3 to 4: normal; 5: hard) | Mean. Co: 3.22; In I: 3.22; In II; 3.11 | SEM = 0.103 (P = 0.6815) |
| Wang et al., 2023 |
Daily, during Ex (n = 10/group) |
FS (1 to 5) (< 2: constipation; 2 to 3: normal; >3: loose stools; 4 to 5: diarrhoea) | Pathological/total stool ratio: mean ± SD§
|
|
| Wang et al., 2024 |
Days 0, 7, 14, 21 and 28 (n = 5/group) |
FS (1 to 5) (1: watery; 3 to 4 normal; 5: hard) | Mean§ ± SD§. Co: 2.66 ± 0.61; In: 2.26 ± 0.38 | MD = −0.40 (P = 0.249) |
| PICO 3 | ||||
| Watson et al., 2019 |
Daily, all study Group A (n = 4/subgroup†) |
See PICO 1 | No comparison reported | |
| Stokes et al., 2017 |
Daily, during Ad and Ex from periods I and II. (n = 16/group ¶) |
Nestlé Purina FS (1 to 7) (1: hard; 2: normal; 7: watery) | Weekly mean ± SD for period I and II
|
No association with the intervention (P = 0.37) or period (P = 0.1) |
| Torres-Henderson et al., 2017 |
Daily, during Ex I and first 4d of Ex II (n = Co: 14; In: 13) |
FS (0 to 7) (0: no stool; 1: hard; 3: normal; 7: liquid) | Pathologic stool/total stool ratio §
|
|
| Whittemore et al., 2018 |
Daily during Ad and Ex (n = 8/group) |
Nestlé Purina FS (1 to 7) (1: hard; 2: normal; 7: watery) |
Weekly mean ± SD
|
P = 0.2 |
- Ad Adaptation phase to diet (without intervention), Co Control group, d Day(s), Ex Experimental phase (intervention or comparator), FS Faecal score, In Intervention group, MD Mean difference (intervention – control), n Sample size by test, ns Not significant (P < 0.05), OR Odds ratio, SD Standard deviation, SEM Standard error of the mean
- † It was assumed that the measurements were performed on all individuals, as it was not indicated otherwise
- ‡ Data obtained after contact with authors
- § Extracted from figures with WebPlotDigitizer
- ¶ Four cats (placebo group) were eliminated from group A during period I
The synthesis of results was based on vote counting (Fig 3), classifying studies according to whether it had a beneficial or detrimental effect on faeces, regardless of their statistical significance: effects were considered harmful if they showed that the intervention group's FS deviated from normality in either direction. Multi-arm or crossover studies with inconsistent direction effects (between supplemented groups or periods), or studies in which consistency remained normal, were classified as mixed or no effects. There was no evidence of benefit in seven of 12 trials. In sick cats, the only study reporting data showed beneficial effects in mild diarrhoea. In healthy cats and in those receiving antibiotics, results were distributed.
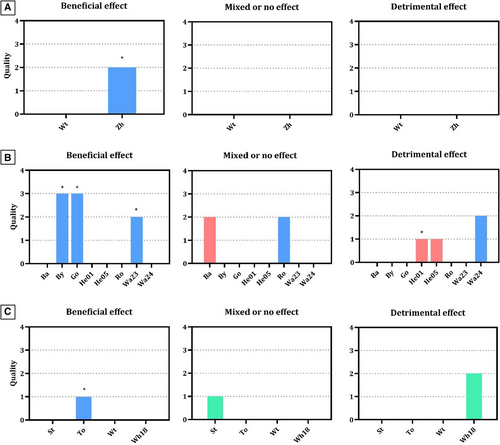
With probiotics, five of the seven trials showed beneficial effects, and the two high-quality studies favoured the intervention. Paradoxically, studies with negative effects showed loosener stools with intervention. Comparison of FS between groups was not reported in a study excluded from synthesis (Watson et al., 2019).
Faecal microbiota
It was described in 286 cats of all ages, corresponding to 15 studies, from all three PICOs (Abecia et al., 2010; Aquino et al., 2012, 2013; Barry et al., 2014; Gookin et al., 2022; Han et al., 2024; Li et al., 2023; Rodrigues et al., 2020; Torres-Henderson et al., 2017; Wang et al., 2023; Wang et al., 2024; Watson et al., 2019; Whittemore et al., 2018, 2019; Zhang et al., 2023). The summary of results is presented in Appendix S4. Mostly, the sequencing of the bacterial 16S rRNA gene was used to assess the abundances after supplementation. Predominant phyla were Bacillota, Actinomycetota, Bacteroidota, Pseudomonadota (Proteobacteria) and Fusobacteriota (Fusobacteria).
