Diagnostic orientation values for ACTH and other parameters for clinically healthy donkeys and mules (insulin, triglycerides, glucose, fructosamines, and ɣ-GT)
Abstract
Pituitary pars intermedia dysfunction is the most prevalent endocrine disease in horses. Although donkeys and mules may also be affected, only a few data have been published. Reference values for diagnostic parameters, such as adrenocorticotropic hormone (ACTH), are especially scarce or even lacking. Therefore, in the present study, available data from the literature have been verified and completed to facilitate a reliable diagnosis. Clinical inspections and haematological and biochemical examinations were carried out four times in a three-month interval (February to November) in 44 donkeys and 31 mules. Data from clinically healthy animals were used as an orientation. Plasma ACTH concentrations showed seasonal changes in both animal groups. However, it was generally higher in donkeys than mules. Although blood glucose (EDTA plasma) showed no difference between groups, serum insulin concentrations were consistently higher in donkeys. Serum fructosamine levels were slightly higher in mules, whereas, in some cases, serum triglyceride levels were considerably higher in donkeys. Serum gamma-glutamyltransferase showed a striking peak in mules in August, whereas the remaining gamma-glutamyltransferase values were lower compared to donkeys. By comparing donkeys and mules, the present work reveals differences in various blood parameters which should be considered for diagnoses and future studies.
1 INTRODUCTION
Pituitary pars intermedia dysfunction (PPID) is the endocrine disease found most frequently in horses (Frank, 2015). In addition to horses, donkeys and mules may also suffer from PPID (Burden, 2012; Mendoza, Toribio, & Perez-Ecija, 2018; Peel, Bouts, Flach, Rivers, & Routh, 2009; Sprayson, 2008). However, only a few publications concerning the latter are available (Schwarz, 2009) and results from horse studies may not simply be transferred to donkeys and mules (Coakley, Peck, Taylor, Matthews, & Mealey, 1999; Duffield, 2008; Levionnois, 2007; Pietta & Bartmann, 2012; Trawford, 2011). Clinical signs of PPID in donkeys and mules, such as chronic laminitis and infections, changes in coat and behaviour, loss of body mass and supraorbital fat pads, are the same as in horses (Burden, 2012; Peel et al., 2009; Sprayson, 2008). Suspected diagnoses of PPID should be verified by determining plasma adrenocorticotropic hormone (ACTH) concentration (Du Toit, Shaw, & Keen, 2011; Du Toit, Trawford, & Keen, 2010; Dugat, Taylor, Matthews, & Gold, 2010; Rickards, 2010; Sprayson, 2008). This method is known to be especially sensitive and specific (Spelta, 2015). Furthermore, a haematology and determination of liver enzyme activities, insulin, glucose, triglycerides and fructosamines provide information on the general state of health and help to differentiate between PPID and equine metabolic syndrome (Dugat et al., 2010; Mattheeuws, Kaneko, Loy, Cornelius, & Wheat, 1966; Schleicher, Olgemöller, Wiedenmann, & Gerbitz, 1993; Schwarz & Anen, 2014; Sprayson, 2008; Staudacher, 1990). However, in many cases, laboratory reference ranges for horses, donkeys and mules differ from each other. Several ranges for diagnostic parameters of PPID in donkeys and mules are contradictory or even lacking (Table 1).
| Horse | Donkey | Mule | |
|---|---|---|---|
| EDTA plasma ACTH (pg/ml) |
Aug–Oct < 47 Nov–July < 291 |
Range 21.3–24.7 |
|
|
37.8–104.8b 36.0–115.0c 67.0d 66.7e, 3 |
|||
|
July–Oct < 45 Nov–June < 354 |
|||
| 40.23e, 5 | |||
| Serum insulin (µU/ml) | <206 |
0–6.3b 0–6.6c 2.1e, 3 |
|
|
1.9–18.3 6.2e, 4 |
|||
| 4.95e, 5 | |||
| Serum triglycerides (mmol/L) | <1.07 | 0.2–4.38 | |
| 1.14–5.7010 |
1.3–6.7 b 1.1–7.9 c , 3 |
||
| 0.11–1.5b, 11 | |||
| Plasma glucose (mmol/L) | 3.5–6.07 |
3.9–4.7 4.4e, 4 |
2.7–6.2 f , 9 |
| 3.1–5.08 | |||
| 3.18–5.01b, 11 | |||
| Serum fructosamines (µmol/L) | <36013 | ||
| Serum ɣ-GT (IU/L) | Up to 2510 | 15–42b, 11 | Up to 2014 |
| 8–49b, 12 | |||
| 13–798 |
Note
- Italicised values indicate that they are converted because a different measuring unit was used in the literature.
