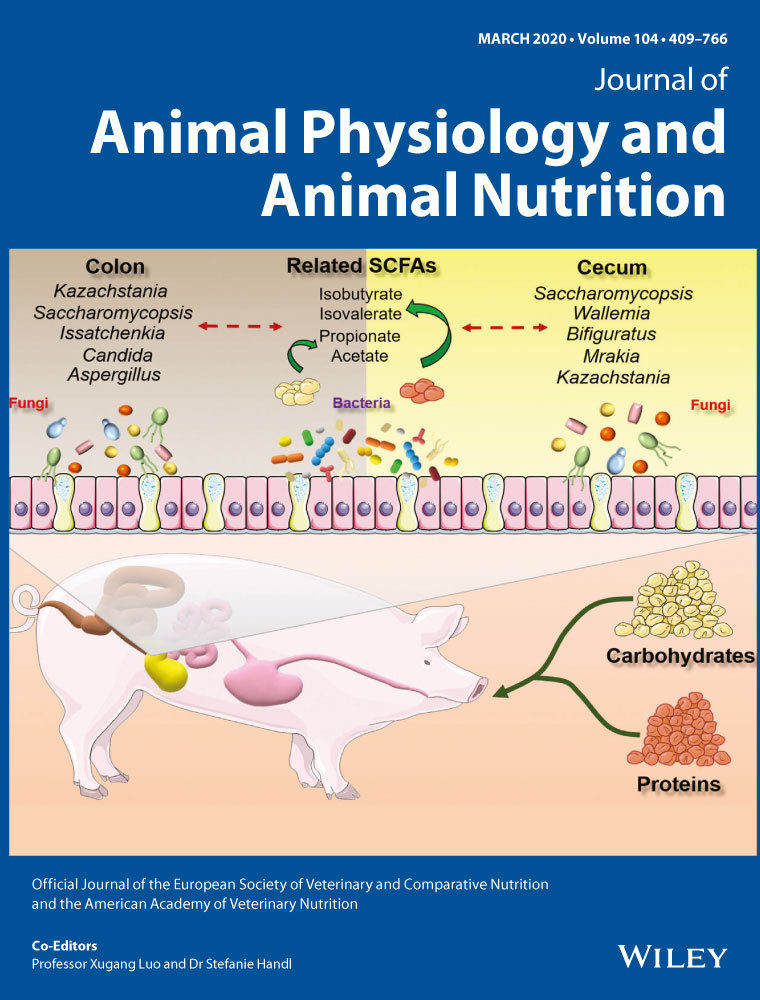
Dietary supplementation of plant essential oil improves growth performance, intestinal morphology and health in weaned pigs
Abstract
The present study was conducted to explore the effect of plant essential oil (PEO) on growth performance, intestinal morphology and health in weaned pigs. Twenty-four weaned pigs were assigned into four groups fed with a basal diet (CON) or basal diet containing PEO at a concentration of 50 (PEO50), 100 (PEO100) or 200 ppm (PEO200). After 21 days, pigs were slaughtered and blood and tissue samples were collected. Result showed that PEO200 group significantly increased the average daily gain (ADG) compared with CON group (p < .05). Moreover, PEO supplementation significantly improved the digestibility of DM (p < .05). However, it significantly decreased the serum triglyceride and cholesterol concentration (p < .05). Interestingly, PEO supplementation significantly increased the activity of sucrase in the duodenal and the activity of lactase in the jejunal mucosa (p < .05). Moreover, PEO supplementation improved the growth of intestinal mucosa. As compared to the CON group, the jejunum and ileum villus height were significantly elevated in the PEO200 group (p < .05). Importantly, the expression levels of critical genes associated with nutrient transportation (i.e., GLUT2 and SGLT1) and barrier function (occludin) were significantly elevated in the PEO200 group (p < .05). Moreover, the PEO100 and PEO200 group had higher propionic acid concentration and higher total bacterial gene copies in colon digesta than the CON group (p < .05) respectively. These results not only suggest that PEO has a positive role in the regulation of growth and intestinal health in weaned pigs, but also offer a potential candidate substituting the conventionally used antibiotics in the livestock industry.
1 INTRODUCTION
In the last decades, antibiotics have been widely utilized in the swine and poultry industries to improve growth rate and efficiency of feed utilization, as well as reduce morbidity and mortality (Cromwell, 2002). However, their continuous or over-dosage utilization not only leads to the risk of drug resistance, but also increases drug residues in livestock products (Cromwell, 2002; Hancock & Sahl, 2006). Therefore, the development of novel alternatives for conventionally used antibiotics has attracted considerable research interest (Hancock & Sahl, 2006; Smith et al., 2011; Walsh, Sweeney, O'Shea, Doyle, & O'Doherty, 2013).
Phytogenic substances are mixtures of lipophilic, volatile, largely terpenoid compounds extracted from plant materials such as buds, leaves, bark, flowers, seed, twigs, roots, woods, herbs and fruits (Brenes & Roura, 2010). According to the European Council (European Commission, 2003), phytogenic substances are categorized as sensory additives and are able to improve feed odour or palatability. In recent years, there has been increased interest in developing phytogenetic feed additives as a potential alternative for antibiotics. Plant essential oils (PEOs) are volatile lipophilic materials extracted from plants, and there are more than one hundred kinds of essential oils (Wei & Shibamoto, 2007). Recently, PEOs have been implicated as a novel source of bioactive compounds exhibiting growth promotional and antimicrobial properties. And a number of studies have showed that certain PEO may improve animal performance and health status by many approaches such as stimulation of digestive secretions, antimicrobial, coccidiostatic, anthelmintic, and anti-inflammatory and antioxidant properties (Berger, 2007; Li et al., 2012; Maenner, Vahjen, & Simon, 2011).
