L-arginine supplementation during the final third of gestation improves litter uniformity and physical characteristics of neonatal piglet thermoregulation
Abstract
The study assessed the effects of dietary L-arginine supplementation from days 85 to 115 of gestation on sow performance, litter quality, piglet physiology and survival variables in the first 24 hr of life. Twenty multiparous sows, with a history of hyperprolificacy (more than 14 piglets per litter), were used. A completely randomized experimental design was used, consisting of two treatments: feed supplemented or not with 1% L-arginine from days 85 to 115 of gestation. The experimental unit consisted of the sow and its respective litter, using 10 replicates per treatment. The sows were distributed into the treatments based on body condition and parity. Supplementation with L-arginine reduced the within-litter standard deviation and the within-litter coefficient of variation of piglet weight at 24 hr by 54 g and 4.14 percentage points respectively (p = .029; p = .035). Supplementation with 1.0% L-arginine decreased the percentages of piglets weighing less than 800 g by 5.60 and 5.08 points at birth and at 24 hr of life respectively. Piglets from sows supplemented with L-arginine had higher (p = .088) average rectal temperatures at birth and lower (p = .030) rectal temperature at 24 hr of life in comparison with control piglets. No significant differences in placental weight or estimated colostrum production and intake were observed in the first 24 hr of life. At 24 hr of life, piglets weighing less than 1,000 g and from supplemented sows had lower (p = .048) surface/mass ratios and higher body mass index (p = .070). Piglets from supplemented sows and who weighed 1601 to 1,800 g had lower body mass index and ponderal index (p = .002; p = .003). Supplementation with L-arginine during the final third of gestation reduces the incidence of unviable piglets (<800 g) and improved litter uniformity and piglets’ body conformation within the first 24 hr of life.
1 INTRODUCTION
Sow prolificacy has markedly increased in the pork supply chain owing to genetic improvement and advances in reproductive biotechniques in recent decades (Knauer, Cassady, Newcom, & See, 2012). One of the main consequences has been increased variation in piglet weights at birth, with a higher proportion of piglets born with low weight in litters (Amdi et al., 2013). This condition causes increased post-natal mortality and worsens productive performance until slaughter (Campos, Silva, Donzele, Oliveira, & Knol, 2012; Quesnel, Brossard, Valancogne, & Quiniou, 2008).
Birthweight and litter uniformity are two characteristics important for predicting litter quality and post-natal survival. Piglets with low birthweights are more likely to die or show worse performance (Panzardi et al., 2013; Quesnel, 2011). This is directly related to the piglets’ pre-natal development and their disadvantages relative to larger piglets, which results in decreased colostrum intake and therefore decreased viability. This inferior condition can be physiologically explained because these piglets have decreased energy reserves, thermoregulatory capacity and passive immunity. In addition, low-weight piglets are more susceptible to being crushed (Quiniou, Dagorn, & Gaudré, 2002; Zindove, Dzomba, Kanengoni, & Chimonyo, 2014).
In addition to birthweight, some authors have suggested and shown the importance of other indices of piglet survival and viability, for example, body conformation indices such as the body mass index and the ponderal index (Baxter et al., 2008, 2009), which consider the piglets’ morphological characteristics. Morphometric characteristics at birth may also help identify early which animals will show below-average performance and be potentially unthrifty (Huting, Sakkas, Wellock, Almond, & Kyriazakis, 2018).
Inadequate sow nutrition during gestation may be a leading cause of piglet birthweight variations in modern sow litters (Yuan et al., 2015). During late gestation, a nutritional deficit generally occurs due to increased nutrient demand for foetal growth and mammary development (Ji, Hurley, & Kim, 2006; McPherson, Ji, Wu, Blanton, & Kim, 2004). Kim, Hurley, Wu, and Ji (2009) reported that weight variations expressed as the coefficient of variation (%) of litters’ foetal weights are lower on day 45 than on day 60 of gestation. In this context, nutritional strategies during gestation can minimize these problems, thereby contributing to abundant, vigorous and uniform litters. One nutritional adjustment that can be implemented is feed supplementation with functional amino acids in pregnant sows. These amino acids have important metabolic functions associated with reproduction and muscle protein synthesis (Mateo et al., 2007).
Arginine is a functional amino acid with multiple metabolic functions that regulate metabolic pathways via cell signalling, including phosphorylation of master regulator of intracellular protein synthesis (mTOR; Wu, 2010). Arginine is also a precursor of molecules that improve nutrient and oxygen flow from the sow to the foetus, thus contributing to foetal development and growth. Understanding the effects of supplementing feed with this functional amino acid during the gestation phase is relevant because arginine may be a viable tool for improving piglet quality and viability (Gao et al., 2012; Li et al., 2015; Quesnel et al., 2014).