- 1Copas and Durham (2012) (chemiluminescent immunoassay—Immulite ACTH), 2Du Toit et al. (2011) (no laboratory methods mentioned), 3Dugat et al. (2010) (Texas Veterinary Medical Diagnostic Laboratory, no more details), 4Schwarz and Anen (2014) (no laboratory methods mentioned), 5Du Toit et al. (2010) (no laboratory methods mentioned), 6Frank (2015) (chemiluminescent assay—Animal Health Diagnostic Laboratory, Cornell), 7Taylor and Hillyer (2001) (no laboratory methods mentioned), 8Svendsen (2008) (no laboratory methods mentioned), 9Scheck et al. (1980) (Merckotest 14055 Gluc. DH, UV), 10Neumann (2014) (photometric), 11Trachsel, Brehm, & Tschudi (2005) (UV/VIS radiation-spectral photometer PU 8605, Phillipps), 12French and Patrick (1995) (Donkey Sanctuary's laboratory, no laboratory methods mentioned), 13Laboklin (2014) photometric), 14Weigert et al. (1980) (Beckman UV-photometer Mod 25; Merckotest 14302).
- a 25%–75%;
- b 5%–95%;
- c 0%–100%;
- d Median;
- e Arithmetic mean;
- f Reference values calculated from mean ± twofold SD.
In most cases, no exact measuring methods for these values are named and they partially dissent. However, we tried to give the best possible overview of all data published. Therefore, the aim of the present study was to verify the existing data from donkeys and mules and to determine orientation values for parameters that have not yet been evaluated.
2 MATERIALS AND METHODS
2.1 Subjects
A total of 44 donkeys and 31 mules were available at the beginning. The numbers were reduced during the study due to sale, death or loss of willingness to co-operate. Finally, the study provided data from 35 donkeys and 30 mules. The donkeys were either owned by private persons (30 donkeys with 16 different owners) or lived in an animal park (13 donkeys). All donkeys were housed indoors and in pastures in varying proportions.
All mules belonged to the Pack Animal Center 230 of the German Armed Forces. They were kept in large paddocks with permanent shelters most of the year with hay and concentrated feed depending on their work.
Twenty-eight of the 44 donkeys were mares, 8 were geldings and 8 were stallions, with one of the stallions being neutered during the study. The mean age in the donkey group was 13.9 years (median: 11 years; range: 3–30 years). Determination was performed by examination of the teeth in cases of missing or disputed information about the age. Accordingly, the mean age is merely a good estimation.
Nineteen of the 31 mules were mares and 12 were geldings. The mean age in this group was 14.0 years (median: 10 years; range: 5–33 years).
Most of the donkeys were common domestic donkeys with only one large breed donkey in the cohort. The mule group comprised heavyweight mules exclusively.
Most of the donkeys (81.8%) and mules (96.8%) had been dewormed regularly. In addition, all mules, except one, had been regularly vaccinated against influenza, equine herpesvirus and tetanus. Regular vaccination in the donkeys was lacking in 95.5% of the cohort.
2.2 Procedure
Samples were taken in a three-month interval from February to November due to known seasonal changes in PPID-specific blood parameters. Each animal was subjected to a clinical examination before blood sampling. In addition to the general part of the clinical examination, several symptoms were known to occur with PPID: abnormal fat depots, pendulous abdomen, hypertrichosis, hyperhidrosis, polyuria, polydipsia, lordosis and muscular atrophy. Moreover, the animal's posture and movement were examined to see whether indications of chronic laminitis were present. A scoring system was used for the PPID-specific examination (Table 2).