The risk of bias from the three studies that addressed PICO 1 was unclear or high (Abecia et al., 2010; Watson et al., 2019; Zhang et al., 2023). The crossover trial (Abecia et al., 2010) found no consistent results between periods, studying the effect of GOS in inflammatory bowel disease (IBD): a washout phase was not reported. In a study on infection with atypical enteropathogenic Escherichia coli (aEPEC) (Watson et al., 2019), its excretion did not decrease after supplementation with Enterococcus hirae. Following supplementation with Saccharomyces boulardii in kittens with diarrhoea, one study did not report significant modifications in the predominant phyla: Actinomycetota increased slightly, and Bacteroidota decreased.
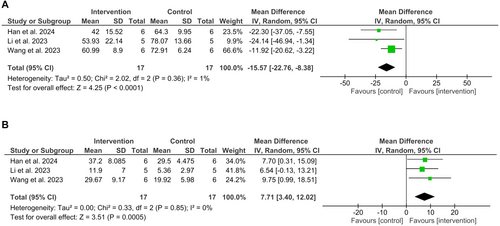
Three parallel trials with probiotics, on 34 healthy young to adult cats (1 to 4 years old) (Han et al., 2024; Li et al., 2023; Wang et al., 2023), were included in a random effects meta-analysis, evaluating the differences in relative abundances of the most abundant phyla after 28 days of supplementation (Fig 4). These studies were at unclear risk of bias. The relative abundance of Bacillota was decreased with supplementation (MD = −15.57; 95% CI −22.76, −8.38; P < 0.0001), and Actinomycetota was increased (MD = 7.71; 95% CI 3.40, 12.02; P = 0.0005). In none of the analyses, neither the P-value of the Chi2 statistic nor the I2 indicated heterogeneity. However, it should be noted that each study used different probiotics (Bacillus, Saccharomyces, Peddiococcus and E. hirae) and that their effects are specific; a sensitivity analysis was performed by omitting each study individually (Appendix S5). After the analysis of the estimated effect for Bacillota, the statistical significance and direction of the effect remained unchanged, but not the magnitude. For Actinomycetota, the results were unmodified. The certainty of evidence was judged to be low in both analyses (Table 5).
| Outcome | MD (95% CI) | Risk of bias | Inconsistency | Indirectness | Imprecision | Other | Certainty of the evidence (GRADE) | |
|---|---|---|---|---|---|---|---|---|
| PICO 2 | ||||||||
|
Population: young to adult cats (1 to 4 years old), both sexes. N = 34 cats from 3 RCT Intervention and comparator: probiotics versus no supplementation |
||||||||
| Relative abundance of Bacillota | Day 28 | −15.57 (−22.76, −8.38) | Serious† | Not serious | Not serious | Serious‡ | None | Low |
| Relative abundance of Actinomycetota | Day 28 | 7.71 (3.40, 12.02) | Serious† | Not serious | Not serious | Serious‡ | None | Low |
| PICO 3 | ||||||||
|
Population: adult to mature cats (3 to 10 years old), both sexes. N = 32 cats from two cross-over RCT Intervention and comparator: synbiotics versus no supplementation |
||||||||
| Reduction in % days vomiting per week | 1st week | −4.14 (−18.78, 10.49) | Very serious§ | Not serious | Not serious | Very serious¶ | None | Very low |
| 2nd week | −12.19 (−28.54, 4.17) | Very serious§ | Not serious | Not serious | Very serious¶ | None | Very low | |
| 3rd week | −8.74 (−19.31, 1.84) | Very serious§ | Not serious | Not serious | Very serious¶ | None | Very low | |
|
GRADE Working Group grades of evidence: High: Further research is very unlikely to change our confidence in the estimate of effect Moderate: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low: We are very uncertain about the estimate |
||||||||
- CI Confidence interval, GRADE Grading of Recommendations Assessment, Development and Evaluation, MD Mean difference (intervention – comparator), RCT Randomised-controlled trial, RIS Review information size
- † Downgraded by one level due to unclear risk of bias (in critical items like baseline characteristics)
- ‡ Downgraded by one level due to small sample size (n < 30 per group). n = 30 was selected due to be considered the necessary sample size to assume normality
- § Downgraded by two levels due to high risk of attrition bias
- ¶ Downgraded by two levels due to 95% CI crossing one threshold corresponding 1 day difference on vomiting (−14%) and a small sample size to detect trivial effect (N < RIS)
Two publications, at unclear and low risk of bias, were not included in the synthesis, as they studied in kittens (<6 months old) (Gookin et al., 2022; Wang et al., 2024). Interestingly, both detected non-significant differences in effect opposed to those detected in adults.