Thus, this study assessed the effects of feed supplementation with L-arginine from days 85 to 115 of gestation on sow and piglet performance, morphological characteristics and piglet viability and survival within the first 24 hr of life.
2 MATERIALS AND METHODS
2.1 Animals and facilities
The Ethics Committee on Animal Use of the Federal University of Lavras (Universidade Federal de Lavras; protocol number 62/16) approved the study procedures. The experiment was conducted on a commercial farm in Lavras, Minas Gerais, Brazil. There were 500 productive sows in a farrow-to-finish system with weekly groups and lactation period of 3 weeks. Twenty multiparous sows from a commercial hybrid line (DB 90), with histories of hyperprolificacy (giving birth to more than 14 piglets at once) and parity from two to seven, were selected and inseminated with semen from the same breeder. The sows were selected based on body condition to keep the body parameters (weight, backfat thickness and loin depth) as homogeneous as possible. The pregnant sows were housed in individually gestation crates and moved to farrowing crates on day 107 of gestation.
2.2 Environmental characterization
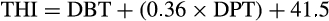
2.3 Experimental design, feed and feed management
The sows were distributed into the treatments considering body condition and birth order. A completely randomized experimental design was used, consisting of two treatments: feed supplemented with 1.0% L-arginine provided 30 days before the expected birthdate or feed without L-arginine supplementation. The L-arginine source was 98.5% pure and purchased from the Division of Animal Nutrition of Ajinomoto do Brasil Indústria e Comércio de Alimentos Ltda, Limeira, São Paulo, Brazil. The experimental unit consisted of a sow and its respective litter, using 10 replicates per treatment.
L-arginine was supplemented in the on-top form and calculated based on the feed quantity provided to account for 1.0% intake. The gestation feed (Table 1) and feed management were those adopted by the farm, supplying 2.8 kg per day from insemination to day 105 of gestation and 5.6 kg per day from day 106 until delivery, divided into two meals (7:00 a.m. and 4:00 p.m.). The sows were given access to water ad libitum throughout the study period.
| Ingredients | % |
|---|---|
| Corn | 57.00 |
| Soybean meal | 15.00 |
| Wheat meal | 24.00 |
| Vitamin–mineral premixb | 4.00 |
| Total | 100.00 |
| Metabolizable energy (kcal/kg)c | 3,035 |
| Crude protein (%)3 | 15.70 |
| Amino acid composition | Total AA (%)c | Digestible AA (%)d |
|---|---|---|
| Arginine | 0.98 | 0.89 |
| Cysteine | 0.22 | 0.18 |
| Phenylalanine | 0.70 | 0.60 |
| Histidine | 0.50 | 0.42 |
| Isoleucine | 0.64 | 0.54 |
| Leucine | 1.36 | 1.15 |
| Lysine | 0.82 | 0.63 |
| Methionine | 0.23 | 0.19 |
| Threonine | 0.58 | 0.43 |
| Tyrosine | 0.52 | 0.45 |
| Valine | 0.75 | 0.62 |
| Sodiumd | 0.21 | – |
| Calciumd | 0.56 | – |
| Total phosphorusd | 0.33 | – |
- a The nutritional requirements of pregnant sows were met according to the recommendation of the genetic analyses used.
- b Quantity per kg of product: 117 g calcium; 33 g phosphorus; 330 mg fluorine; 48 g sodium; 10.5 international units (IU) choline; 255,000 IU vitamin A; 62,500 IU vitamin D3; 1,750 IU vitamin E; 100 mg vitamin K3; 62.5 mg vitamin B1; 200 mg vitamin B2; 87.5 mg vitamin B6; 750 mg Vitamin B12; 500 mg pantothenic acid; 50 mg folic acid; 1,000 mg niacin; 8.75 mg biotin; 2,500 mg iron; 375 mg copper; 1,250 mg manganese; 3,125 mg zinc; 35 mg iodine; 7.5 mg selenium; 5 mg chromium; Saccharomyces cerevisiae; 12 SFTU/g phytase.
- c Values analysed: the percentage of crude protein (CP) was assessed using the Kjeldahl method (AOAC 2000), and the amino acid content was determined via high-performance liquid chromatography (HPLC).
- d Estimated value.
2.4 Sow and litter variables
The sows were not induced to deliver. At birth, the piglets were weighed and tagged, and each piglet's body length was measured with a tape measure from the medial portion of the skull at the height of the base of the ear to the first coccygeal vertebra. Each piglet's body temperature (rectal) was measured at birth and after 24 hr. The numbers of stillborn, mummified and live piglets 24 hr after calving were calculated, and the total placental weight and parturition duration were assessed.