| 0 | 1 | 2 | 3 | |
|---|---|---|---|---|
| Behaviour and posture | Unremarkable (attentiveness) | Slightly depressive | Lethargic | Apathetic |
| Abnormal fat depots/allocation | no | One body region (affected) (e.g., mane-comb/crest or supraorbital) | Two body regions (affected) | Three or more body regions (affected) |
| Hanging belly | No | Mild | Intermittently | Severe |
| Hypertrichosis | No | (Too) long coat (may be limited to single regions) | (Too) long coat all over (may be wavy and curly) | (Too) long coat and curls all over |
| Hyperhidrosis | No | Sporadic sweating limited to one region, or excessive sweating after physical strain | Sporadic to frequent sweating limited to two regions | Generalised severe hyperhidrosis |
| PU | No | Yes | – | – |
| PD | No | Yes | – | – |
| Swayback | No | Mild | Intermittently | Severe |
| Muscular atrophy | No | One body region | Two body regions | Generalised |
| Previous bouts of laminitis/founder | No | One | Two | Three or more |
| Hoof shape | Normal | Weak/mild founder rings | Founder rings; slightly dished (concave) dorsal hoof wall | Prominent founder rings; distinctly concave; protrusion of sole |
| Digital pulse(s) | Negative | Positive | – | – |
| Palpable drop of coffin bone at coronary band | No | Yes | – | – |
| Horn quality | Good | Poor | – | – |
| Expansion of white line (white line) | No | Slight expansion | Intermittent expansion | Distinct expansion, and hollow horny walls (chronic founder) |
| Hoof testing | Negative | Slightly sensitive to knocking touch | Intermittently sensitive to knocking touch or slightly pressure-sensitive | Highly sensitive to knocking touch and/or pressure |
| Classic laminitis shortened stride/changed foot lifting | No | Yes | – | – |
Blood samples were processed to perform a differential blood count and to determine concentrations of plasma ACTH, plasma glucose, serum insulin, triglycerides, gamma-glutamyltransferase (ɣ-GT) enzyme activity and serum fructosamines. Food restriction was limited to concentrated feed because overnight fasting before blood collection was not practicable for all animals. Consequently, all animals had access to straw, hay and grass but not to concentrated feed before blood collection.
Blood samples for the plasma ACTH values were collected with ethylenediaminetetraacetic acid (EDTA) and stored for no longer than 3 hr before being centrifuged (15 min at 3,000 Cl/min). Subsequently, the cooled plasma, the EDTA plasma stabilised sodium fluoride, the EDTA blood and the serum samples were shipped to another laboratory (Laboklin, Bad Kissingen, Germany) and examined not later than the day after collection. Different analytical methods and equipment were used in the laboratory: for ACTH: chemiluminescence immunoassay, Immulite 2000 XPi (Siemens); for insulin: chemiluminescence immunoassay, ADVIA Centaur XPT (Siemens); and for gamma-glutamytransferase, fructosamines, glucose and triglycerides: photometry, cobas c701 (Roche).
2.3 Statistics
Statistical analyses and graphics were performed with SPSS (version 22.0, SPSS Inc.) or Excel (version 2010, Microsoft) respectively. The non-parametric Kolmogorov–Smirnov test was used to determine whether sample data have been drawn from a normally distributed population. If the data passed the test for normality (p ≥ .05) and the Levene test for homogeneity of variance, two independent samples were compared with Student's t test. If the variances were unequal, the Welch test was applied, whereas the non-parametric Mann–Whitney U test was used in the case of abnormally distributed data. The Friedman test was used for more than two independent samples that were abnormally distributed. Correlations between two parameters were determined by calculating the Spearman's rank correlation coefficient (r). Results were rated as follows: r < .2 very low correlation, r = .2–.5 low, r > .5 and <.7 moderate, r = .7–.9 high and r > .9 very high.
Two-tailed tests were generally used to test for significant differences. Only values derived from clinically healthy animals were used for the determination of the reference ranges. The highest and lowest values were defined as the upper and lower limit of the range respectively. Only extreme outliers were excluded.
3 RESULTS
Only values from obviously clinically healthy animals were used for the determination of the orientation values. An animal that was excluded once could not return in the next examinations. Mean, median, standard deviation (SD), and the highest and lowest value of the data were used for the orientation values, which are listed in Table 3. Additionally, the number of samples used for each orientation value is also listed.
