Contamination With Antimicrobial-Resistant Escherichia coli, Salmonella, and Enterococcus in Raw Meat-Based Diets for Pets
ABSTRACT
Raw meat-based diets (RMBDs) for pets, which contain raw meat from livestock, aquatic species, or wild-captive animals, are known to harbour feed-borne pathogens and antimicrobial-resistant (AMR) bacteria. This study investigated the contamination of extended-spectrum β-lactamase (ESBL)-producing Escherichia coli, Salmonella, Enterococcus faecalis, and E. faecium in Thai RMBD products, focusing on their AMR phenotypic and genotypic properties. A total of 50 RMBD samples and five fresh-cooked diets (FCDs) were analysed for total aerobic colony counts (ACC) and the presence of third-generation cephalosporins (3GC)-resistant E. coli, Salmonella, E. faecalis, and E. faecium. The AMR phenotypes of isolated bacteria were determined using antimicrobial susceptibility testing and ESBL production, while blaCTX-M and mcr genes were detected by PCR assays. ACCs in RMBD samples were higher than those in FCD samples. The RMBD products tested positive for 3GC-resistant E. coli (n = 40, 80.0%), Salmonella spp. (n = 35, 70.0%), E. faecalis (n = 50, 100%), and E. faecium (n = 42, 84.0%). All 3GC-resistant E. coli isolates were ESBL producers carrying blaCTX-M group 1 and group 9. Among the Salmonella isolates, five ESBL-producing S. Infantis isolates carried blaCTX-M group 9. Two ESBL-producing E. coli and seven Salmonella isolates exhibited colistin resistance. No vancomycin resistance was observed in the enterococcal isolates. These findings underscore the high prevalence of Salmonella and ESBL-producing E. coli in RMBD products, emphasising the potential health risks associated with RMBD consumption for pets. This study highlights the need for awareness and preventive measures to mitigate AMR spread through pet foods.
1 Introduction
Raw meat-based diets (RMBDs), also known as biologically ‘appropriate’ raw food or BARF, consist of uncooked animal materials combined with fruits and vegetables to mimic a ‘natural’ diet (Davies et al. 2019). Many pet owners claim anecdotal health benefits from feeding RMBDs, including shinier coats, increased muscle mass, and cleaner teeth (Morelli et al. 2019). However, scientific evidence supporting claims such as immune system enhancement, allergy prevention, extended lifespan, and improved coat and skin condition remains limited (Algya et al. 2018; Anturaniemi et al. 2020; Hiney et al. 2021). Despite their popularity, studies have highlighted the nutritional imbalances in RMBDs, including significant deficiencies in essential nutrients and excessive fat content (Dillitzer et al. 2011; Vecchiato et al. 2022). Additionally, studies have conclusively demonstrated the risks associated with RMBDs, particularly the transmission of zoonotic bacterial pathogen to both pets and humans (Groat et al. 2022; Nüesch-Inderbinen et al. 2019).
The transmission routes include direct contact with contaminated RMBDs, handling of infected food during preparation, and contact with pets that shed pathogens through feces or licking. These routes pose a significant risk to young children, elderly individuals, and immunocompromised persons (Finley et al. 2006; Freeman et al. 2013; Stull et al. 2013). RMBDs are particularly prone to contamination due to inadequate hygiene practices during processing and transportation. Additionally, the inability to inactivate pathogens through freezing and the absence of heat treatment contribute to the persistence of microbial contaminants (Behravesh et al. 2010; Freeman et al. 2013).
Escherichia coli, Salmonella spp., and Enterococcus spp. are key bacteria in antimicrobial resistance (AMR) surveillance due to their ability to develop multidrug resistance (MDR) and their pathogenic potential (FAO 2019). In E. coli and Salmonella, the production of extended-spectrum β-lactamases (ESBLs), which confer resistance to third-generation cephalosporins (3GCs), along with colistin resistance, poses significant public health concerns by limiting treatment options for Gram-negative bacterial infections. The major mechanisms of 3GC and colistin resistance are mediated by the mobilisable blaCTX-M and mcr genes, respectively, which are located on mobile genetic elements (MGEs) such as plasmids (McGann et al. 2016). These MGEs promote the accumulation and spread of AMR genes by genetic recombination and horizontal gene transfer, thereby driving the development of MDR.