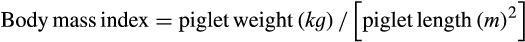
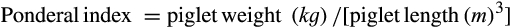
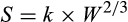
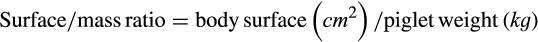
Subsequently, all piglets were packed into the creep, measuring the time elapsed between birth and first breastfeeding. The first breastfeeding was defined as the first time that the piglet suckled the sow's teat for 30 s. The litters were not standardized during the first 24 hr post-partum period. Piglet colostrum intake was estimated using the equation proposed by Devillers, VanMilgen, Prunier, and Dividich (2004); colostrum production per sow was calculated based on each piglet's intake.
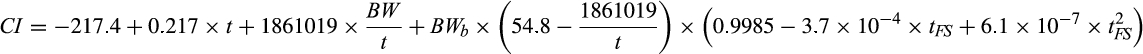
2.5 Litter uniformity evaluation
Variability of the piglets’ weights at birth and 24 hr was compared using the coefficient of variation by weight percentage stratification into seven weight classes: <800 g, 801 to 1,000 g, 1,001 to 1,200 g, 1,201 to 1,400 g, 1,401 to 1,600 g, 1601 to 1,800 g and > 1,800 g and by graphical analysis of the normal piglet weight distribution. Criteria for using these classes were based on Bérard, Pardo, Bethaz, Kreuzer, and Bee (2010), who stated that piglets weighing less than 800 g have retarded intrauterine growth, and on Pettigrew et al. (1986), Roehe and Kalm (2000) and Quiniou et al. (2002), who observed that the probability of post-weaning survival is less than 75% for piglets weighing less than 1,000 g and higher than 95% for piglets weighing more than 1,800 g.
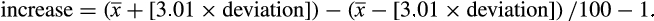
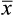 = average piglet birthweight; and
= average piglet birthweight; and 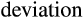 = standard deviation of the average piglet birthweight.
= standard deviation of the average piglet birthweight.Subsequently, the probability of each weight in the normal distribution was calculated using the = DIST.NORM.N function within the range of the mean to 3.01 times the standard deviation of the mean, thereby generating the chart in the “insert area chart” option, dispersion option for each treatment. By overlapping the normal distribution curves of the weights of piglets from sows supplemented or not with 1.0% L-arginine, the intersection points (weights) of these curves and thus the probabilities of the areas representing differences between the curves were determined using the = DIST.NORM.N function.
2.6 Statistical analysis

where in Yij = observations of the effects of 1.0% L-arginine i, replicate j and experiment k; μ = overall mean; Ǥi = fixed effect of supplementation with 1.0% L-arginine; and εij = random error associated with each observation, considered independent, identically distributed, and normal with mean 0 and variance σ.
3 RESULTS
The average temperature inside the gestation sheds and farrowing pens during the study period was 18.9 ± 2.73°C, with a maximum of 24°C and minimum of 15.4°C. The average relative humidity was 72.6 ± 4.90%, with a maximum of 78.6% and minimum of 62.2%. The calculated mean temperature–humidity index was 64.79 ± 4.16.
The sows’ body conditions (body weight, backfat thickness and loin depth) were not significantly affected (p > .05) by L-arginine supplementation in the final third of gestation (Table 2).
| Parameters | Control | L-arginine | p-value | SD |
|---|---|---|---|---|
| Weight at 85 days (kg) | 262.00 | 250.91 | .426 | 27.78 |
| Average backfat thickness at 85 days (mm) | 14.85 | 14.10 | .214 | 2.28 |
| Average loin depth at 85 days (mm) | 52.74 | 51.01 | .225 | 3.89 |
| Weight at 105 days (kg) | 271.00 | 266.40 | .657 | 20.11 |
| Average backfat thickness at 105 days (mm) | 12.99 | 13.22 | .406 | 1.46 |
| Average loin depth at 105 days (mm) | 50.49 | 50.72 | .811 | 6.84 |
| 85-d weight gain at 105 days (kg) | 9.00 | 15.49 | .753 | 19.05 |
- Abbreviation: Standard deviation.
Supplementation with L-arginine did not significantly affect (p > .05) the sows’ productive performances or their litters at birth (Table 3), although supplementation reduced (p < .05) the within-litter standard deviation and the within-litter coefficient of variance of the piglet weight at 24 hr by 54 g and 4.14 percentage points respectively. Supplementation with 1.0% L-arginine decreased (p < .05) the percentages of piglets weighing less than 800 g by 5.60 and 5.08 points at birth and at 24 hr of life respectively (Figure 1a and 1b). Live-born piglet weight and weight at 24 hr did not significantly differ between treatments (Table 3).