| Season | Donkey | Mule | ||
|---|---|---|---|---|
| Plasma ACTH (pg/ml) | August | Median | 49.55 | 31.25 |
| Mean | 62.93 | 34.38 | ||
| SD | 37.40 | 16.21 | ||
| Minimum | 19.50 | 9.82 | ||
| Maximum | 143 | 68.70 | ||
| Number of samples | 20 | 20 | ||
| Plasma ACTH (pg/ml) | February, May and November | Median | 15.10 | 8.99 |
| Mean | 20.06 | 10.12 | ||
| SD | 14.06 | 6.64 | ||
| Minimum | 5 | 2.50 | ||
| Maximum | 72.60 | 37.10 | ||
| Number of samples | 74 | 66 | ||
| Serum Insulin (µU/ml) | February, May, August and November | Median | 2.85 | 3.15 |
| Mean | 4.16 | 3.55 | ||
| SD | 3.46 | 1.72 | ||
| Minimum | 0.70 | 1 | ||
| Maximum | 14.40 | 8.50 | ||
| Number of samples | 94 | 85 | ||
| Serum Triglycerides (mmol/L) | February, May, August and November | Median | 0.80 | 0.53 |
| Mean | 0.86 | 0.54 | ||
| SD | 0.50 | 0.13 | ||
| Minimum | 0.20 | 0.30 | ||
| Maximum | 2.20 | 0.90 | ||
| Number of samples | 96 | 88 | ||
| Plasma Glucose (mmol/L) | February, May, August and November | Median | 4.40 | 4.40 |
| Mean | 4.44 | 4.56 | ||
| SD | 0.68 | 0.44 | ||
| Minimum | 3 | 3.90 | ||
| Maximum | 6.30 | 6.30 | ||
| Number of samples | 95 | 88 | ||
| Serum Fructosamines (µmol/L) | February, May, August and November | Median | 294.60 | 332.45 |
| Mean | 292.45 | 331.85 | ||
| SD | 32.47 | 26.71 | ||
| Minimum | 208.30 | 270.10 | ||
| Maximum | 357.60 | 385.60 | ||
| Number of samples | 95 | 88 | ||
| Serum ɣ-GT (IU/L) | February, May, August and November (donkeys)/ February, May and November (mules) | Median | 16.70 | 7.85 |
| Mean | 18.75 | 10.33 | ||
| SD | 7.78 | 5.54 | ||
| Minimum | 7.20 | 4.90 | ||
| Maximum | 43.40 | 30 | ||
| Number of samples | 91 | 65 | ||
| Serum ɣ-GT (IU/L) | August (mules only) | Median | 27.90 | |
| Mean | 35.39 | |||
| SD | 25.97 | |||
| Minimum | 9.10 | |||
| Maximum | 117.70 | |||
| Number of samples | 22 |
3.1 Adrenocorticotropic hormone (ACTH)
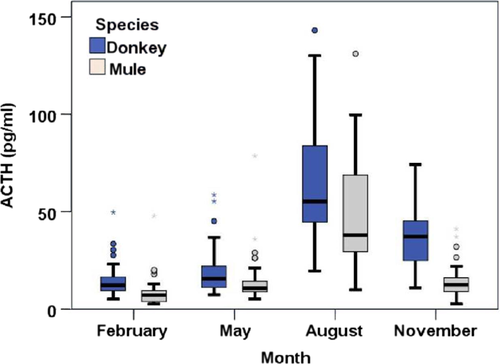
Seasonal changes were found in ACTH plasma concentrations (EDTA plasma) from both donkeys and mules (p < .001, respectively; Figure 1). Values of the clinically healthy animals were the lowest in February. The values were slightly higher in May, followed by massively increased concentrations in August. The ACTH then showed lower values again in November. However, these concentrations were comparable to the low level in February only in mules. By contrast, the donkey group still showed several higher values (Table 4). The ACTH concentrations were generally higher in donkeys than mules (mean in pg/ml [donkey/mule]: February 14.3/8.9, p < .001; May 19.4/15.2, p = .006; August 81.9/61.2, p = .017; November 60.0/14.4, p < .001). The corresponding orientation values are shown in Table 4.
| Donkey | Mule | |
|---|---|---|
| ACTH (pg/ml) |
Aug–Oct 19.5–143 Nov–July 5.0–55.4 |
Aug–Oct 9.8–68.7 Nov–July < 5–37.1 |
| Insulin (µU/ml) | 0.7–14.4 | 1.0–8.5 |
| Triglycerides (mmol/L) | 0.2–2.2 | 0.3–0.9 |
| Glucose (mmol/L) | 3.0–6.3 | 3.9–6.3 |
| Fructosamines (µmol/L) | 208.3–357.6 | 270.1–385.6 |
| ɣ-GT (IU/L) | 7.2–43.4 | 4.9–26.4–67.7 (summer) |
The ACTH values from February and August were used to examine whether there is a relationship between ACTH concentrations and the age of the animals. There was no significant correlation between ACTH level and age in donkeys (r = .247 and r = .239; p = .105 and p = .154 respectively). Although significant results were found in mules, the correlation is low for both February (r = .383; p = .034) and August (r = .492; p = .006). Nevertheless, it is noteworthy that the upper limit of the orientation values did not derive from animals older than 11 years.