The popularity of feeding RMBDs has been increasing among pet owners and breeders in Asian countries, including Thailand (Kananub et al. 2020). A recent study reported contamination of RMBD products in Thailand with E. coli, Salmonella spp., Listeria monocytogenes, and Staphylococcus aureus, highlighting RMBDs as potential sources of feed-borne pathogens for both pets and their owners. In Thailand, commercial RMBDs are widely available in pet shops and through online marketplaces. However, the lack of specific regulations or risk assessments for establishing quality standards in RMBD production has resulted in limited investigations into their safety and quality. This study aimed to evaluate the presence of 3GC-resistant E. coli, Salmonella spp., and Enterococcus spp. in RMBD products in Thailand, focusing on their AMR profiles.
2 Materials and Methods
2.1 Ethical Approval and Sample Collection
This study was approved by the Institutional Biosafety Committee of Faculty of Veterinary Science, Chulalongkorn University (CU-VET-IBC), under Protocol No. IBC 2431002. The sample size for RMBD was calculated using WinEpiscope 2.0, based on a reported prevalence of Salmonella spp. in a previous study (4.0%) (Groat et al. 2022), with a 95.0% confidence interval and a 10.0% desired precision. A minimum of 38 samples was determined to be necessary, assuming a binomial distribution. To ensure an adequate sample size, a total of 50 frozen-mixed RMBD products, comprising five samples from each of 10 different commercial brands, were obtained by purchasing from pet shops in Bangkok or through online retailers in Thailand between May and November 2023. Additionally, five frozen-cooked diet (FCDs) products were included for comparison. All frozen-mixed food samples were transported under temperature-controlled conditions, maintaining temperatures below 4°C, and delivered to the microbiology laboratory within 6 h. Upon arrival, the sample were stored in a –20°C freezer until further processing. Details such as meat sources, packaging type, package size, production and expiry dates, hygienic handling advice, and feeding instructions provided on the labels were recorded, following the guidelines outlined in Commission Regulation No 767/2009 (EC 767/2009 2009).
2.2 Aerobic Colony Count (ACC)
A sample of 25 g of RMBD and FCD products was mixed with 225 mL of 0.85% normal saline and subjected to a serial 10-fold dilution ranging from 10−1 to 10−4. From each dilution, a 100 µL aliquot was spread onto Plate Count Agar (Difco, France) in triplicate and incubated overnight at 37°C. Plates containing 30–300 colonies were used to calculate colony-forming units per gram (CFU/g). An acceptable ACC of less than 5 × 106 or 6.69 log10 CFU/g was established based on the acceptance criteria for minced meat from Commission Regulation No 2073/2005 by the European Committee on microbiological criteria for foodstuffs (EC 2073/2005 2005).
2.3 Isolation and Identification of 3GC-Resistant E. coli, Salmonella, and Enterococcus Spp.
Two pre-enrichment media were used for the selective and non-selective culture of 3GC-resistant bacteria. For selective enrichment, 3GC-resistant E. coli and 3GC-resistant Salmonella spp. were pre-enriched from 25 g of RMBD samples using 225 mL of buffered peptone water (BPW) supplemented with 2 µg/mL cefotaxime (CTX). For non-selective enrichment, non-3GC-resistant Salmonella spp. and Enterococcus spp. were pre-enriched in BPW without antimicrobials. Subsequently, 3GC-resistant E. coli were cultured on MacConkey agar (Difco) supplemented with 2 µg/mL CTX. Both 3GC-resistant and non-3GC-resistant Salmonella spp. were cultured following the ISO 6579-1:2017 protocol, using modified semisolid Rappaport-Vassiliadis agar (Difco) and Xylose Lysine Deoxycholate agar (Difco). Colonies from enriched 3GC-resistant Salmonella were selected if both 3GC- and non-3GC-resistant isolates were recovered from the same sample. E. faecalis and E. faecium in BPW were further enriched in Brain Heart Infusion containing 6.5% sodium chloride and plated onto m-Enterococcus agar (Difco). Bacterial species were identified using matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry (MALDI-TOF MS) (Bruker Daltonics GmbH, Germany). Salmonella serovars were identified by slide agglutination tests based on the protocol described in ISO/TR 6579-3:2014, with results interpreted according to the Kauffmann-White scheme for Salmonella serotyping (Grimont and Weill 2007).