The PEOs are commonly composed of cinnamaldehyde, thymol, citral, carvacrol and other volatile components (Berger, 2007). Previous studies indicated that cinnamaldehyde and thymol possessed antibacterial in vitro (Burt, 2004; Di et al., 2007; Lee & Ahn, 1998). For instance, the cinnamaldehyde and thymol directly inhibited the growth of Salmonella Heidelberg in crops and reduced the risk infections in broilers after ingestion of such diet (Alali, Hofacre, Mathis, & Faltys, 2013). Moreover, dietary supplementation of cinnamaldehyde and thymol improved broiler weight gain and ileal nitrogen digestibility in both ground form- and whole form-based diets (Amerah, Peron, Zaefarian, & Ravindran, 2011). In contrast, Kirkpinar et al. failed to observe a beneficial effect of PEO supplementation on broiler growth (Kırkpınar, Ünlü, & Özdemir, 2011), which is probably due to the many reasons such as different experimental conditions, composition of essential oils used and the dose employed (Berger, 2007; Li et al., 2012). Thus, both the roles and application strategies, as well as the regulatory mechanisms of PEO, should be further explored.
In pig production, the efficient and profitable operation of commercial swine units is often limited by high mortality, morbidity and poor performance in the nursery phase of production. Previous study indicated that the addition of antimicrobial products to nursery feeds is especially effective with typical improvements in growth rates and feed conversion efficiencies of up to 16% and 6% respectively (Cromwell, 2001). Because of the concerns on drug resistance and residues, novel alternatives including the PEO have attracted considerable research interests in the last decade. However, there are only few studies investigating the effects of PEO supplementation on pigs. In this study, our aim was to explore the effect of PEO supplementation on growth performance, nutrient digestibility and intestinal mucosa development in weaned pigs. This study will facilitate the application of PEO as an alternative for conventionally used antimicrobial drugs.
2 MATERIALS AND METHODS
2.1 Animals and experimental design
A total of 24 commercial crossing DLY (Duroc × Landrace × Large White) male pigs weaned at 28 days. And then according to the weight (9.19 kg [SEM 0.34]), all pigs were randomly assigned to four groups: (a) CON (control; a basal diet), (b) PEO50 (basal diet containing 50 ppm PEO), (c) PEO100 (basal diet containing 100 ppm PEO) and (d) PEO200 (basal diet containing 200 ppm PEO). The level of PEO supplementation was according to previous studies (Cho et al., 2006; Li et al., 2012; Manzanilla et al., 2004). PEO was provided by Cheng Du Hua Luo Bio-Tech Col., the active ingredient of PEO was 13.5% thymol and 4.5% cinnamaldehyde, and carrier was dextrin.
2.2 Diets and feeding management
Diets were corn–soybean-based diets and formulated according to National Research Council 2012 requirements. Ingredients and nutrient composition of experimental diets are shown in Table 1. Diets were fed in mash form throughout the experiment. The experiment was carried out at the Research Base of the Institute of Animal Nutrition of Sichuan Agricultural University. All pigs were housed individually in an environmentally controlled nursery room and had free access to feed and water throughout the 3-week feeding trial. The temperature of the house was controlled between 25 and 32°C, and the humidity was controlled between 50% and 60%. Feed intake was recorded daily. Pigs were weighed at 08:00 on an empty stomach on days 1 and 21. Faeces were collected from day 18 to 21, and hydrochloric acid insoluble ash (AIA) as an indicator. The apparent digestibility of DM, CP, EE, GE and ash in feed was measured with reference to Mccarthy (Mccarthy, Aherne, & Okai, 1974). Body weight was weighed, feed intake was recorded, and average daily gain (ADG) and the ratio of feed intake:gain (F:G) were calculated.
| Composition | Nutrient level | ||
|---|---|---|---|
| Ingredients | Proportion (%) | Items | Content (%) |
| Maize | 30.00 | CP | 19.80 |
| Extruded maize | 26.00 | Ca | 0.91 |
| Soya bean meal, dehulled | 10.50 | Total P | 0.55 |
| Puffed soya bean | 4.53 | P, available | 0.37 |
| Fishmeal | 4.90 | Lys | 1.41 |
| Soya protein concentrate | 8.00 | Met | 0.47 |
| Whey powder | 8.00 | Thr | 0.79 |
| Glucose | 3.00 | Trp | 0.22 |
| Soya oil | 2.50 | DE, MJ/kg | 14.02 |
| Calcium carbonate | 1.20 | ||
| Calcium phosphate | 0.20 | ||
| L-Lys HCl | 0.38 | ||
| NaCl | 0.25 | ||
| Choline chloride | 0.10 | ||
| DL-Met | 0.15 | ||
| Trp | 0.01 | ||
| L-Thr | 0.03 | ||
| Vitaminsb | 0.05 | ||
| Mineralsc | 0.20 | ||
- a Dietary nutrient level value is calculated.