3.2 Insulin and triglycerides
Concentrations of insulin and triglycerides (measured in blood serum) did not show seasonal differences in donkeys (p = .066 and p = .789). In mules, however, seasonal changes were found for both insulin (p = .013) and triglycerides (p = .009). Nevertheless, the changes were too small to affect the definition of the reference values (February/May/August/November; insulin [µU/ml] 1.2–8.5/1.0–6.6/1.6–7.5/1.5–7.2, respectively; triglycerides [mmol/L] 0.3–0.9/0.4–0.9/0.3–0.8/0.3–0.7 respectively). The high variation of values in donkeys resulted in orientation values of 0.7–14.4 µU/ml for insulin and 0.2–2.2 mmol/L for triglycerides, whereas the orientation values for insulin in mules were 1.0–8.5 µU/ml and for triglycerides were 0.3–0.9 mmol/L. Despite the tendency towards higher insulin levels in donkeys compared to mules (Table 4), the difference reached significance only in May (p = .006). By contrast, concentrations of triglycerides were always significantly higher in donkeys than in mules (p < .05 respectively).
3.3 Glucose
Glucose concentrations measured in EDTA plasma stabilised with sodium fluoride showed a high variability in both donkeys and mules but without any seasonal trend. Orientation values were similar for both donkeys (3.0–6.3 mmol/L) and mules (3.9–6.3 mmol/L).
3.4 Fructosamines
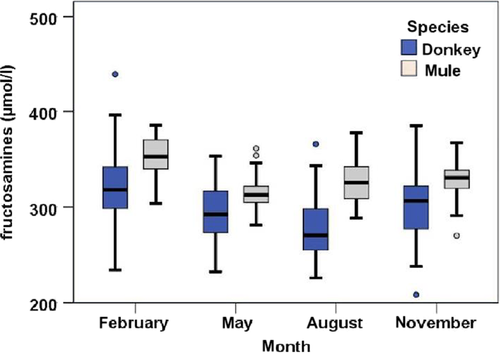
Fructosamine levels (measured in blood serum) for both donkeys and mules were elevated in February compared to the other three measurement times (p < .001). However, the small difference did not affect the orientation values. Notably, the values from the donkeys showed a tendency towards a wider distribution compared to the mules (Figure 2). Furthermore, fructosamine concentrations were generally lower in donkeys than mules (mean, donkeys/mules: February 322.7/351.6, p < .001; May 293.6/313.0, p = .002; August 279.2/318.9, p = .001; November 299.6/329.2 µmol/L, p < .001). Orientation values of 208.3–357.6 µmol/L and 270.1–385.6 µmol/L were defined for donkeys and mules respectively.
3.5 ɣ-GT
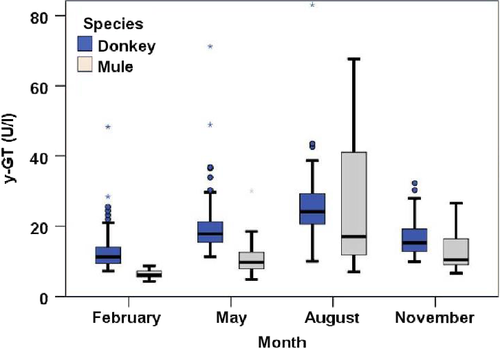
In blood serum, concentrations of ɣ-GT showed clear seasonal changes with the lowest values in February and highest values in August in both donkeys and mules (p < .001, each). However, ɣ-GT levels were higher in donkeys compared to mules (p ≤ .001, Figure 3, Table 5), except for August (p = .284).
| Donkey | Mule | |
|---|---|---|
| February | 15.2 | 6.4 |
| May | 21.5 | 10.6 |
| August | 26.1 | 28.8 |
| November | 19.7 | 1.9 |
The variability of values from mules was considerably increased in August compared to the remaining time points. Whereas the median (17.1 IU/L) was clearly below that of the donkeys (24.1 IU/L), the mean value of the two groups was comparable (Table 5). The single reference range for donkeys was 7.2–43.4 IU/L. However, two different ranges arose out of the data for mules, that is one for February, May and November (4.9–26.4 IU/L) and a second one with a much higher upper limit (67.7 IU/L) for August.