2.4 ESBL Phenotypic Detection and Antimicrobial Susceptibility Testing (AST)
3GC-resistant E. coli and Salmonella isolates from the selective culture were tested for ESBL production using a combination disk test. Four antimicrobial discs were used: a 30-µg CTX disk, a 30-µg ceftazidime (CAZ) disk, a 30-µg CTX disk combined with 10-µg clavulanic acid (CA), and a 30-µg CAZ disk combined with 10-µg CA. Isolates showing an increase of ≥ 5 mm in the zone diameter for either CTX or CAZ when combined with CA, compared to the zone diameter of the drugs alone, were classified as ESBL producers (CLSI 2023). The minimum inhibitory concentration of antimicrobials for phenotypic AMR detection in 3GC-resistant E. coli and Salmonella isolates was determined using the broth microdilution method with Sensititre ASSECAF/ASSECB customised plates (Thermo Fisher Scientific, USA). The list of antimicrobials, concentration ranges, and interpretive criteria are provided in Supporting Information S1: Table 1 (CLSI 2023; EUCAST 2024). MDR was defined as resistance to three or more antimicrobial classes. Additionally, the disk diffusion method was used for AMR detection in E. faecalis and E. faecium isolates. The list of antimicrobial disks and interpretive criteria are provided in Supporting Information S1: Table 2 (CLSI 2023; EUCAST 2024).
2.5 blaCTX-M and mcr Gene Detection and E. coli Phylogrouping
Genomic DNA of 3GC-resistant and/or colistin-resistant E. coli and Salmonella isolates was extracted using the G-Spin Total DNA extraction kit (iNtRON Biotechnology, South Korea) and used as a DNA template in PCR-based assays. All PCR reactions were conducted in a total volume of 25 µL using 5X FIREPol Master Mix Ready to Load PCR (Solis Biodyne, Estonia). The blaCTX-M group 1, 2, 9, and 8/25 were detected by multiplex and simplex PCR with specific primers and conditions (Dallenne et al. 2010). Two multiplex PCRs were performed to detect mcr variants: multiplex PCR I for mcr-1 to mcr-5 detection, and multiplex PCR II for mcr-6 to mcr-9 detection (Borowiak et al. 2020; Rebelo et al. 2018). The E. coli phylogroups were identified using quadruplex PCR to amplify chuA, yjaA, TspE4.C2, and arpA genes (Clermont et al. 2013). Additionally, allele-specific trpA and arpA genes were detected to distinguish between phylogroups C and E, respectively (Lescat et al. 2013). PCR products were separated by 1.5% Tris-acetate EDTA agarose gel electrophoresis and visualised using the iBright CL1500 imaging system (Thermo Fisher Scientific).
2.6 Data Analysis
The mean of total ACC levels in log10 CFU/g for each RMBD and FCD brand was calculated and presented in a bar chart using Microsoft Excel. One-way Analysis of Variance (ANOVA) was used to compare the ACC levels among the brands and was followed by Tukey HSD to observe the significant differences. In addition, an independent t-test was used to analyse the association between the ACC levels and the characteristics of RMBD. All statistical analyses were conducted using IBM SPSS Statistics Version 29.0.1. Moreover, descriptive statistical analysis was used to illustrate the presence and frequency of 3GC-resistant E. coli, Salmonella spp., E. faecalis, and E. faecium, as well as the frequency of AMR phenotypes and AMR genes. For all statistical analyses, p < 0.05 was considered significant.
3 Results and Discussion
3.1 RMBD Characteristics and ACCs
Commercial RMBDs have gained popularity as an alternative pet food due to their availability, convenience, and ready-to-use format. The product characteristics and ACC of the RMBD samples included in this study are presented in Table 1. All samples were labelled as complete pet food, indicating their suitability as a primary food source for daily consumption. Thirty-one and nineteen samples were intended for dogs and cats, respectively. Only RMBD products for dogs contained vegetables. Additionally, the majority of RMBD samples were formulated for both growth and maintenance life stages, making them appropriate for animals of all ages. Although hygienic handling advice and feeding instructions, which provide guidelines how to prepare RMBDs and recommended daily amount, are essential for proper food handling, storage, and preparation, nearly half of the samples lacked these instructions. Most samples consisted of chicken, duck meat, and beef, with some also containing meat from fish and wild-captive animals (Supporting Information S1: Table 3). This finding is consistent with the fact that meat from food-producing animals is a common primary ingredient in RMBDs for pets (Morelli et al. 2019).