| Parameters | Control | L-arginine | p-value | SD |
|---|---|---|---|---|
| Total born piglets (n) | 15.43 | 16.20 | .484 | 2.95 |
| Live-born piglets (n) | 14.57 | 14.89 | .686 | 1.07 |
| Stillborn piglets (n) | 0.86 | 0.64 | .09 | 3.12 |
| Mummified piglets (n) | 0.00 | 0.18 | .341 | 0.34 |
| Total placental weight (kg) | 3.66 | 3.60 | .904 | 0.98 |
| Average litter birthweight (kg) | 20.48 | 20.46 | .988 | 3.13 |
| Average live-born piglet birthweight (kg) | 1.411 | 1.382 | .775 | 0.195 |
| Within-litter standard deviation of birthweight (kg) | 0.292 | 0.253 | .201 | 0.06 |
| Within-litter coefficient of variation of birthweight (%) | 19.76 | 18.23 | .355 | 3.77 |
| Interval from birth to first breastfeeding (min) | 24.51 | 22.52 | .665 | 8.57 |
| Number of piglets at 24 hr (n) | 14.29 | 14.56 | .361 | 1.31 |
| Average litter weight at 24 hr (kg) | 21.21 | 21.49 | .859 | 2.99 |
| Average piglet weight at 24 hr (kg) | 1.426 | 1.474 | .493 | 0.13 |
| Within-litter standard deviation of weight at 24 hr (kg) | 0.311 | 0.257 | .029 | 0.05 |
| Within-litter coefficient of variation of weight at 24 hr (%) | 21.64 | 17.50 | .035 | 4.07 |
| Colostrum production per sow (kg) | 4.711 | 4.843 | .709 | 0.67 |
| Colostrum intake per piglet (g) | 336.01 | 307.48 | .439 | 72.04 |
- Abbreviation: Standard deviation.
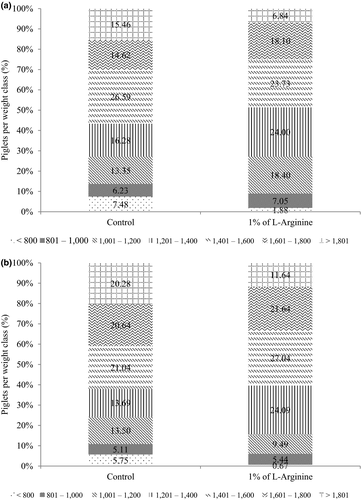
At birth, piglets from sows supplemented with L-arginine had numerically higher (p = .088) average rectal temperatures than did piglets from non-supplemented sows (Table 4). However, at 24 hr of life, the opposite was observed which piglets from the L-arginine group had lower rectal temperature in comparison with control piglets. In Table 5 and for the statistical analysis, evaluation of the indices in the different weight classes required clustering the data from the lower weight class (<800 g) with the data from the next weight class (<1,000 g) because the lower weight class had insufficient data. Piglets from sows supplemented with L-arginine in the final third of gestation and weighing 1,401 to 1,600 g had higher rectal temperatures at birth than did piglets from un supplemented sows (p < .05; Table 5).
| Parameters | Control | L-arginine | p-value | SD |
|---|---|---|---|---|
| 0 hr | ||||
| Rectal temperature (°C) | 38.23 | 38.40 | .088 | 1.51 |
| Body mass index (kg.m−2) | 20.85 | 21.42 | .223 | 3.48 |
| Ponderal index (kg.m−3) | 85.27 | 82.39 | .165 | 16.29 |
| Surface/mass ratio (cm2.kg−1) | 636.34 | 634.34 | .713 | 57.59 |
| 24 hr | ||||
| Rectal temperature (°C) 24h | 38.25 | 38.06 | .030 | 0.88 |
| Body mass index (kg.m−2) 24 hr | 21.53 | 21.71 | .654 | 3.33 |
| Ponderal index (kg.m−3) 24 hr | 82.77 | 84.45 | .408 | 16.06 |
| Surface/mass ratio (cm2.kg−1) 24 hr | 621.91 | 618.00 | .568 | 51.36 |
| Variables | 0 hr | 24 hr | p-value | SD | ||||
|---|---|---|---|---|---|---|---|---|
| Control | L-arginine | Control | L-arginine | 0 hr | 24 hr | 0 hr | 24 hr | |
| <1000 g | ||||||||
| Number of piglets (n) | 15 | 14 | 11 | 7 | ||||
| Rectal temperature (°C) | 37.68 | 37.83 | 38.14 | 38.2 | 0.849 | 0.893 | 1.83 | 0.78 |
| Body mass index (kg.m−2) | 15.61 | 18.04 | 15.00 | 16.87 | 0.098 | 0.070 | 3.89 | 2.02 |
| Ponderal index (kg.m−3) | 70.50 | 83.21 | 66.70 | 72.36 | 0.136 | 0.464 | 22.38 | 14.35 |