3.6 Mule with suspected PPID
Only one mule showed signs of PPID during all the examinations. The first two clinical examinations (February, May) in a female mule (33 years old; Figure 4a,b) revealed hypertrichosis (Figure 4c), a slight lordosis and muscular atrophy at the croup. Moreover, a slight corneal opacity in the left eye was noticed. Slightly intensified breathing sounds were found only during the first examination. An additional hyperhidrosis was observed in May. Furthermore, ACTH concentrations, unlike the other blood parameters, were increased in February (47.7 pg/ml) and May (78.4 pg/ml). However, the levels of glucose (3.7 mmol/L) and ɣ-GT (4.3 IU/L) were slightly below the respective reference range. The mule died prior to the third blood collection. She had to be euthanised after an accident, which happened when the mule, once again, tried to escape from the field. Attempts to escape have been preceded by repeated self-selected separations from another mule it had accompanied for years. These behavioural abnormalities were reported by its owner.

4 DISCUSSION
Samples of at least 120 individuals should generally be used to determine meaningful reference values (Horowitz, 2008). However, the total number of donkeys and especially of mules in Germany is quite low (Starkey & Starkey, 2004). The 40 mules of the training centre for pack animals (Bad Reichenhall) represent the biggest German population (Bartmann & Henseler, 2014). Therefore, it was impossible to follow the recommendation of a minimum sample number of 120.
However, the minimum and maximum value is indicated in the present study as recommended for orientation values based on less than 80 samples (Schwendenwein, 2014).
4.1 Adrenocorticotropic hormone (ACTH)
The ACTH represents the primary biomarker for PPID (Lee, Zylstra, & Haritou, 2010). The ACTH concentrations in horses may be used for diagnosis regardless of the time of the year, given that the appropriate reference range is considered. In comparison with the rest of the year, ACTH levels are increased between August and October (Copas & Durham, 2012; Donaldson et al., 2005; Lee et al., 2010). It is also known that ACTH concentrations for donkeys show distinct seasonal changes (Du Toit et al., 2011), which was confirmed by the present results. Additionally, the present study revealed seasonally changing ACTH values in mules. The fact that their ACTH values in November were again as low as in February and May resembles the findings in horses. Unlike horses and mules, the donkeys in the present study showed ACTH values in November that were already decreased compared to the high concentrations in August but still higher than those of February and May. There were no major differences in housing conditions between donkeys and mules compared to the other seasons. Thus, housing conditions were excluded as the reason for this phenomenon. Accordingly, the question arises whether the ACTH decrease after the peak in summer is generally decelerated in donkeys compared to horses. However, an earlier study in horses demonstrated relatively high ACTH concentrations until December, which were only slightly below the values from September (Lee et al., 2010). The authors speculated about potential causes, such as seasonal differences in food quality (for details, see Hoffman et al., 2001; Watts, 2005), seasonal changes in lipid metabolism (Robie, Janson, Smith, & O'Connor, 1975) and the decreasing photoperiod in general (see Beech, Boston, McFarane, & Lindborg, 2009). Indeed, these factors might explain still elevated ACTH levels during autumn. However, since the mules in the present study showed ACTH values in November that were as low as the values from February and May, species-dependent differences seem to exist between mules, donkeys and horses. The assumption is confirmed by comparing the orientation values for donkeys and mules from the present study with the established reference ranges for horses (Copas & Durham, 2012; also see Table 1). The results from horses and mules are comparable for winter, spring and summer, but ACTH values in healthy mules during autumn may be considerably higher than in horses. The ACTH concentrations in donkeys generally tend to be higher compared to mules and horses. The orientation values (see Table 4) indicate that clinically healthy donkeys may exhibit ACTH values in autumn that are about twice as high as those in mules at the same time. The differences were less striking for the rest of the year. Surprisingly, earlier published reference ranges for clinically unremarkable donkeys (July–October, 28.9–36.9 pg/ml; Nov–June, 16.5–19.5 pg/ml; Du Toit et al., 2011) are based on considerably higher ACTH values compared to the present study, although the animals' ages are comparable between the two studies. It is important to note, however, that the retrospective study by Du Toit et al. (2011) was based on results from routine examinations and that the lack of adiposity and laminitis was sufficient to exclude PPID and to include the animals' data. Another donkey study also revealed higher ACTH values (May/June, 37.8–104.8 pg/ml; Dugat et al., 2010) compared to the present study. However, large breed donkeys (n = 44, and one miniature donkey) were used almost exclusively, whereas the group in the present study comprised only common domestic donkeys and one large breed donkey. Furthermore, Dugat et al. (2010) merely stated the body condition score (ideal or moderate) without initial clinical examination. Neither of the studies revealed a correlation between the age of the donkeys and their ACTH level, which is why the data of all suspected clinically healthy animals, regardless of age, were used to determine the orientation values respectively.