| Brand | Sample | Meat sources | ACC (log10 CFU/g) | RMBD characteristics | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Meat composition | Meat source | Target pet consumer | Manufacturer typea | Packaging | Presence of hygiene and/or handling advice | Feeding instructionsb | |||||||||||
| Single meat | Multiple meat | Poultry | Non-poultry | Cat | Dog | Company | Non-company-related | Plastic bowl | Plastic bag | Yes | No | Yes | No | ||||
| A | A1 | Beef; Lamb; Duck; Venison | 4.45 | + | + | + | + | + | + | + | |||||||
| A2 | Chicken; Salmon; Duck; Venison | 5.01 | + | + | + | + | + | + | + | ||||||||
| A3 | Duck | 5.38 | + | + | + | + | + | + | + | ||||||||
| A4 | Chicken | 4.46 | + | + | + | + | + | + | + | ||||||||
| A5 | Crocodile | 4.61 | + | + | + | + | + | + | + | ||||||||
| B | B1 | Quail | 4.31 | + | + | + | + | + | + | + | |||||||
| B2 | Chicken | 5.38 | + | + | + | + | + | + | + | ||||||||
| B3 | Duck; Chicken | 3.96 | + | + | + | + | + | + | + | ||||||||
| B4 | Rabbit | 4.29 | + | + | + | + | + | + | + | ||||||||
| B5 | Venison | 3.82 | + | + | + | + | + | + | + | ||||||||
| C | C1 | Chicken | 5.05 | + | + | + | + | + | + | + | |||||||
| C2 | Duck | 4.36 | + | + | + | + | + | + | + | ||||||||
| C3 | Salmon | 3.49 | + | + | + | + | + | + | + | ||||||||
| C4 | Beef | 4.38 | + | + | + | + | + | + | + | ||||||||
| C5 | Crocodile | 5.04 | + | + | + | + | + | + | + | ||||||||
| D | D1 | Duck | 6.11 | + | + | + | + | + | + | + | |||||||
| D2 | Chicken | 6.34 | + | + | + | + | + | + | + | ||||||||
| D3 | Mackerel | 5.09 | + | + | + | + | + | + | + | ||||||||
| D4 | Salmon | 5.61 | + | + | + | + | + | + | + | ||||||||
| D5 | Chicken; Beef | 6.05 | + | + | + | + | + | + | + | ||||||||
| E | E1 | Chicken; Salmon | 3.14 | + | + | + | + | + | + | + | |||||||
| E2 | Beef; Salmon | 3.90 | + | + | + | + | + | + | + | ||||||||
| E3 | Chicken; Tuna | 3.20 | + | + | + | + | + | + | + | ||||||||
| E4 | Duck | 5.39 | + | + | + | + | + | + | + | ||||||||
| E5 | Chicken | 3.69 | + | + | + | + | + | + | + | ||||||||
| F | F1 | Duck | 6.40 | + | + | + | + | + | + | + | |||||||
| F2 | Beef | 6.42 | + | + | + | + | + | + | + | ||||||||
| F3 | Chicken | 6.21 | + | + | + | + | + | + | + | ||||||||
| F4 | Mackerel | 6.19 | + | + | + | + | + | + | + | ||||||||
| F5 | Salmon | 6.46 | + | + | + | + | + | + | + | ||||||||
| G | G1 | Beef | 4.91 | + | + | + | + | + | + | + | |||||||
| G2 | Crocodile | 4.79 | + | + | + | + | + | + | + | ||||||||
| G3 | Tuna | 2.47 | + | + | + | + | + | + | + | ||||||||
| G4 | Grouper | 4.55 | + | + | + | + | + | + | + | ||||||||
| G5 | Chicken | 4.08 | + | + | + | + | + | + | + | ||||||||
| H | H1 | Beef | 4.91 | + | + | + | + | + | + | + | |||||||
| H2 | Salmon | 4.39 | + | + | + | + | + | + | + | ||||||||
| H3 | Chicken | 5.54 | + | + | + | + | + | + | + | ||||||||
| H4 | Tuna | 5.43 | + | + | + | + | + | + | + | ||||||||
| H5 | Duck | 4.36 | + | + | + | + | + | + | + | ||||||||
| I | I1 | Chicken | 5.07 | + | + | + | + | + | + | + | |||||||
| I2 | Beef | 5.61 | + | + | + | + | + | + | + | ||||||||
| I3 | Beef; Chicken | 5.15 | + | + | + | + | + | + | + | ||||||||
| I4 | Sardine | 4.19 | + | + | + | + | + | + | + | ||||||||
| I5 | Duck | 4.65 | + | + | + | + | + | + | + | ||||||||
| J | J1 | Duck | 4.78 | + | + | + | + | + | + | + | |||||||
| J2 | Rabbit | 4.59 | + | + | + | + | + | + | + | ||||||||
| J3 | Chicken | 5.12 | + | + | + | + | + | + | + | ||||||||
| J4 | Beef | 5.64 | + | + | + | + | + | + | + | ||||||||
| J5 | Sardine | 3.32 | + | + | + | + | + | + | + | ||||||||
| Total | 42 | 8 | 26 | 24 | 19 | 31 | 25 | 25 | 10 | 40 | 31 | 19 | 20 | 30 | |||
| Percentage (%) | 84.0 | 16.0 | 52.0 | 48.0 | 38.0 | 62.0 | 50.0 | 50.0 | 20.0 | 80.0 | 62.0 | 38.0 | 40.0 | 60.0 | |||
- a Company manufacturer type had significantly higher ACC levels (p < 0.05, ANOVA).