Other studies answering PICO 2 were not included in the synthesis due to different measurement methods. A study (Abecia et al., 2010) found a more stable response between periods to supplementation in healthy cats than those found with IBD. Two of the studies that used cultures for the characterisation found no significant differences between lactic, enteric and clostridial bacteria after yeast cell wall (YCW) administration (Aquino et al., 2012, 2013), and one (Rodrigues et al., 2020) detected a significant increase in lactic bacteria after supplementation with Lactobacillus spp. A trial using qPCR, found that E. coli decreased its expression in faeces from the group that received FOS and inulin (Barry et al., 2014).
In cats receiving antibiotics, the microbiota was significantly modified after treatment (Torres-Henderson et al., 2017; Whittemore et al., 2018, 2019), but supplementation did not alleviate the changes. One study (Watson et al., 2019) found that E. hirae was not present in faeces after administration, as it did in the group that did not receive antibiotics.
Vomiting
The effect of biotic supplementation on vomiting was studied in 59 adult-mature cats, receiving antibiotics, corresponding to three publications (PICO 3) (Stokes et al., 2017; Torres-Henderson et al., 2017; Whittemore et al., 2018). The results of the studies are presented in Appendix S6. Antibiotics and dosage varied between studies. The biotic was administered 2 hours before (Torres-Henderson et al., 2017), 2 hours after (Stokes et al., 2017) or together with the antibiotic (Whittemore et al., 2018). Risk of bias was unclear or high; however, the expected direction of bias due to attrition and insufficient washout period (Stokes et al., 2017) leans towards the null effect, due to the elimination of seriously ill cats (placebo group) and a possible residual effect of the synbiotics (period effect).
The frequency of vomiting increased significantly after the antibiotic, but no significant association was found with the intervention. One of the studies (Torres-Henderson et al., 2017) found a non-significant increase in the incidence of cats vomiting at least once with the intervention, but no data on vomiting frequency was presented. A random-effects meta-analysis was performed including two studies, only considering data from the first period from the crossover trial (Stokes et al., 2017; Whittemore et al., 2018) (Fig 5). The estimated effect did not show a clinically relevant reduction on percentage of days vomiting per week (week 1: MD (%) −4.14; 95% CI −18.78, 10.49; P = 0.58; week 2: MD (%) −12.19; 95% CI −28.54, 4.17; P = 0.14; week 3: MD (%) −8.74; 95% CI −19.31, 1.84; P = 0.11). The P-values of the Chi2 statistic and the results of I2 showed no important heterogeneity in the effect. The certainty of the evidence was very low, due to high risk of bias, CI crossing a clinical threshold (reduction of vomiting in 1 day) and a small sample size to detect a trivial effect (Table 5).
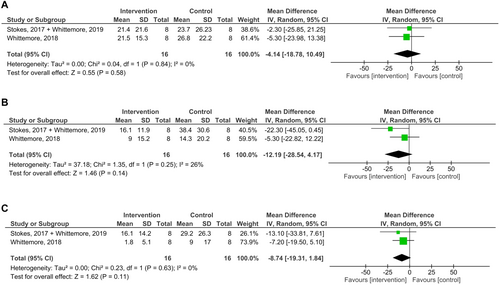
Other results
The summary of secondary outcomes is presented in Appendix S7. Weight and appetite, studied in 249 cats (from nine trials) and 164 cats (11 trials), respectively, remained stable in all treatment groups (Aquino et al., 2012; Barry et al., 2014; Gookin et al., 2022; Hesta et al., 2001, 2005; Torres-Henderson et al., 2017; Verbrugghe et al., 2009; Wang et al., 2024; Watson et al., 2019; Whittemore et al., 2018; Zhang et al., 2023). In cats receiving antibiotics, two studies (Stokes et al., 2017; Whittemore et al., 2018) found significantly decreased intake during antibiotic treatment, and although it increased with supplementation, it was only significant in one of them.