4.2 Glucose, insulin and fructosamines
In addition to the specific symptoms of PPID, there are some comorbidities that must also be considered. Many horses, for instance, suffering from PPID also show changes in glucose homeostasis (Burns & Toribo, 2015; Keen, McLaren, Chandler, & McGorum, 2004) with increased values of blood glucose, insulin and fructosamines. However, these values could also be increased in animals with equine metabolic syndrome. A differentiation between PPID and equine metabolic syndrome is only possible by measuring the ACTH or dynamic testing, such as thyrotropin-releasing hormone stimulation tests (Schwarz & Anen, 2014).
Although insulin represents a sensitive parameter for the diagnosis of PPID (Gracia & Beech, 1986; van der Kolk, Wensing, Kalsbeek, & Breukink, 1995), it is not a specific one (Reeves, Lees, & McGowan, 2001). Not all horses with PPID show insulin resistance or dysregulation. Hyperinsulinaemia, for example, may occur during fasting and after food intake. Insulin values in horses are considered more reliable for diagnosing a dysregulation if blood samples are taken from a fed animal (Frank, 2010). However, differences in food composition complicate the determination of reference values further. In the present study, animals had access to straw, hay and grass but not to concentrated feed before blood collection. Nevertheless, the donkeys, unlike the mules, were not provided by one but eighteen different animal owners with differences in feeding. The corresponding diversity of housing conditions and food composition might have affected glucose levels and, thus, might be one reason for the broad distribution of insulin and triglyceride concentrations in the donkeys. Blood glucose concentrations and, thereby, insulin and triglyceride values may also be affected by other factors, such as stress, pregnancy, adiposity-derived insulin resistance and drugs, such as corticosteroids and α2 agonists (Taylor & Hillyer, 2001). That adiposity and medical treatment previously falsified the results can be excluded for the present study. Attempts were made to minimise stress during blood collection. Additionally, each time an animal was obviously stressed during the blood collection, it was noted on the examination sheet. The values of these animals did not influence the diagnostic orientation values. The small sample size might have been another reason for a broad distribution of values and the lack of significant seasonal changes in insulin and triglycerides.
The orientation values for glucose in the present study are similar for both animal groups (Table 4), which is in good agreement with earlier results published by Scheck, Weigert, Lemmer, and Noreisch (1980). They did not find any differences in glucose levels between Haflinger horses and mules. Several glucose values in donkeys from the literature are slightly below the orientation values given here (Table 1).
The reference range for insulin in horses, regardless of being in a fed or fasting state, is considerably higher compared to donkeys and mules. If compared between mules and donkeys, the present study revealed lower insulin levels in mules. Reference ranges for insulin in donkeys are quite different in the literature. In one study, values did not exceed 6.6 µU/ml, although the donkeys had free access to grass and small amounts of grain (Dugat et al., 2010). By contrast, physiological insulin concentrations of up to 18.3 µU/ml are mentioned in a medical handbook about donkeys (Schwarz & Anen, 2014). In view of the present results (0.7–14.4 µU/ml), it appears to be advisable to follow the higher values.