- b RMBD samples with lacking feeding instructions had significantly higher ACC levels (p < 0.001, independent t-test).
ACC levels in the RMBD samples ranged from 2.47 to 6.46 log10 CFU/g, with a median value of 4.78 log10 CFU/g. The ACCs in RMBDs from all commercial brands were higher than those in FCDs (Figure 1). Brand D and F had significantly higher ACCs (p < 0.001), indicating a high level of contamination in these brands. Statistical analysis revealed a significant association (p < 0.05) between the type of manufacturer and ACCs (Table 1). RMBD samples produced by companies had higher contamination levels than those from non-company-related production, which refers to small-scale commercial or homemade producers rather than large manufacturers (Figure 1 and Table 1). Large production scales and meat from various sources could contribute to cross-contamination, increasing the overall contamination levels in the final RMBD products from companies. Notably, microbial loads in RMBDs can increase significantly after storage at 2°C for 3 days (Morelli et al. 2020). Moreover, RMBD samples lacking feeding instructions on their packaging had significantly higher ACCs (p < 0.001), which may lead to food spoilage if the products are not properly stored and prepared before feeding. To prevent RMBD spoilage and reduce the risk of bacterial contamination in households, it is essential to mandate hygienic handling advice and feeding instructions for all RMBD products (EC 767/2009 2009). The absence of feeding instructions not only compromises the safety of these diets for companion animals but also increases the risk of bacterial transmission to humans. This underscores the need to enhance regulations regarding proper sourcing of raw ingredients, hygienic manufacturing practices, and labelling requirements, as well as to conduct comprehensive safety assessments to evaluate microbiological quality and the presence of harmful pathogens in Thailand's raw pet food industry.
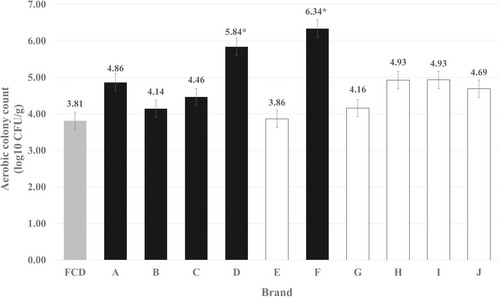
The use of raw meat as an ingredient in pet food has been directly linked to high total ACCs. However, the ACCs in the RMBD samples in this study were lower than those reported in previous studies (8.25–8.41 log10 CFU/g) (Kananub et al. 2020; Vecchiato et al. 2022). Although total contamination levels here are below the acceptable threshold, it is important to note that this reference range applies to raw meat intended for human consumption, which is typically subjected to thermal processing before consumption. In contrast, RMBDs are fed to pets without any heat treatment, allowing bacterial contamination to remain at high levels upon ingestion. Moreover, RMBDs can serve as a source of psychotropic bacteria that can proliferate at low temperatures (Lenz et al. 2009). Consequently, pets fed RMBDs may be directly exposed to significant bacterial loads, posing health risks from feed-borne and AMR bacteria.
3.2 3GC-Resistant E. coli
The high prevalence of 3GC-resistant E. coli at 80.0% (n = 40) in this study was comparable to findings from a study in the Netherlands (van Bree et al. 2018). In contrast, lower prevalence rates, ranging from 15.0% to 40.0%, have been reported in other European countries (Davies et al. 2019). In addition to the misuse and overuse of 3GCs in the poultry industry, the intestinal carriage of ESBL-producing Enterobacteriaceae can contribute to contamination in meat products through leakage from the gastrointestinal tract during slaughtering processes (García-Béjar et al. 2021). This is further supported by our finding of a high proportion of commensal-associated E. coli phylogroups A and B1 (Table 2), which are predominantly found in the intestine of healthy chickens (Wang et al. 2021). Conversely, RMBDs may not serve as a significant source of AMR extraintestinal pathogenic E. coli, as evidenced by the absence of phylogroup B2, D, and F in this study.
