Deletion of ferritin H in neurons counteracts the protective effect of melatonin against traumatic brain injury-induced ferroptosis
Abstract
Accumulating evidence demonstrates that ferroptosis may be important in the pathophysiological process of traumatic brain injury (TBI). As a major hormone of the pineal gland, melatonin exerts many beneficial effects on TBI, but there is no information regarding the effects of melatonin on ferroptosis after TBI. As expected, TBI resulted in the time-course changes of ferroptosis-related molecules expression and iron accumulation in the ipsilateral cortex. Importantly, we found that treating with melatonin potently rescued TBI induced the changes mentioned above and improved functional deficits versus vehicle. Similar results were obtained with a ferroptosis inhibitor, liproxstatin-1. Moreover, the protective effect of melatonin is likely dependent on melatonin receptor 1B (MT2). Although ferritin plays a vital role in iron metabolism by storing excess cellular iron, its precise function in the brain, and whether it involves melatonin's neuroprotection remain unexplored. Considering ferritin H (Fth) is expressed predominantly in the neurons and global loss of Fth in mice induces early embryonic lethality, we then generated neuron-specific Fth conditional knockout (Fth-KO) mice, which are viable and fertile but have altered iron metabolism. In addition, Fth-KO mice were more susceptible to ferroptosis after TBI, and the neuroprotection by melatonin was largely abolished in Fth-KO mice. In vitro siFth experiments further confirmed the results mentioned above. Taken together, these data indicate that melatonin produces cerebroprotection, at least partly by inhibiting neuronal Fth-mediated ferroptosis following TBI, supporting the notion that melatonin is an excellent ferroptosis inhibitor and its anti-ferroptosis provides a potential therapeutic target for treating TBI.
1 INTRODUCTION
As a critical health concern worldwide, traumatic brain injury (TBI) causes significant morbidity, mortality, and financial cost worldwide, and more than 50 million new TBI cases occurring annually.1, 2 Despite the high burden of severe TBI, no effective pharmaceutical intervention or treatment is available for TBI patients as determined by randomized controlled trials,3 indicating that the crucial mechanisms that contribute to cell death and functional deficits following TBI are still elusive. During the pathophysiological process of TBI, various forms of cell death occur, including apoptosis, necrosis, necroptosis, autophagy, pyroptosis, and ferroptosis.4 However, the mechanism of ferroptosis has not been exploited greatly in the context of TBI. Given that ferroptosis may play an important role in the pathophysiological process of TBI, to find new approaches, aiming to block ferroptosis-related cell death, may help decrease the risk of TBI and increase the chances of recovery.
Ferroptosis, a recently described form of iron and lipid oxidation products-dependent regulated cell death, is involved in a variety of brain disorders or injuries, including ischemia stroke,5 intracerebral hemorrhage,6 and TBI.4, 7 Dysregulation of key components of ferroptosis machinery includes iron homeostasis, GSH depletion, and lipid peroxidation. Iron is essential for various fundamental metabolic processes and is incorporated into many proteins in the form of cofactors such as heme and iron-sulfur clusters. Iron is also required for the accumulation of lipid peroxides and the execution of ferroptosis. Abnormal iron homeostasis has been implicated in ferroptosis, resulting in central nervous system (CNS) pathological conditions. Patients with inherited disorders that develop abnormal iron accumulation share motor, cognitive, and intellectual disability.8 Notably, altered iron levels were observed in TBI patients supporting that iron homeostasis seems to be an important process in the pathobiology of TBI.9, 10 Several proteins have been shown to play a role in maintaining cell iron homeostasis by regulating iron uptake, transport, and storage. Iron uptake is controlled by the regulation of transferrin receptor-1 (Tfr1),11 and iron exporter is controlled through a unique iron exporter ferroportin (Fpn).12 As the major iron-storage protein, elevated ferritin levels in serum are associated with lower hospital admission Glasgow Coma Scale (GCS) scores and higher intensive care unit (ICU) mortality in patients with severe TBI.13
Ferritin is a heteropolymer composed of 24 subunits of heavy (Fth, also known as Fth1) and light (Ftl) types which can store up to 4500 atoms of iron. Importantly, Fth has ferroxidase activity, catalyzing the conversion of the ferrous form (Fe2+) to the ferric form (Fe3+) for storage in ferritin nanocages, thereby attenuating the iron-mediated catalysis of reactive oxygen species (ROS). Although the iron was overloaded and Fth expression was upregulated both in the experimental animal and human cortex after TBI,14 the role of Fth in brain remains unexplored. Global loss of Fth in mice causes early embryonic lethality,15 but Ftl deletion is not embryonically lethal in mice,16 suggesting that the latter is not a functionally redundant gene. In brain tissue, microglia cells are rich in Ftl-chains, whereas Fth is expressed predominantly in the neurons.17, 18 Notably, we and others previously tried to generate conditional Fth knockout mice, which develop tissue damage in solid organs, including the liver,19 kidneys,20 and heart.21 However, the precise role of Fth in brain is poorly understood. To determine the role of Fth and TBI-induced ferroptosis in brain, we generated mice that lack Fth selectively in adult neurons by crossing Fth-floxed mice (Fthflox/flox) with mice that express Cre recombinase driven by the neuronal Map2 promoter; the offspring are referred to hereafter as Fthflox/flox; Map2-Cre mice (FthMap2/Map2). Following tamoxifen treatment, a novel neuron-specific Fth knockout (Fth-KO) mouse model was then generated.