| Surface/mass ratio (cm2.kg−1) | 765.31 | 743.16 | 765.38 | 725.04 | 0.234 | 0.048 | 54.36 | 39.15 |
| 1001–1200 g | ||||||||
| Number of piglets (n) | 13 | 30 | 11 | 14 | ||||
| Rectal temperature (°C) | 39.05 | 38.24 | 38.03 | 38.15 | 0.111 | 0.754 | 1.28 | 0.64 |
| Body mass index (kg.m−2) | 19.46 | 19.85 | 18.78 | 18.88 | 0.575 | 0.796 | 2.11 | 2.2 |
| Ponderal index (kg.m−3) | 81.54 | 83.92 | 77.19 | 78.03 | 0.769 | 0.546 | 13.05 | 13.27 |
| Surface/mass ratio (cm2.kg−1) | 674.58 | 673.67 | 670.82 | 673.98 | 0.827 | 0.434 | 12.25 | 8.63 |
| 1201–1400 g | ||||||||
| Number of piglets (n) | 17 | 37 | 15 | 27 | ||||
| Rectal temperature (°C) | 37.97 | 37.52 | 38.05 | 38.14 | 0.919 | 0.461 | 1.99 | 1.17 |
| Body mass index (kg.m−2) | 19.85 | 20.80 | 21.54 | 19.83 | 0.164 | 0.193 | 2.38 | 3.12 |
| Ponderal index (kg.m−3) | 77.72 | 83.30 | 87.80 | 77.47 | 0.177 | 0.087 | 13.7 | 19.2 |
| Surface/mass ratio (cm2.kg−1) | 640.27 | 639.25 | 636.55 | 638.01 | 0.740 | 0.373 | 10.01 | 9.87 |
| 1401–1600 g | ||||||||
| Number of piglets (n) | 27 | 35 | 21 | 37 | ||||
| Rectal temperature (°C) | 38.02 | 39.02 | 38.20 | 38.09 | 0.006 | 0.640 | 1.33 | 0.98 |
| Body mass index (kg.m−2) | 22.90 | 22.44 | 22.24 | 21.33 | 0.586 | 0.127 | 3.05 | 2.28 |
| Ponderal index (kg.m−3) | 90.87 | 87.33 | 85.56 | 80.67 | 0.345 | 0.181 | 17.92 | 13.05 |
| Surface/mass ratio (cm2.kg−1) | 612.44 | 610.32 | 607.62 | 609.54 | 0.254 | 0.315 | 7.40 | 6.96 |
| 1601–1800 g | ||||||||
| Number of piglets (n) | 15 | 28 | 21 | 31 | ||||
| Rectal temperature (°C) | 38.50 | 38.81 | 38.27 | 37.89 | 0.290 | 0.052 | 0.96 | 0.73 |
| Body mass index (kg.m−2) | 23.44 | 23.15 | 24.75 | 22.64 | 0.777 | 0.002 | 2.57 | 2.30 |
| Ponderal index (kg.m−3) | 88.45 | 86.40 | 94.90 | 82.77 | 0.963 | 0.003 | 14.61 | 13.37 |
| Surface/mass ratio (cm2.kg−1) | 589.39 | 587.80 | 585.07 | 584.23 | 0.505 | 0.633 | 6.26 | 6.13 |
| >1800 g | ||||||||
| Number of piglets (n) | 15 | 11 | 20 | 34 | ||||
| Rectal temperature (°C) | 38.49 | 38.68 | 38.61 | 38.06 | 0.501 | 0.083 | 0.87 | 0.73 |
| Body mass index (kg.m−2) | 23.55 | 24.47 | 24.33 | 23.88 | 0.433 | 0.288 | 2.73 | 2.27 |
| Ponderal index (kg.m−3) | 82.20 | 88.81 | 85.97 | 92.76 | 0.260 | 0.087 | 13.79 | 16.28 |
| Surface/mass ratio (cm2.kg−1) | 559.74 | 565.93 | 558.79 | 597.03 | 0.215 | 0.001 | 10.44 | 40.26 |
- Abbreviation: Standard deviation.
A trend towards a higher body mass index at birth (0.05 < p<.10) was observed among piglets weighing less than 1,000 g and from sows supplemented with L-arginine. At 24 hr old, piglets from this class also had lower surface/mass ratio (p < .05) than did piglets from the control group. Piglets from supplemented sows and who weighed 1,601–1,800 g had lower body mass index and ponderal index (p < .05). Piglets from the control group weighing more than 1,800 g at 24 hr old showed higher surface/mass ratio.
Analysis of the probability distribution for piglet birthweight showed that L-arginine supplementation decreased the probability of piglets being born weighing less than 1,019 g by 1.40 percentage points and increased the probability of piglets being born weighing 1,019–1,587 g by 7.85 percentage points (Figure 2a). Concurrently, L-arginine supplementation decreased the probability of piglets weighing more than 1,587 g at birth by 6.45 percentage points.