- b The vitamin and mineral premix (maize powder as diluent) provided the following amounts per kg complete diet: retinol, 8.4 mg; cholecalciferol, 0.008 mg; vitamin E, 20 mg; menadione, 1 mg; vitamin B12, 0.03 mg; riboflavin, 5 mg; niacin, 20 mg; pantothenic acid, 15 mg; folic acid, 0.5 mg; thiamin, 1.5 mg; pyridoxine, 2 mg; biotin, 0.1 mg.
- c The mineral premix provided the following amounts per kg complete diet: Fe, 100 mg (FeSO4·7H2O); Cu, 6 mg (CuSO4·5H2O); Zn, 100 mg (ZnSO4·7H2O); Mn, 4 mg (MnSO4·H2O); Se, 0.3 mg (Na2SeO3·5H2O); I, 0.14 mg (KI).
2.3 Sample collection
Blood samples were collected from the portal vein precava into vacuum pick blood vessels (Axygen Biotechnology) on 8:00 in the morning of day 21. Serum was prepared by centrifuging the blood (3,500 g, 4°C, 10 min) and immediately stored at −20°C. All pigs were slaughtered by exsanguination according to protocols approved by the Sichuan Agricultural University Animal Care Advisory Committee. Mucosa of the duodenum, jejunum, ileum and chyme in these small intestines was collected and snap-frozen in liquid N2 and then stored at −80°C for assay. PH of chyme in the jejunum, caecum and colon was measured with a pH meter (PHS-3D, Sanxin). Segments of the duodenum (10 cm from the stomach), the middle section of the jejunum and the ileum (10 cm from the caecum) were collected quickly and then fixed in 4% paraformaldehyde for villus height (VH), crypt depth (CD), villus width (VW), goblet cell (GC)and columnar cell (CC) measurements.
2.4 Intestinal histomorphology
The preserved segments (duodenum, jejunum and ileum) were prepared using standard paraffin-embedding techniques. The samples were sectioned at 5 μm thickness and stained with haematoxylin and eosin. Measurements of fifteen well-orientated and intact villi, crypts, GC and CC were performed for each segment. Cell density was expressed as number of total stained cells per 1,000 µm2. Images of the sections were captured under an Olympus BX51 microscope (Olympus, Tokyo, Japan) equipped with DP70 digital camera. The VH, CD and VW were measured with JD801 morphologic image analysis software, GC and CC were counted and the VH:CD ratio was calculated. All the morphometric analyses were conducted by the same person, who was blinded to the treatments.
2.5 Analysis of serum biochemistry and digestive enzyme
The levels of triglyceride (TG) and total cholesterol (TC) in serum were examined using a Hitachi 7020 Automatic Analyzer. Mucosa of duodenum and jejunum was homogenized (1:10, w:v) in glass homogenizer with ice-cold 0.9% normal saline. Homogenate was collected by centrifuging 3,500 g, 4°C, 15 min. Total protein (TP), the activity of maltase, lactase, sucrase in duodenum mucosa and jejunal mucosa were measured by assay kits A045-3, A082-3, A082-1 and A082-2 from Nanjing Jiancheng Bioengineering Institute. The methods were according to the manufacturer's instructions. A 96-well microtitre plate reader Spectramax M2 (Molecular Devices) was commonly used in enzyme measurement.
2.6 RNA isolation and real-time quantitative PCR
Total RNA was extracted from samples of duodenum, jejunum and ileum samples using TRIzol reagent (TaKaRa). RNA concentration was measured by NanoDrop 2000 (Thermo Fisher Scientific). The integrity of RNA was verified by electrophoretic analysis. Reverse transcription was run with the PrimeScript™ RT Reagent Kit (TaKaRa) with 2 mg RNA sample according to the manufacturer's instructions. The final reaction volume of 20 μl cDNA was then adjusted to 250 μl using nuclease-free water and stored at −20°C. The cDNA was used as the template for PCR. Real-time quantitative PCR was performed on cDNA using the ABI PRISM 7500 Fast Sequence Detection System for ninety-six-well plates (Applied Biosystems). The primers were synthesized commercially by Invitrogen, and the primers of β-actin, occludin (OCLN), claudin-1 (CLDN1), glucose transporter 2 (GLUT2), sodium–glucose cotransporter 1 (SGLT1) and Zonula occludens 1 (ZO-1) are shown in Table 2. The gene β-actin was used as house-keeping gene. The melting peaks of the amplification products were determined by melting curve which indicated only one expected amplification products had been generated. Each primer pair used yielded a single peak in the melting curve and a single band with the expected size in agarose gel. The relative gene expressions compared with the house-keeping gene β-actin were calculated by 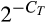 (Livak & Schmittgen, 2001).