The effect of probiotic supplementation on serum cytokine levels was studied in 42 young cats and kittens, from three publications (Wang et al., 2023; Wang et al., 2024; Zhang et al., 2023): following vote counting based on the direction of effect, proinflammatory cytokines tended to decrease, while anti-inflammatory ones increased (Fig 6). Microbiota-derived metabolites were studied in 121 cats of all ages, from eight trials (Aquino et al., 2012; Barry et al., 2014; Han et al., 2024; Li et al., 2023; Wang et al., 2023; Whittemore et al., 2018, 2019; Zhang et al., 2023). Faecal fatty acids were widely studied, although some studies also assessed the complete faecal metabolomic profile. Results for the main SCFAs (acetic, propionic and butyric) were synthesised by vote counting based on the direction of effect (Fig 6). Acetic, propionic and butyric acids each increased in three of six studies. In sick cats, supplementation only increased acetic acid. In healthy ones, it increased butyric and propionic acids concentration in three of five trials, while acetic acid decreased in three of five. Butyric acid increased in all three studies evaluating probiotics. Concentrations were not significantly modified by synbiotics in cats receiving antibiotics. These studies only reported data for those acetic, propionic and butyric acid-derived SCFAs that differed significantly, so their results were not combined in a synthesis.
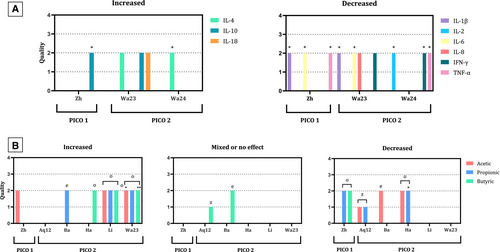
No additional unintended adverse effects were reported, except for hyperaemic intestinal lesions with high dosages of YCW (Aquino et al., 2013).
DISCUSSION
The majority of trials studied the effect of biotics in healthy cats (14 trials). Few studies evaluated the response in sick cats (three trials) and in cats receiving antibiotics (four trials). It is a reasonable hypothesis that healthy cats will not benefit as much from supplementation as the sick. This review also recovered few trials using prebiotics: a limitation was the exclusion of those including them in substitution for another dietary component. These assays may be valuable in detecting its benefits compared to other ingredients, improving the quality of diets. Furthermore, the sample size by intervention group was generally low (four to six cats in half of the trials), limiting the precision of the results.
Additionally, the quality of included reports revealed that half of the items specified on quality checklists were not completed. This created an additional limitation on the interpretation of results. Furthermore, no protocol from the included trials was recovered, and no registration was informed. This complicated the risk of reporting bias assessment: although it was judged to be low after comparing methods and results sections, this interpretation must be taken with caution. Seven included reports declared to be financed by animal nutrition private companies (Abecia et al., 2010; Aquino et al., 2013; Bybee et al., 2011; Stokes et al., 2017; Verbrugghe et al., 2009; Whittemore et al., 2018, 2019). Worth noting, despite not applying a year restriction, only publications from 2001 onward were ultimately eligible, with half of them published after 2018.
Vote counting based on the direction of effect was used as a preferred method over narrative synthesis, when meta-analysis could not be performed. This method allows for a comparison between positive and negative effects, and stratification according to the intervention and the quality of the study. Even so, results obtained from this approach should be interpreted with caution, as it is less powerful than other methods (McKenzie & Brennan, 2023).
In certain trials, supplementation worsened faecal consistency: in some of them, high dosages were used. Compared to the recommended dose in humans, 5 to 8 g FOS daily (ISAPP, 2021), these individuals could had been receiving doses up to 10 times higher (Hesta et al., 2001, 2005). Adverse results could be due to an osmotic effect (Guarino et al., 2020) and lesions to the mucosa, as disruption of the intestinal barrier due to high concentrations of butyrate (Liu et al., 2018). The effectiveness of biotics can also be affected by duration of administration, which was 28 days (mode) in this review: comparing trials that found an effect after supplementation with those without effect, there was no clear association in duration of supplementation. Four trials showed significant effects with short administration times (7 to 17 days) (Bybee et al., 2011; Gookin et al., 2022; Hesta et al., 2001; Torres-Henderson et al., 2017). In acute pathologies, short supplementation times could shorten diarrhoea in children, according to a previous meta-analysis (Van Niel et al., 2002), but not in dogs (Scahill et al., 2024). In this review, no trials studied acute pathologies, except for one publication that did not report data comparing supplementation groups (Watson et al. 2019). Moreover, reports suggest the existence of a medium-term effect after discontinuation of supplementation (Stokes et al., 2017; Whittemore et al., 2019). With the available evidence, the duration of the effects provided with biotics is uncertain, so cross-over trials may be less reliable.
Only three studies reported significant differences in microbiota after supplementation, detecting the presence of probiotic species in faeces or a reduction in pathogenic ones (Barry et al., 2014; Rodrigues et al., 2020; Watson et al., 2019). The manipulation of microbiome is only part of the effects of biotics, and they can be effective even without modifying it: probiotics do not have to colonise the intestine, but they do displace pathogens, promoting colonisation of beneficial communities (Chandrasekaran et al., 2024; Monteagudo-Mera et al., 2019).