| Brand | Sample | Meat sources | E. coli phylogroup | Salmonella serovar | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B1 | C | E | Infantis | Livingstone | Subsp III | Enteritidis | II | Rissen | Agona | Abortusequi | Mura | Typhimurium | Virchow | Saintpaul | Reading | Stanley | Ruzizi | Others | |||
| A | A1 | Beef; Lamb; Duck; Venison | + | + | ||||||||||||||||||
| A2 | Chicken; Salmon; Duck; Venison | + | + | |||||||||||||||||||
| A3 | Duck | + | ||||||||||||||||||||
| A4 | Chicken | + | ||||||||||||||||||||
| A5 | Crocodile | + | + | |||||||||||||||||||
| B | B1 | Quail | + | + | ||||||||||||||||||
| B2 | Chicken | + | + | |||||||||||||||||||
| B3 | Duck; Chicken | + | + | |||||||||||||||||||
| B4 | Rabbit | + | ||||||||||||||||||||
| B5 | Venison | + | ||||||||||||||||||||
| C | C1 | Chicken | + | + | ||||||||||||||||||
| C2 | Duck | + | + | |||||||||||||||||||
| C3 | Salmon | + | + | |||||||||||||||||||
| C4 | Beef | + | + | |||||||||||||||||||
| C5 | Crocodile | + | + | |||||||||||||||||||
| D | D1 | Duck | + | + | ||||||||||||||||||
| D2 | Chicken | + | + | |||||||||||||||||||
| D3 | Mackerel | + | ||||||||||||||||||||
| D4 | Salmon | + | + | |||||||||||||||||||
| D5 | Chicken; Beef | + | ||||||||||||||||||||
| E | E1 | Chicken; Salmon | + | + | ||||||||||||||||||
| E2 | Beef; Salmon | + | ||||||||||||||||||||
| E3 | Chicken; Tuna | |||||||||||||||||||||
| E4 | Duck | + | ||||||||||||||||||||
| E5 | Chicken | + | ||||||||||||||||||||
| F | F1 | Duck | + | + | ||||||||||||||||||
| F2 | Beef | + | + | |||||||||||||||||||
| F3 | Chicken | + | + | |||||||||||||||||||
| F4 | Mackerel | + | + | |||||||||||||||||||
| F5 | Salmon | + | + | |||||||||||||||||||
| G | G1 | Beef | + | + | ||||||||||||||||||
| G2 | Crocodile | + | + | |||||||||||||||||||
| G3 | Tuna | |||||||||||||||||||||
| G4 | Grouper | + | ||||||||||||||||||||
| G5 | Chicken | + | + | |||||||||||||||||||
| H | H1 | Beef | + | + | ||||||||||||||||||
| H2 | Salmon | + | + | |||||||||||||||||||
| H3 | Chicken | + | + | |||||||||||||||||||
| H4 | Tuna | + | ||||||||||||||||||||
| H5 | Duck | + | + | |||||||||||||||||||
| I | I1 | Chicken | + | |||||||||||||||||||
| I2 | Beef | + | ||||||||||||||||||||
| I3 | Beef; Chicken | + | ||||||||||||||||||||
| I4 | Sardine | |||||||||||||||||||||
| I5 | Duck | + | ||||||||||||||||||||
| J | J1 | Duck | + | |||||||||||||||||||
| J2 | Rabbit | + | ||||||||||||||||||||
| J3 | Chicken | + | + | |||||||||||||||||||
| J4 | Beef | + | + | |||||||||||||||||||
| J5 | Sardine | |||||||||||||||||||||
| Total | 15 | 17 | 4 | 4 | 5 | 4 | 4 | 3 | 3 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | ||
- Note: +, presence of the third-generation cephalosporin (3GC)-resistant Escherichia coli phylogroup and Salmonella serovar in the RMBD samples; Grey shade, 3GC-resistant E. coli and/or Salmonella were not detected in the samples.
All 3GC-resistant E. coli isolates displayed ESBL phenotypes and carried the blaCTX-M gene. Figure 2a illustrates the frequencies of AMR among the 3GC-resistant E. coli isolated in this study. None of the isolates exhibited resistance to meropenem. Resistance rates to colistin and tigecycline were low (≤ 5.0%), while resistance rates to other antimicrobials ranged from 17.5%−100.0%. Notably, 95.0% (38/40) of the 3GC-resistant E. coli isolates carried blaCTX-M group 1, while the remaining 5.0% (2 isolates) carried blaCTX-M group 9. The predominance of blaCTX-M group 1 in ESBL-producing E. coli in RMBDs aligns with findings from previous studies (Nüesch-Inderbinen et al. 2019). ESBL-producing E. coli are commonly isolated from poultry and pigs, as well as their meat products (García-Béjar et al. 2021; Lay et al. 2021). Additionally, 92.5% (37/50) of the ESBL-producing E. coli isolates were MDR. The association between ESBL-producing E. coli and MDR phenotypes is often due to the accumulation of multiple AMR genes, alongside clonal selection and spread (Bush and Jacoby 2010). Such MDR characteristics could limit antimicrobial treatment options for opportunistic infections in both animals and humans (Fayez et al. 2023).