(Livak & Schmittgen, 2001).
| Genes/micro-organism | Primer sequences (5′-3′) | Product size (bp) |
|---|---|---|
| β-actin | F:TCTGGCACCACACCTTCT | 114 |
| R:TGATCTGGGTCATCTTCTCAC | ||
| CLDN1 | F:GCCACAGCAAGGTATGGTAAC | 140 |
| R:AGTAGGGCACCTCCCAGAAG | ||
| OCLN | F:CTACTCGTCCAACGGGAAAG | 158 |
| R:ACGCCTCCAAGTTACCACTG | ||
| ZO-1 | F:TGGCATTATTCGCCTTCATAC | 171 |
| R:AGCCTCATTCGCATTGTTT | ||
| SGLT1 | F:TGTATTTGAGGCCAGTGTCA | 198 |
| R:GGGCGACCACAACTCTTAAA | ||
| GLUT2 | F:TGGAATCAGCCAACCTGTTT | 165 |
| R:ACAAGTCCCACCGACATGA | ||
| Total bacteria | F: ACTCCTACGGGAGGCAGCAG | 200 |
| R: ATTACCGCGGCTGCTGG | ||
| Lactobacillus | F: GAGGCAGCAGTAGGGAATCTTC | 126 |
| R: CAACAGTTACTCTGACACCCGTTCTTC | ||
| P: AAGAAGGGTTTCGGCTCGTAAAACTCTGTT | ||
| Escherichia coli | F: CATGCCGCGTGTATGAAGAA | 96 |
| R: CGGGTAACGTCAATGAGCAAA | ||
| P: AGGTATTAACTTTACTCCCTTCCTC | ||
| Bifidobacterium | F: CGCGTCCGGTGTGAAAG | 121 |
| R: CTTCCCGATATCTACACATTCCA | ||
| P: ATTCCACCGTTACACCGGGAA | ||
| Bacillus | F: GCAACGAGCGCAACCCTTGA | 92 |
| R: TCATCCCCACCTTCCTCCGGT | ||
| P: CGGTTTGTCACCGGCAGTCACCT |
- Abbreviations: CLDN1, claudin 1; F, forward; GLUT2, glucose transporter 2; OCLN, occludin; P, probe; R, reverse; SGLT1, sodium–glucose cotransporter 1; ZO-1, Zonula occludens 1.
2.7 Intestinal microflora
Microbial genomic DNA was extracted from chyme samples using a QIAamp DNA stool kit (Qiagen) according to the manufacturer's instructions. Concentration and quality of DNA were measured with a NanoDrop 2000 (Thermo Fisher Scientific). The integrity of DNA was verified by denaturing agarose gel electrophoresis. Standard curves were generated according to the method described by O'Shea et al. (2012).
Primers and fluorescent oligonucleotide probes (Table 2) were designed with Primer Premier 5.0 (Premier Bio International) and followed 16S rRNA sequences of maximum species of each genus homology downloaded from GenBank database, European Molecular Biology Laboratory and DNA Data Bank of Japan to obtain specific amplification, and the sequences of all the genera taken from the database were submitted to DNAStar (MegAlign) program (DNASTAR), as described by Wan, Jiang, Xu, Chen, and He (2016). qPCR was performed in a final mastermix volume of 10 μl containing 1.5 μl of template DNA, 0.5 μl of forward (100 pM) and 0.5 μl of reverse primers (100 pM), 5 μl SYBR Green PCR Master Mix (Applied Biosystems) and 2.5 μl nuclease-free water. All amplification programmes included an initial denaturation step at 98°C for 5 min followed by forty cycles of 95°C for 15 s and 65°C for 1 min. Dissociation analyses of the qPCR product were performed to confirm the specificity of the resulting qPCR products. All samples repeated twice. The mean cycle threshold (Ct) values of duplicates of each sample were used for calculations, and results were expressed as log copies number/g of fresh matter.
2.8 Analysis of volatile fatty acids
Volatile fatty acids (VFA) concentrations were determined using a modified gas chromatographic method (Franklin, Mathew, Vickers, & Clift, 2002; Playne, 1985). Briefly, 1.2 ml of supernatant was mixed with 240 μl of 25% metaphosphoric acid (5:1 ratio) and incubated at 4°C for 30 min, and then centrifuged at 12,000 g for 10 min at 4°C. 1.2 ml of supernatant was mixed with 23.3 μl 210 mmol/L crotonic acid, picked up 0.3 ml mixture and mixed with 0.9 ml methyl alcohol (1:3). The supernatant was filtered using a 0.22-μm filter (Millipore), and 1 μl was used to determine the concentrations of VFA with flame ionization detector (FID) in Varian CP 3,800 gas chromatograph, equipped with a CP-FFAP 25-m × 0.32-mm × 0.3-μm capillary column. The temperature programming starts at an initial temperature of 100°C for 1 min, increasing to 190°C at a rate of 20°C/min and holding at 190°C for 3 min to insure complete VFA volatilization. Injection port temperature was 220°C. The split ratio was 50:1, and FID detector temperature was 250°C. Nitrogen was used as a carrier gas with a flow rate of 35 ml/min. Air and hydrogen gas were used for combustion with a flow rate of 450 and 40 ml/min respectively. The results were expressed in μmol/g.
2.9 Statistical analysis
All data were analysed using SPSS 20.0 software (SPSS) by a one-way ANOVA procedure. Differences between means were tested using Duncan's range test. The significance level for all analyses was set at p < .05, with a trend of .05 ≤ p < .10.