The fructosamine concentrations measured, unlike blood glucose which represents the instantaneous situation, allow conclusions to be made regarding the blood glucose level of the preceding 2–3 weeks (Mattheeuws et al., 1966; Schleicher et al., 1993; Staudacher, 1990). Furthermore, direct effects on the fructosamine level by factors such as stress for the animal before and during blood collection can be ruled out (Staudacher, 1990). Increased fructosamine concentrations have been described for mares during late gestation and lactation (Filipovic, Stojevic, & Pravanovic, 2010) as well as for PPID (Keen et al., 2004) and (equine) laminitis (Knowles, Withers, & Mair, 2012). Values up to 360 µmol/L are considered to be physiological (Laboklin, 2014). Reference ranges for donkeys or mules have not been found in the literature. The reference range for donkeys in the present study is comparable to that for horses, whereas the range in mules is shifted slightly upwards. Since no mule showed any clinical sign of laminitis, was pregnant or lactating, the higher fructosamine values compared to donkeys and horses are likely to be specific for mules.
4.3 Triglycerides
Triglyceride concentrations in horses with adequate feeding should be below 1 mmol/L (Taylor & Hillyer, 2001). Correspondingly, the present study revealed orientation values of 0.3–0.9 mmol/L for mules. The triglyceride values measured in donkeys, however, were more than twice as high as in the mules, which is comparable to the values in ponies (Pongratz, Junge, Riond, & Schwarzwald, 2016). As described above, it cannot be ruled out that different feeding of the donkeys affected the determined reference range of 0.2–2.2 mmol/L. By contrast, other factors that are known to affect triglyceride levels, such as early lactation (Taylor & Hillyer, 2001; Watson, Mury, & Love, 1992) and treatment with glucocorticoids (Bagdade & Biermann, 1970), can be excluded. Regarding gestation, which also belongs to these factors, early gestation cannot be ruled out. However, with a view to the even higher values from the literature (see also Table 1; Dugat et al., 2010; Svendsen, 2008), the present results for triglyceride levels in donkeys are likely to represent physiological values.
4.4 ɣ-GT
The present results of ɣ-GT concentrations from mules are very similar to published reference values in horses (up to 25 IU/L; Neumann, 2014). The mules showed considerably higher values only in August. The second reference range was indispensable, since 50% of values from August were above the first range. This might have been due to the fact that the mules were kept within a grazing area at this time, which was likely to be linked to changes in food quality. Earlier published values (up to 20 U/L; Weigert, Scheck, Lemmer, & Noreisch, 1980) are slightly lower compared to the present findings from outside the grazing period. The reference range for donkeys has been adjusted upwards in the literature in recent years. Only earlier published ɣ-GT values (8–49 IU/L; Svendsen, 1997) are comparable to the present results in donkeys. The ɣ-GT levels mentioned in the newer literature are even higher (13–79 IU/L; Svendsen, 2008) than the present peak values from August in mules.
4.5 Mule with PPID
The diagnosis of PPID was based on clinical symptoms and blood parameters. Unfortunately, post-mortem examination to confirm diagnosis was not possible. Although the diagnosis was clear, the behavioural abnormalities, which are described above, remain intriguing. Indeed, PPID-related behavioural changes in horses have been described, but the animals affected were lethargic rather than active (Frank, 2015). The data of the mule suffering from PPID were not used for the determination of the orientation values.
5 CONCLUSION/PERSPECTIVE
The reliable diagnosis of PPID in donkeys is more difficult than in horses. Neither clinical examination for a tentative diagnosis nor blood values provide inevitably unambiguous results. Thanks to the present data, reference ranges from the literature may be complemented and adjusted. Orientation value for fructosamines was even lacking. Future studies might answer the question whether the autumnal decrease in ACTH is decelerated in donkeys compared to horses and mules. Regarding the diagnosis of PPID in mules, the present study not only describes a case example, but also provides the first orientation values for ACTH, insulin, triglycerides and fructosamines.
ACKNOWLEDGEMENTS
We thank all the people who were involved in conducting the present study. We are also grateful to Boehringer Ingelheim for financial support, the laboratory Laboklin (Bad Kissingen, Germany), the Pack Animal Center 230 (Bad Reichenhall, Germany), the animal park Sababurg and all animal owners and keepers.
ANIMAL WELFARE STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. No ethical approval was required as all data were acquired from samples taken for routine diagnostic procedures. According to the German welfare law, additional ethical approval is not required in this case. The Animal Welfare Officer of our University confirmed this.