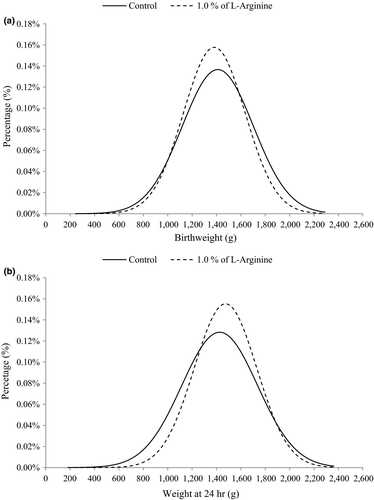
Assessing the weight probability distribution at 24 hr showed that L-arginine supplementation decreased the probability of piglets weighing less than 1,283 g by 9.40 percentage points and increased the probability of piglets weighing 1,283–1,889 g by 10.92 percentage points (Figure 2b). However, the probability of piglets weighing more than 1,889g decreased by 1.51 percentage points when the sows were supplemented. Considering the total gains from L-arginine supplementation in the final third of gestation, the live-born piglet weights at 24 hr improved by 18.83 percentage points.
4 DISCUSSION
The average ambient temperature in the sheds was near the ideal range suggested by Campos et al. (2008) of 12–18°C. However, between 11:00 a.m. and 11:00 p.m., the sows were subjected to higher temperatures, with a maximum of 24°C at 3:00 p.m., which is considered the upper critical temperature per Costa and Felicidade (2009). The relative humidity ranged from 50% to 70%, which is considered ideal (Campos et al., 2008; Figure 1). The calculated temperature–humidity index value of 64.79 can be considered thermal comfort for pigs at this stage (Hahn, 1985).
L-arginine supplementation did not significantly affect the pregnant sows’ body conditions (weight and backfat thickness at days 85 and 105 of gestation), possibly because the feeding regimen at the farm provided a high quantity of feed in the final third of gestation. This was also observed by Wu et al. (2013), and Gao et al. (2012) found that sows supplemented with 1.0% L-arginine from days 22 to 110 of gestation did not significantly differ from sows in the control group in body weight or backfat thickness at 110 days.
L-arginine supplementation did not affect the number of total live-born piglets. This parameter is established mainly in the initial third of gestation; therefore, later nutritional strategies may not affect this variable because embryonic losses occur within the first 30 days of gestation (Ford, Vonnahme, & Wilson, 2002; Wu et al., 2013). Studies have reported significant increases (0.05 < p<.10) in the numbers of live-born piglets upon supplementation in the final third of gestation (Che, Yang, Fang, Lin, & Wu, 2013; Liu et al., 2012; Wu et al., 2012); however, the current study showed no significant improvement in this parameter.
Piglet birthweight is thought to be directly related to piglet survival (Quiniou et al., 2002; Roehe & Kalm, 2000). The effect of L-arginine supplementation on piglet birthweight remains unclear, and the literature presents conflicting results (Palencia et al., 2017). L-arginine supplementation at 0.5% in the last third of gestation increased piglet birthweights by approximately 100 g, thus suggesting increased placental blood flow and improved foetal development compared with non-supplemented sows (Nuntapaitoon, Muns, Theil, & Tummaruk, 2018). Studies have also reported improved litter uniformity after amino acid supplementation (Che et al., 2013). Some studies found results similar to the results herein, observing no supplementation effect on piglet birthweight (Bass et al., 2011; Quesnel et al., 2014). This may be related to the sows’ uterine capacity, which limits foetal growth. This limitation remains to be overcome through nutritional strategies (Quesnel et al., 2014).
Variations in birthweight may result from increased placental weight or efficiency (Wilson, Biensen, & Ford, 1999). Sows supplemented with L-arginine showed no significant difference in total average placental weight, which may explain the findings for piglet birthweight. Dallanora et al. (2017) also observed no significant effects of L-arginine supplementation on placental weight and efficiency, when sows were supplemented in the middle third of gestation.
Knight, Bazer, Thatcher, Franke, and Wallace (1977) found that sow placental weight did not change considerably after 70 days of gestational supplementation in sows. Conversely, other studies showed that dietary supplementation with L-arginine benefitted placental weight, especially when performed in the initial third of gestation, near the time of implantation (Gao et al., 2012; Li et al., 2010; Seo et al., 2014). When supplemented in other phases, L-arginine, as a precursor of polyamines and nitric oxide, positively affects vascular growth and blood flow in the final third of gestation (Reynolds et al., 2006; Wu,et al. 2013).