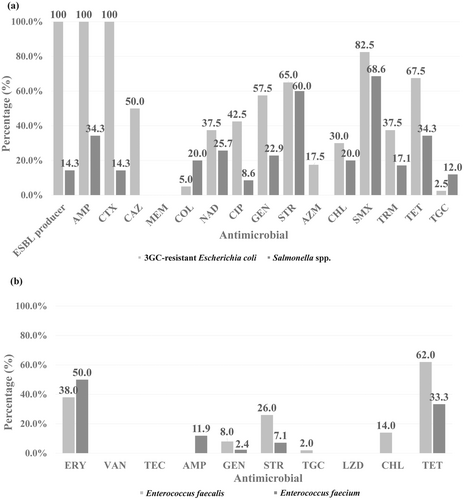
Two ESBL-producing E. coli isolates (5.0%) also showed resistance to colistin and tested positive for mcr-1 (Supporting Information S1: Figure 1). Since the emergence of plasmid-mediated colistin resistance, mcr genes have been identified in E. coli colonising livestock and contaminating animal products worldwide, including in Thailand (Lay et al. 2021). Therefore, RMBDs could serve as a transmission source of mcr-carrying E. coli to pets. Healthy pets can act as reservoirs of ESBL-producing E. coli, potentially facilitating further transmission to humans and the environment (Kidsley et al. 2020). Thus, feeding pets with RMBDs poses a risk of transmitting ESBL-producing E. coli and significant AMR genes from livestock to pets within the community.
3.3 Salmonella spp.
A high prevalence of Salmonella contamination (70.0%; n = 35) in RMBDs was observed in this study. This prevalence exceeded that reported in a recent study in Thailand (52.9%) (Kananub et al. 2020), and the contamination rates found in other countries, such as in the USA and the Netherlands, which they range from 5.0% to 20.0% (Lenz et al. 2009; van Bree et al. 2018). Salmonella is also one of the most commonly reported zoonotic bacterial pathogens in humans worldwide (Abebe et al. 2020). Furthermore, Salmonella is the primary microbiological hazard in pet foods, making pet foods a significant carrier and posing a considerable risk of zoonotic transmission to humans.
A variety of Salmonella serovar were identified among the RMBD samples in this study, as shown in Table 2. Figure 2a presents the frequencies of AMR in the Salmonella isolates. While resistance to CAZ, meropenem, and azithromycin was not detected, resistance to sulfamethoxazole and streptomycin was most prevalent, observed in 68.6% and 60.0% of isolates, respectively. Furthermore, 42.9% of the Salmonella isolates were classified as MDR. Of particular concern, five RMBD samples (10.0%) tested positive for ESBL-producing S. Infantis and carried blaCTX-M group 9 gene (Supporting Information S1: Figure 2). In Thailand, several studies have reported low but increasing rates of ESBL-producing Salmonella carrying blaCTX-M group 1. In contrast, blaCTX-M group 9 was relatively rare and was previously detected in E. coli isolated from fresh pork meat (Kanokudom et al. 2021). However, high rates of resistance to ampicillin, chloramphenicol, nalidixic acid, sulfamethoxazole, and tetracycline in Salmonella isolates have been frequently reported from various livestock sources (Luk-in et al. 2021; Noenchat et al. 2023; Trongjit et al. 2017). S. Infantis is increasingly recognised as an emerging foodborne and zoonotic Salmonella serovar due to its contamination in chicken and other foods worldwide. Notably, S. Infantis isolates carrying blaCTX-M-65 and multiple AMR genes located on conjugative pESI-like mega-plasmids have raised concerns because of their potential to facilitate AMR transmission (Aviv et al. 2014). Seven Salmonella isolates (20.0%) exhibited colistin resistance, but none harboured the mcr gene, suggesting that the resistance may be mediated by alternative mechanisms. These mechanisms potentially involve chromosomal mutations in genes associated with lipopolysaccharide and outer membrane synthesis and modification, as well as components of multidrug efflux pumps (Fortini et al. 2022). The colistin-resistant Salmonella isolates belonged to serovar Enteritidis (n = 3), subsp. III (n = 3), and Mura (n = 1). Despite the absence of mobilisable colistin resistance genes, the presence of colistin-resistant Salmonella poses a significant concern due to the clinical importance of colistin as a last-resort drug for treating of carbapenem-resistant Salmonella infections in humans. These findings underscore the importance of monitoring of RMBD products for AMR pathogens, especially those with resistance to medically important antimicrobials.