3 RESULTS
3.1 Effects of PEO on growth performance and nutrient digestibility
No significant difference was observed in feed intake:weight gain (F:G) between pigs fed PEO and CON diet (Table 3). However, average daily gain (ADG) of pigs receiving 200 ppm PEO diet was greater than those pigs fed CON diet (p < .05). Amongst the PEO groups, pigs fed 200 ppm PEO diet tended to increase the ADG when compared with other two groups (0.05 ≤ p < .10). Moreover, PEO supplementation did not improve the nutrient digestibility of CP, EE, GE and ash amongst the treatments. However, the PEO100 and PEO200 group had higher apparent digestibility of DM than the PEO50 group (p < .05).
| Items | CON | PEO50 | PEO100 | PEO200 | SEM | p |
|---|---|---|---|---|---|---|
| ADG (kg/day) | 0.45b | 0.45ab | 0.46ab | 0.50a | 0.01 | .09 |
| F:G | 1.47 | 1.55 | 1.43 | 1.34 | 0.04 | .36 |
| DM (%) | 81.95a | 80.39b | 81.96a | 82.38a | 0.24 | .01 |
| CP (%) | 83.23 | 82.79 | 83.57 | 85.14 | 0.42 | .29 |
| EE (%) | 83.23 | 82.79 | 83.57 | 85.14 | 0.75 | .29 |
| GE (%) | 81.28 | 80.70 | 81.67 | 82.35 | 0.32 | .36 |
| Ash (%) | 40.91 | 40.85 | 41.87 | 43.71 | 1.16 | .89 |
Note
- Mean values within a row with unlike superscript letters (a, b) were significantly different (p < .05). n = 6.
- Abbreviations: ADG, average daily gain; CP, crude protein; DM, dry matter; EE, ether extract; F:G, feed:gain; GE, gross energy.
3.2 Effects of PEO on serum biochemical parameters
The effects of dietary treatments on the blood biochemical parameters are presented in Figure 1. As compared to the CON group, the concentration of serum triglyceride (TG) in PEO50 and PEO200 group was significantly decreased (p < .05). However, the concentration of serum total cholesterol (TC) was greater in PEO50 group than in the CON group (p < .05). Interestingly, the PEO100 group tended to reduce the concentration of serum TG and TC when compared with CON group (0.05 ≤ p < .10). As compared to the PEO50 group, the concentration of serum TC in PEO200 was significantly decreased (p < .05).

3.3 Effect of PEO on intestinal morphology
As shown in Table 4, no significant differences were observed in villus height (VH), crypt depth (CD), VW (villus width), villus height:crypt depth (VH:CD), goblet cell (GC) and columnar cell (CC) in the duodenum (p > .05). However, pigs receiving 100 and 200 ppm PEO tended to increase the VH in jejunum (0.05 ≤ p < .10). In addition, the ileal VH in the PEO200 group was significantly greater than the CON group (p < .05). As compared to the CON group, pigs fed 100 ppm PEO diet tended to increase the villus height in the ileum (0.05 ≤ p < .10). The ileal VH:CD in the PEO100 and PEO200 group was higher than the CON group (p < .05). Interestingly, the jejunal villus width (VW) in PEO100 group and PEO200 group was significantly greater than the CON group (p < .05); however, ileal VW in PEO200 group was significantly lower than the PEO50 group (p < .05). As compared to the CON group, pigs receiving 200 ppm PEO tended to increase the VW in the duodenum (0.05 ≤ p < .10). Moreover, the jejunal GC in PEO200 group was significantly higher than the CON group (p < .05).
| Items | CON | PEO50 | PEO100 | PEO200 | SEM | p |
|---|---|---|---|---|---|---|
| Duodenum | ||||||
| VH (μm) | 433.29 | 437.87 | 454.68 | 468.17 | 12.30 | .78 |
| CD (μm) | 264.76 | 275.92 | 250.55 | 264.61 | 5.53 | .49 |
| VW (μm) | 119.01 | 122.68 | 116.52 | 118.75 | 2.28 | .86 |
| VH:CD | 1.65 | 1.59 | 1.83 | 1.79 | 0.06 | .48 |
| GC | 16.00 | 29.00 | 15.25 | 17.25 | 1.99 | .15 |
| CC | 126.00 | 129.50 | 114.00 | 122.75 | 0.48 | .85 |
| Jejunum | ||||||
| VH (μm) | 253.31ab | 227.68b | 316.70ab | 326.06a | 13.30 | .13 |
| CD (μm) | 150.85 | 143.75 | 144.67 | 142.44 | 7.53 | .98 |
| VW (μm) | 109.59b | 114.63ab | 121.35a | 130.39a | 2.95 | .08 |
| VH:CD | 1.87 | 1.78 | 1.95 | 1.96 | 0.06 | .68 |
| GC | 4.50b | 3.50b | 5.25b | 15.00a | 0.82 | .01 |
| CC | 119.00 | 118.33 | 131.25 | 120.67 | 0.43 | .33 |
| Ileum | ||||||
| VH (μm) | 239.41b | 255.20ab | 252.35ab | 276.34a | 7.00 | .22 |
| CD (μm) | 144.75 | 141.73 | 123.81 | 129.04 | 4.28 | .25 |
| VW (μm) | 92.09ab | 105.09a | 103.49ab | 87.45b | 2.89 | .07 |
| VH:CD | 1.62b | 1.89ab | 2.03a | 2.15a | 0.07 | .06 |
| GC (μm) | 3.25 | 5.50 | 3.25 | 4.00 | 0.41 | .41 |
| CC (μm) | 91.00 | 112.50 | 94.00 | 92.50 | 0.38 | .55 |
Note
- Mean values within a row with unlike superscript letters (a, b) were significantly different (p < .05). n = 6.