The decreased values for within-litter standard deviation and within-litter coefficient of variation of piglet birthweights highlight the potential of L-arginine supplementation to improve litter uniformity and lower the incidence of piglets with low viability (Madsen, Pardo, Kreuzer, & Bee, 2017; Palencia et al., 2017; Shi et al., 2018). Quesnel et al. (2014) observed a decreased coefficient of variation and improved uniformity in piglet birthweights after L-arginine supplementation during the final third of gestation.
Dallanora et al. (2017) reported a decrease in the number of low-weight piglets, with a significant decrease in the number of piglets weighing less than 1,000 g from sows supplemented with 1.0% L-arginine in the middle third of gestation. Nuntapaitoon et al. (2018) supplemented the diets of sows with 0.5% L-arginine in the final third of gestation and observed a decreased incidence of piglets weighing less than 1,000 g and an increased number of piglets weighing >1,350 g. A decrease (4%) in the number of piglets weighing less than 1,000 g was also reported after dietary L-arginine supplementation in sows from 30 to 114 days of gestation (Che et al., 2013). These studies indicate that L-arginine supplementation at the end of gestation may effectively modulate piglet birthweights (Nuntapaitoon et al., 2018) and determine which gestational nutritional strategies may selectively improve foetal development and growth, thereby favouring low-weight piglets (Dwyer, Stickland, & Fletcher, 1994; Quesnel et al., 2014). Sow nutrition may alter piglet birthweight by modulating the lipid metabolism and placental energy and may explain the mechanism that enables increased foetal weight uniformity (Che et al., 2017).
Decreased numbers of low-weight piglets are of great interest because low-birthweight piglets have significantly lower daily weight gains until weaning and during subsequent stages than do high-birthweight piglets (Beaulieu, Aalhus, Williams, & Patience, 2010; Fix et al., 2010; Lanferdini et al., 2018), who also show higher mortality rates (Hales, Moustsen, Nielsen, & Hansen, 2014) and need more time to reach market weight (Beaulieu et al., 2010; López-Vergé et al., 2018). Dietary supplementation with L-arginine may help increase the nitric oxide content in endothelial cells that line blood vessels, thus increasing the blood flow, causing vasodilatation and decreasing weight variations and the incidence of low-weight piglets (Campos et al., 2008; Madsen et al., 2017; Mateo, Wu, Moon, Carroll, & Kim, 2008). Genetic improvement may also increase weight-at-birth uniformity and can be performed indirectly by selecting for survival, birthweight and uterine capacity (Kapell, Ashworth, Knap, & Roehe, 2011).
At birth and 24 hr post-partum, sows supplemented with L-arginine showed significantly lower within-litter coefficient of variation and within-litter standard deviation of piglet weights. Colostrum intake may be related to weight uniformity at 24 hr of life; however, no significant differences in intake between treatments were found in this study. Furthermore, no treatment effect on colostrum production was found, similar to the results of Nuntapaitoon et al. (2018). The relative weight calculated within the different classes was higher for lighter weight classes, indicating a lower survival capacity. However, absolute weight is the best indicator of piglet viability at birth (Hales et al., 2014).
Body temperature in the first hours of life is affected by the piglet's body weight among other factors. Low-weight piglets are less able to maintain body temperature and need more time to reach the udder; therefore, they consume less colostrum (Herpin et al., 1996; Lay Júnior, Matteri, Carroll, Fangman, & Safranski, 2002). Furthermore, piglets’ body conformation affects their thermoregulation capacity in the first hours of life (Caldara et al., 2014). In this study, dietary L-arginine supplementation increased piglets’ average rectal temperature at birth. According to Baxter et al. (2008), rectal temperature correlates positively with piglet vitality and reflects the intrauterine condition. The authors reported a positive correlation between room temperature (rectal temperature) at birth and vitality; piglets housed at higher temperature in the first hours of life also showed greater vigour for breastfeeding.
Panzardi et al. (2009) indicate that body temperature at 24 hr post-partum is a good predictor of the survival rate during the first week of life. Rectal temperature at 24 hr was significantly associated with mortality rate (Nuntapaitoon et al., 2018) and weight gain until weaning (Pedersen, Schild, & Malmkvist, 2015), being identified as an indicator of piglet growth at weaning (Panzardi et al., 2013). However, the rectal temperature of piglets within the first 24 hr of life is influenced by environmental factors as reported by Pedersen et al. (2015). In the current study, the piglets from the L-arginine group had lower rectal temperature at 24 hr of life in comparison with control piglets. The reduction in body temperature within the first 24 hr was already expected, as reported by Baxter et al. (2008). Physiological and environmental factors may be related to the fact that control piglets did not suffer this reduction at 24 hr. However, the average values observed at 24 hr (38.25ºC and 38.06ºC) for both groups are within the normal range for 24-hr-old piglets. These temperatures also are above the average temperature where the best daily weight gain during lactation is observed (>37.3ºC) and above the temperature of piglets with high mortality potential (37.58ºC) (Pedersen et al., 2015).