The risks of Salmonella infections from RMBD feeding practices are well documented, especially among cats and dogs with immunocompromised conditions (Giacometti et al. 2017). The detection of ESBL-producing S. Infantis and other significant serovars in RMBDs in this study highlights a pressing concern regarding the potential circulation of AMR Salmonella in households. Healthy pets can also act as asymptomatic carriers, shedding Salmonella into the environment and thereby increasing the risk of zoonotic transmission to humans (Ribeiro-Almeida et al. 2024). ESBL-producing E. coli and Salmonella can be transmitted not only through direct contact with pets but also during pet food handling and shared activities. Moreover, these bacteria may disseminate beyond the household, potentially entering wastewater systems or being spread by insects or birds (Antunes et al. 2024). This raise a significant One Health concern, as the interconnectedness of human, animal, and environmental health becomes apparent.
3.4 E. faecalis and E. faecium
A high prevalence of E. faecalis (100%; n = 50) and E. faecium (84.0%; n = 42) found in RMBD samples emphasises the likelihood of intestinal contents being the primary source of contamination with these Gram-positive commensal floras. These prevalences align with the findings of a 100% prevalence in frozen raw meat for dogs (Finisterra et al. 2021) but exceeded the rates (24.0%−30.0%) reported in raw chicken meat intended for human consumption in Thailand (Klaharn et al. 2022; Noenchat et al. 2022). E. faecalis and E. faecium are well-recognised as frequent causative agents of urinary tract infections in both pets and humans (Clark et al. 2023). Additionally, the enterococcal isolates in this study displayed AMR, as shown in Figure 2b. The development of AMR likely arises from the acquisition of AMR genes from other intestinal bacteria under selective pressure from antimicrobial use (Ramos et al. 2020). While enterococcal species are typically commensal, the presence of AMR strains in RMBDs poses risks of colonisation in pets and humans after exposure or consumption, potentially making them asymptomatic carriers of MDR enterococcal strains.
Although studies on the contamination of AMR enterococci in RMBDs and their transmission to pets and humans remain limited, some evidence indicates a concerning spread of AMR genes. Enterococcus spp. from RMBDs in Germany and Switzerland exhibited a high proportion of chloramphenicol and linezolid resistance, associated with the carriage of the cfr gene (Nüesch-Inderbinen et al. 2023). In this study, linezolid resistance in enterococci was absent, while chloramphenicol resistance was observed at a low frequency. Nonetheless, the cfr gene has been detected in S. aureus from livestock in Thailand (Chanchaithong et al. 2019). Monitoring of AMR to last-resort antimicrobials, such as linezolid and vancomycin, remains essential due to the potential transmission of significant AMR genes among Gram-positive bacterial genera of clinical and public health importance.
4 Conclusion
This study highlights the high prevalence of 3GC-resistant E. coli, Salmonella spp., E. faecalis, and E. faecium in commercial RMBD products in Thailand, underscoring the significant public health risks associated with their AMR properties. The detection of significant mobilisable AMR genes, such as blaCTX-M and mcr, in E. coli isolates further emphasises the clinical and public health importance of these bacteria. Additionally, the contamination of Salmonella serovars, including ESBL-producing S. Infantis and colistin-resistant S. Enteritidis, S. Typhimurium, and S. Virchow, highlights the role of RMBDs as potential sources of feed-borne pathogen transmission from livestock to pets. As frontline professionals, veterinarians play a critical advisory role in informing about the safety of RMBD feeding practices. They can also guide pet owners toward safer dietary choices, diagnose feed-borne bacterial infections, and manage AMR-related health issues. The awareness and actions of both veterinarians and pet owners are essential in mitigating the risks of AMR transmission through RMBDs.
RMBDs could serve as an unnoticed and silent route of AMR transmission from livestock production to pets and humans within the One Health framework. Effective and immediate interventions should include the establishment of regulatory standards specifically targeting RMBD quality, covering production, handling and labelling requirements. Regular quality assessments and monitoring of RMBD products can enhance safety by identifying contamination sources and establishing a baseline for ongoing improvements.
Acknowledgements
This study is supported by the 90th Anniversary of Chulalongkorn University Scholarship under the Ratchadapisek Somphot Endowment Fund, Chulalongkorn University−Veterinary Research Fund RI 2/2568, and the National Research Council of Thailand (NRCT) Project ID N42A660897. Rusmin Indra received the Second Century Fund (C2F) from Chulalongkorn University.
Ethics Statement
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. No ethical approval was required as this study did not involve live animals. No animal testing and handling was conducted as part of this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