In this review, the microbiota in healthy control cats was composed mostly of Bacillota (25% to 78%), followed by Actinomycetota (5% to 70%), or Bacteroidetes (1% to 12%). The population studied in two trials by the same team (Stokes et al., 2017; Whittemore et al., 2018, 2019) was markedly different when compared to the other trials (Gookin et al., 2022; Han et al., 2024; Li et al., 2023; Wang et al., 2023, 2024). It is known that the microbiota is modulated due to geography, environment, previous treatments, diet, age (mainly early life) and the individual (Desai et al., 2009; Farhana Binti, 2015; Jia Jie et al., 2011; Palmer et al., 2007; Senghor et al., 2018; Tiihonen et al., 2010; Zakošek Pipan, 2023). This may explain part of the heterogeneity of the results. It would therefore be useful to encourage researchers to report in detail the previous history and origin of animals used, with particular attention to variables that influence microbiome, and to conduct baseline microbiological characterisations.
The modulation of the ratio F/B in humans has been associated with various diseases (An et al., 2023; Stojanov et al., 2020). The role of Bacillota/Actinomycetota ratio (the most prevalent phyla in this review) in cat health is less known, but Actinomycetota is composed by organisms of probiotic interest, like Bifidobacteriaceae, Propionibacteriaceae, Corynebacteriaceae and Streptomycetaceae (Bernal et al., 2017; Binda et al., 2018; Cuozzo et al., 2023; Ghosh et al., 2023; Menberu et al., 2022; Thierry et al., 2011). In this review, some probiotics used in healthy adult cats decreased the Bacillota/Actinomycetota ratio (Han et al., 2024; Li et al., 2023; Wang et al., 2023), and others appeared to increase it in healthy young ones (Gookin et al., 2022; Wang et al., 2024). Different probiotic species have a specific role in modulating the microbiome (Stojanov et al., 2020), but results vary in function of the diseases studied (Azad et al., 2018): so, the effect of biotics also depends on the biotic balance of the host prior to supplementation (Hou et al., 2020).
Lastly, causes of diarrhoea include factors common in cats housed in shelters and kittens (stress, infectious diseases, dysbiosis, etc.). Studies that evaluated the effect of probiotics in those (Bybee et al., 2011; Gookin et al., 2022) detected a significant decrease in the prevalence of diarrhoea. Finding preventive treatments for cats in these groups at risk is of significant importance, as diarrhoea can lead to serious crises in shelters, due to its contagious nature (delays in adoption and veterinary expenses) and high mortality in kittens (due to their immature immune system) (Cave et al., 2002; Ghosh et al., 2013; Nutter et al., 2004). With the current evidence, it would be necessary to explore the benefit of biotics in further research to defend their use against other therapies. In other groups at risk, such as cats receiving antibiotics, supplementation seemed to reduce vomiting only slightly, but the certainty of the evidence was very low: there was high risk of attrition bias that leans to the null effect. Additionally, probiotics seemed to improve inflammatory profile in three trials (Wang et al., 2023; Wang et al., 2024; Zhang et al., 2023), and may be associated with less damage from inflammation (Hiippala et al., 2018); and increased production of butyrate (Han et al., 2024; Li et al., 2023; Wang et al., 2023), the main energy source of colonocytes (Zeng et al., 2019).
Current evidence does not allow to evaluate with high confidence the efficacy of biotics in the treatment or prevention of gastroenteropathies, due to the few trials conducted. Further research is needed before defending its use, evaluating its use in sick patients and as a preventive for groups at risk. Probiotics showed, with low certainty evidence, a significant reduction in the Bacillota/Actinomycetota ratio in healthy adult cats. Synbiotics presented no clinically relevant effect in reducing antibiotics-associated vomiting, with very low certainty. Some probiotics improved the inflammatory profile in young cats and kittens, and they increased the production of butyric acid in healthy cats.
Author contributions
Á. López Martí: Conceptualization (lead); data curation (lead); formal analysis (lead); investigation (equal); methodology (lead); project administration (lead); visualization (lead); writing – original draft (lead). C. Montero Palma: Conceptualization (supporting); data curation (supporting); investigation (equal); writing – review and editing (equal). H. López Martí: Investigation (equal); writing – review and editing (equal). A. Ranchal Sánchez: Conceptualization (supporting); methodology (supporting); supervision (lead); writing – review and editing (equal).
Conflict of interest
None of the authors of this article has a financial or personal relationship with other people or organisations that could inappropriately influence or bias the content of the paper.
Open Research
Data availability statement
The data that support the findings of this study are available from the first author, [ALM], upon reasonable request.