The energy demand for homeothermia preservation mainly depends on glycogen reserves or colostrum intake to increase the metabolic rate (Mount, 1966) . Arginine is a precursor to the synthesis of several important metabolic molecules, including nitric oxide (Bazer, Wang, Johnson, & Wu, 2015; Wu et al., 2013), which has been associated with increased blood flow (Reynolds et al., 2006), thereby improving nutrient distribution to foetuses. Baxter et al. (2008) highlighted the importance of the uterine environment for post-natal piglet physiological and environmental adaptation, which may be improved through L-arginine supplementation.
The indices assessed (Table 5) showed a trend towards improved body conformation in piglets from supplemented sows, especially in piglets weighing less than 1,000 g. Douglas, Edwards, and Kyriazakis (2016) suggest that piglets with normal birthweights (>1,250 g) but with low pre-weaning growth result from post-partum factors other than morphological characteristics. Conversely, post-natal growth of low-weight piglets is predicted by morphological characteristics, such as body mass index and abdominal circumference; therefore, disproportionately elongated and thin piglets have a greater risk of dying. Thus, the observation of higher body mass index values in low-weight classes suggests L-arginine's potential to modulate body mass index values in the pre-natal period and improve the body mass index of low-weight piglets. This is reflected in a higher pre-weaning survival capacity (Hales, Moustsen, Nielsen, & Hansen, 2013).
At the same time, heavy piglets (1,601–1,800 g) from supplemented sows had lower body mass index and ponderal index. That observation was unexpected; however, this weight class is not a concern for the swine industry. Piglets weighing more than 1,600 are not considered low-weight piglets and therefore generally do not suffer impairment on growing performance, as observed in low-birthweight piglets (Pardo, Müller, Bérard, Kreuzer, & Bee, 2013). Lanferdini et al., (2018) reported that piglets born weighing over 1,500 g do not have their performance influenced until marketed and do not have the physiological limitations and low efficiency observed in low-birthweight piglets.
The relationship between piglet body mass index and physiology remains unclear. Hales et al. (2013) assessed body mass index and other survival-related variables and observed that piglets with a high body mass index could adequately nourish themselves and were not subjected to physiological challenges, such as hypothermia, and that the piglets that died on days 0 or 1 after birth had lower body mass indexes. These authors also observed that piglets with the same birthweight but different body mass index values differed in mortality risk.
The mechanisms involved in this possible modulation of piglet conformation and viability are unknown. The known functions of L-arginine as a functional amino acid are potentially related. In addition to improving nutrient flow, L-arginine may help increase the piglets’ energy reserves (Amdi et al., 2013) and pre-natal differentiation of secondary muscle fibres (Bérard et al., 2010; Dwyer, Fletcher, & Stickland, 1993; Town, Putman, Turchinsky, Dixon, & Foxcroft, 2004; Tse et al., 2008).
Low-weight piglets generally dissipate more heat in the first hours of life owing to their high surface/mass ratio (Theil, Lauridsen, & Quesnel, 2014). Piglets weighing less than 1,000 g from sows supplemented with L-arginine showed lower surface/mass ratio (p < .05) at 24 hr than did control piglets, indicating decreased heat dissipation in the first hours of life and susceptibility to hypothermia (Herpin et al., 2002).
The mechanisms through which L-arginine decreased the occurrence of low-weight piglets at birth and at 24 hr are related to the functional activity directed to reproduction (Wu et al., 2013). L-arginine is a precursor of nitric oxide and polyamines, which are important molecules that actively participate in foetal development (Bazer et al., 2015). Upon supplementation in the final third of gestation, L-arginine, via nitric oxide and polyamines, improves placental function by enhancing nutrient and oxygen flow to the foetus, thereby improving foetal development (Reynolds et al., 2006; Wu, Bazer, Hu, Johnson, & Spencer, 2005) and reducing foetal weight variations, thus directly affecting subsequent phases.
Among the post-partum indices related to piglet survival, viability and uniformity evaluated in this study, the results indicated that supplementing sows with L-arginine improved piglet quality and viability.
5 CONCLUSION
Supplementation with L-arginine during the final third of gestation reduces the incidence of unviable piglets (<800 g) and improved litter uniformity and piglets’ body conformation within the first 24 hr of life.
ACKNOWLEDGEMENTS
The authors want to acknowledge the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Instituto Nacional de Ciência e Tecnologia de Ciência Animal (INCT-CA/CNPq), the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
CONFLICT OF INTEREST
None.
ANIMAL WELFARE STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to and the appropriate ethical review committee approval has been received. The authors confirm that they have followed EU standards for the protection of animals used for scientific purposes.