- Abbreviations: CC, columnar cell; CD, crypt depth; GC, goblet cell; VH, villus height; VH:CD, villus height:crypt depth; VW, villus width.
3.4 Effect of PEO on mucosa digestive enzyme activities
The activity of maltase, lactase and sucrase in duodenal and jejunal mucous is shown in Table 5. No significant difference was observed in total protein (TP), maltase and lactase in duodenal mucosa amongst treatments. However, the sucrase activity of the duodenal mucosa in the PEO100 and PEO200 groups was significantly higher than the CON group (p < .05). Moreover, the lactase and sucrase activities in the jejunal mucosa of PEO200 group were significantly higher than PEO50 group (p < .05).
| Items | CON | PEO50 | PEO100 | PEO200 | SEM | p |
|---|---|---|---|---|---|---|
| Duodenum | ||||||
| TP (gprot/L) | 77.09 | 78.38 | 78.30 | 76.29 | 3.45 | 1.00 |
| Maltase (U/gprot) | 74.67 | 77.96 | 95.28 | 77.19 | 9.71 | .91 |
| Lactase (U/gprot) | 25.79 | 25.59 | 23.76 | 25.20 | 1.29 | .96 |
| Sucrase (U/gprot) | 5.73b | 8.44ab | 11.38a | 10.41a | 0.78 | .02 |
| Jejunum | ||||||
| TP (gprot/L) | 83.91 | 85.10 | 83.61 | 84.48 | 4.03 | 1.00 |
| Maltase (U/gprot) | 148.74 | 130.60 | 153.15 | 180.51 | 13.61 | .46 |
| Lactase (U/gprot) | 4.23b | 4.11b | 4.29b | 5.75a | 0.21 | .02 |
| Sucrase (U/gprot) | 37.59ab | 29.01b | 33.17ab | 39.67a | 1.70 | .98 |
Note
- Mean values within a row with unlike superscript letters (a, b) were significantly different (p < .05). n = 6.
- Abbreviation: TP, total protein.
3.5 Effects of PEO on expression of critical genes associated with transportation and barrier functions
The expression levels of claudin 1 (CLDN1), occludin (OCLN), Zonula occludens 1 (ZO-1), sodium–glucose cotransporter 1 (SGLT1) and glucose transporter 2 (GLUT2) of intestinal mucosa were determined (Table 6). We found that the expression levels of OCLN, SGLT1 and GLUT2 in duodenal mucosa were significantly higher in the PEO200 group than in the CON group (p < .05). As compared to the CON group, pigs receiving 200 ppm PEO diet tended to increase the expression level of OCLN in duodenal mucosa (0.05 ≤ p < .10). Interestingly, the expression levels of GLUT2 in jejunal and ileal mucosa were lower in the PEO50 group than in the CON group (p < .05). However, the expression levels of OCLN in the PEO 100 and PEO200 were both significantly elevated in ileal mucosa (p < .05).
| Items | CON | PEO50 | PEO100 | PEO200 | SEM | p |
|---|---|---|---|---|---|---|
| Duodenum | ||||||
| OCLN | 1.00ab | 0.57b | 1.27a | 1.45a | 0.11 | .01 |
| CLDN1 | 1.00 | 1.07 | 0.97 | 1.17 | 0.08 | .81 |
| ZO-1 | 1.00 | 1.22 | 1.20 | 1.32 | 0.08 | .77 |
| GLUT2 | 1.00b | 1.44b | 1.58b | 2.96a | 0.21 | <.01 |
| SGLT1 | 1.00b | 1.23b | 1.46b | 2.04a | 0.21 | .03 |
| Jejunum | ||||||
| OCLN | 1.00 | 0.95 | 1.33 | 1.56 | 0.16 | .50 |
| CLDN1 | 1.00 | 0.95 | 1.01 | 0.96 | 0.08 | 1.00 |
| ZO-1 | 1.00 | 1.14 | 1.10 | 1.13 | 0.07 | .94 |
| GLUT2 | 1.00a | 0.66b | 1.02a | 1.02a | 0.06 | .08 |
| SGLT1 | 1.00 | 0.89 | 1.02 | 1.48 | 0.14 | .45 |
| Ileum | ||||||
| OCLN | 1.00b | 1.81ab | 2.60a | 3.11a | 0.26 | .06 |
| CLDN1 | 1.00 | 0.99 | 0.95 | 1.05 | 0.07 | .99 |
| ZO-1 | 1.00 | 0.93 | 0.93 | 1.00 | 0.05 | .96 |
| GLUT2 | 1.00a | 0.91b | 1.35a | 2.24a | 0.13 | <.01 |
| SGLT1 | 1.00 | 0.89 | 1.16 | 1.26 | 0.08 | .66 |
Note
- Mean values within a row with unlike superscript letters (a, b) were significantly different (p < .05). n = 6.
- Abbreviations: CLDN1, claudin 1; GLUT2, glucose transporter 2; OCLN, occludin; SGLT1, sodium–glucose cotransporter 1; ZO-1, Zonula occludens 1.
3.6 Effects of PEO on pH, bacterial metabolites and microbial community
As shown in Table 7, the pH in caecum and colon was not affected by PEO supplementation. As compared to the CON group, pigs fed 50 ppm PEO diet tended to decrease the propionic acid concentration in caecum (0.05 ≤ p < .10). However, the caecum propionic acid concentration was significantly higher in the PEO100 and PEO200 groups than in the PEO50 group (p < .05). Additionally, the colon propionic acid concentration was significantly higher in the PEO100 group than in the CON group (p < .05). Both the concentrations of acetic acid and butyric acid were not affected by PEO supplementation. However, the PEO50 and PEO100 groups tended to increase the total volatile fatty acids (TVFAs) in caecum digesta (0.05 ≤ p < .10). The PEO200 group also tended to have a higher TVFA concentration than the CON group in colon digesta (0.05 ≤ p < .10). In addition, the Bifidobacterium, Bacillus, Lactobacillus and Escherichia coli in caecum and colon were not significantly affected upon PEO supplementation. However, the PEO100 and PEO200 groups had significantly higher total microbial count than in the CON group (p < .05) in the caecum. Moreover, pigs on 100 ppm PEO diet also elevated the total microbial count in the colon (p < .05). As compared to the CON group, the number of Lactobacillus in the PEO200 group tended to increase in the colon (0.05 ≤ p < .10).
| Items | CON | PEO50 | PEO100 | PEO200 | SEM | p |
|---|---|---|---|---|---|---|
| Caecum | ||||||
| pH | 5.51 | 5.39 | 5.33 | 5.48 | 0.09 | .64 |
| Acetic acid, μmol/g | 42.81 | 46.83 | 50.01 | 49.20 | 2.40 | .78 |
| Propionic acid, μmol/g | 23.60ab | 16.24b | 27.33a | 25.84a | 1.59 | .04 |
| Butyric acid, μmol/g | 10.82 | 9.55 | 11.15 | 10.51 | 0.71 | .88 |
| TVFA, μmol/g | 77.23 | 72.61 | 88.49 | 85.55 | 2.93 | .20 |
| Bifidobacterium | 6.66 | 6.71 | 6.87 | 6.92 | 0.10 | .79 |
| Bacillus | 8.96 | 9.10 | 9.17 | 9.18 | 0.08 | .76 |
| Lactobacillus | 7.82 | 8.05 | 8. 39 | 8.49 | 0.15 | .37 |
| Escherichia coli | 8.27 | 8.86 | 8.57 | 8.49 | 0.13 | .52 |
| Total count | 10.93ab | 10.87b | 11.14a | 11.12a | 0.04 | .04 |
| Colon | ||||||
| pH | 6.16 | 5.96 | 6.06 | 6.01 | 0.06 | .80 |
| Acetic acid, μmol/g | 28.30 | 32.37 | 36.04 | 33.94 | 2.19 | .71 |
| Propionic acid, μmol/g | 11.50b | 13.68ab | 18.50a | 15.65ab | 1.09 | .14 |
| Butyric acid, μmol/g | 7.37 | 8.06 | 9.05 | 8.21 | 0.43 | .63 |
| TVFA, μmol/g | 47.70 | 54.10 | 63.59 | 57.80 | 3.16 | .37 |
| Bifidobacterium | 7.10 | 7.05 | 7.35 | 7.65 | 0.17 | .63 |
| Bacillus | 10.26 | 10.31 | 10.61 | 10.66 | 0.08 | .22 |
| Lactobacillus | 8.11 | 8.16 | 8.26 | 9.02 | 0.19 | .33 |
| Escherichia coli | 7.46 | 8.26 | 7.45 | 7.26 | 0.19 | .30 |
| Total count | 10.80b | 10.82b | 10.87ab | 11.02a | 0.03 | .08 |
Note
- Mean values within a row with unlike superscript letters (a, b) were significantly different (p < .05). n = 6.
- Abbreviation: TVFAs, total volatile fatty acids.
4 DISCUSSION
The present study evaluated the effects of PEO supplementation on growth performance, and intestinal health, as well as its possibility to serve as an alternative for conventionally used antibiotics in weaned pigs. According to the current data, dietary supplementation of PEO at a dosage of 200 ppm significantly improved the growth performance of pigs after weaning. This result was consistent with previous report that dietary supplementation of thymol and cinnamaldehyde has resulted in 10% improvement of ADG and F:G in weaned pigs (Zeng et al., 2015). However, several studies did not find a positive effects on growth performance in pigs (Manzanilla et al., 2004; Namkung et al., 2004). The differences in the responses of growth performance to PEO may result from the compositions of the additives, dosage of essential oils, the type of basal diets and the approaches of administration (Zeng, Zhang, Wang, & Piao, 2016).
Moreover, dietary supplementation of PEO at 200 ppm significantly decreased the concentration of serum triglyceride (TG) and total cholesterol (TC), suggesting that PEO may affect the lipid metabolism. Previous study has indicated that the PEOs were absorbed quickly after ingestion, which significantly increased secretions of saliva and bile (Jang et al., 2004), and it is well known that the bile plays a critical role in the metabolism of lipids (Trauner, Claudel, Fickert, Moustafa, & Wagner, 2010).
Villus and crypts are two important components of the small intestine, and their geometry provides an indicator of the absorptive capacity of the small intestine. Turnover of the intestinal epithelium reflects a dynamic equilibrium between the production of enterocytes in the crypts and their subsequent desquamation from the villus (Pluske, Hampson, & Williams, 1997). The villus height:crypts depth (VH:CD) ratio is an available criterion for evaluating intestinal health and function. The results obtained from the present study showed that PEO supplementation significantly increased the villus height in the jejunum and ileum, and VH:CD ratio in the ileum. Similarly, positive impacts on the intestinal morphology have been reported in birds (Hong, Steiner, Aufy, & Lien, 2012) and weaned pigs (Sehm, Lindermayer, Dummer, Treutter, & Pfaffl, 2007) by previous studies. Moreover, dietary PEO supplementation significantly increased the number of goblet cells (GC), which secretes mucus and plays a critical role in maintaining the mucous membranes and preventing pathogens invasion (Blomberg, Krivan, Cohen, & Conway, 1993; Reisinger, Steiner, Nitsch, Schatzmayr, & Applegate, 2011).
The activity of critical enzymes in the intestinal mucosa was measured. Interestingly, we found that the activity of sucrase in the duodenal and jejunal mucosa, and the activity of lactase in jejunal mucosa were significantly improved by 200 ppm PEO supplementation. Small intestine is the main site for nutrients digestion and absorption. Previous study indicated that spices and herbs from which essential oil are extracted could stimulate the secretion and enhance activity of digestive enzymes in the small intestine in rats (Platel & Srinivasan, 1996). Importantly, essential oils have been reported to increase the activity of digestive enzymes in swine and poultry, resulting in enhanced nutrients absorption and an improved feed conversion ratio (Windisch, Schedle, Plitzner, & Kroismayr, 2008). Moreover, the positive effect of PEO supplementation on the activity of intestinal mucosa enzymes was also observed in birds (Jang et al., 2004).
Sodium–glucose cotransporter 1 (SGLT1) and glucose transporter 2 (GLUT2) are two important transporters in intestine and are responsible for transporting glucose from the intestinal lumen to the enterocyte and then to the blood stream (Gorboulev et al., 2012). In the present study, the expression levels of GLUT2 and SGLT1 in duodenum mucosa were significantly up-regulated by dietary PEO supplementation. The expression level of GLUT2 was also up-regulated in the jejunum and ileum mucosa. The results obtained in this study were consistent with previous report that both the SGLT1 and GLUT2 genes in the intestinal mucosa were significantly up-regulated by oregano essential oils in laying hens (He et al., 2016). These results provided a molecular basis for improved nutrient absorption after dietary PEO supplementation. The tight junction proteins (i.e., ZO-1 and OCLN) play a critical role in maintaining the intestinal barrier integrity, which efficiently prevent the paracellular diffusion of intestinal bacteria and other antigens across the epithelium (Furuse et al., 1993). We found that PEO markedly up-regulated the expression levels of OCLN in the duodenum and ileum, which was consistent with previous report using a pig model (Zou, Xiang, Wang, Peng, & Wei, 2016). The positive effects of PEO on tight junction proteins suggested that PEO maintained the integrity of intestinal barrier and reduced the enteric pathogen burden in the gastrointestine of weaning pigs (Lange et al., 2010).
Volatile fatty acids (VFAs) are the major end products of bacterial metabolism in the large intestine of swine (Bergman, 1990). Production of VFA participates in maintaining the intestinal pH which is required for optimum activity of digestive enzymes. Furthermore, increasing beneficial VFA production could stabilize the microbial eubiosis in the gastrointestinal tract (Roth & Kirchgessner, 1998). However, previous report showed that the total colonic VFAs were diminished linearly by plant extract mixture inclusion in weaned pigs and propionic acid in the caecum and colon was not affected (Manzanilla et al., 2004). In the present study, we found that PEO supplementation significantly increased the concentration of propionic acid in the caecum and colon, which may be helpful to reduce the level of total serum cholesterol and improve the rate of weight gain (Thacker & Bowland, 1981; Young, Brown, & Sharp, 1970). The quantity of VFA produced in the large intestine depends on the amount and composition of the substrate and the microflora (Macfarlane & Macfarlane, 2003). We found that PEO supplementation observably increased the total bacterial amounts in the caecum and colon and tended to increase the number of Lactobacillus in colon. The Lactobacillus are a major part of the lactic acid bacteria group and constitute a significant component of the beneficial microbiota in the gastrointestine. Similar results were observed in previous studies using models (Li et al., 2012; Zhang, Jung, Kim, Kim, & Kim, 2012). Both studies suggested positive effects of PEO on intestinal bacterial community. Although the PEO components such as the cinnamaldehyde and thymol were found to directly inhibit some harmful micro-organisms (Burt, 2004; Di et al., 2007; Lee & Ahn, 1998), the molecular mechanisms operating in the PEO-modulated gut microflora still need to be further investigated.
5 CONCLUSION
Dietary supplementation of PEO improves the growth performance and intestinal mucosa growth in weaned pigs. The beneficial effects of PEO appear to be mediated through improvement in intestinal integrity and function. These results also indicated that the PEO has potential benefits as an alternative to conventionally used antibiotics in the pork industry.
ACKNOWLEDGEMENTS
We would like to thank Fei Jiang and Lan Wang for help with animal trial and statistic analysis. This work was supported by the Special Fund for Agro-scientific Research in the Public Interest (201403047) and the Fok Ying Tung Education Foundation (141027).
ANIMAL WELFARE STATEMENT
The experimental procedures followed the actual law of animal protection were approved by the Animal Care Advisory Committee of Sichuan Agricultural University (no. 20160709) and were performed in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals, and that the research meets EU standards for the protection and use of animals for scientific purposes and/or feed legislation.