Melatonin (N-acetyl-5-methoxytryptamine) is synthesized by the pineal gland and other organs. Intensive research by us and others has indicated melatonin's benefits in both the experimental models22-24 and clinical treatments of TBI.25, 26 Although melatonin has diverse biological functions in treating TBI, including anti-apoptotic, anti-oxidative, and anti-inflammatory properties,24, 27 to our knowledge, no report about the effect of melatonin on ferroptosis after TBI has been published. Most of melatonin's physiological functions are mediated through its two receptors: melatonin receptor 1A (MT1) and melatonin receptor 1B (MT2), which are seven transmembrane G-protein-coupled receptors. An antagonist of MT1/MT2 Luzindole and a selective MT2 antagonist 4P-PDOT are frequently used as melatonin receptor antagonists.28 Decreased expression of MT1 and MT2 was observed within the frontal cortex during the acute (6 and 24 hours) period post-TBI in rats.29 However, whether the neuroprotective ability of melatonin in the experimental TBI model dependent upon the presence and activation of melatonin receptors is not known.
In the present study, using liproxstatin-1 as a positive control (a specific inhibitor of ferroptosis), we first investigated whether ferroptosis is involved in the effects of melatonin's neuroprotection following TBI. We then exploited whether melatonin's neuroprotection against ferroptosis is dependent on MT1/MT2 in a mouse TBI model, using melatonin receptor antagonists (Luzindole and 4P-PDOT). To determine whether Fth mediates the neuroprotection of melatonin against TBI-induced ferroptosis, we generated neuron-specific Fth conditional knockout (Fth-KO) mice, and then detected several biomarkers associated with ferroptosis and melatonin receptors in these mice with or without TBI. We also showed that treatment with melatonin attenuated TBI-induced ferroptosis in these KO mice. Finally, using Fth siRNAs, we further investigated the underlying mechanisms of melatonin against TBI in vitro.
2 MATERIALS AND METHODS
2.1 Animals
All animal experiments were reviewed and approved by the Ethics Committee of Soochow University and Zhejiang University. All animal procedures were conducted in strict compliance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals. Adult male C57BL/6J mice (20-25 g) were obtained from the Animal Center of the Chinese Academy of Sciences (Shanghai, China). Fth-floxed (Fthflox/flox) mice were purchased from the Jackson Laboratory (stock numbers 018063).21 Map2-Cre transgenic mice (C57BL/6J-Map2em1(2A-CreERT2)Smoc), which express Cre recombinase specifically in neurons, were purchased from Shanghai Biomodel Organism (Shanghai, China). All animals were housed in a temperature and humidity-controlled room under a 12-hour light/dark cycle, and maintained in standard cages with free access to food and water.
Fth-floxed mice were crossed with Map2-Cre transgenic mice (C57BL/6J background) to obtain Fthflox/flox; Map2-Cre mice (or FthMap2/Map2). Mice carrying the Fthflox/flox and Map2-Cre alleles were genotyped by PCR (Figure S1). The primers are presented in Table S1. A tamoxifen stock solution (2.5 mg/mL) was then prepared by dissolving and sonicating tamoxifen in autoclaved vegetable oil. To obtain neuron-specific Fth knockout mice (Fth-KO), FthMap2/Map2 mice and Fthflox/flox (control) mice were injected intraperitoneally once a day for five consecutive days with 100 mg/kg of tamoxifen, and a mouse TBI model was performed in the following day.
2.2 Mouse TBI model
Eight-week-old male C57BL/6J, Fthflox/flox, and Fth-KO mice weighing 20-25 g were subjected to controlled cortical impact (CCI) to establish a TBI model. Before the operation, mice were coded and randomly divided. Afterward, animals were anesthetized using pentobarbital sodium (50 mg/kg, intraperitoneally), followed by fixation in a prone position in a stereotaxic apparatus (RWD Life Science Co., Shenzhen, China). Subsequently, a skin incision was conducted to expose the skull, and a 3-mm craniotomy was located approximately midway between bregma and lambda lateral to the sagittal suture on the left side, the skull disk was then removed carefully without disruption of the dura. The procedure was described in detail previously.30, 31 TBI was generated in a direction perpendicular to the brain surface, using a pneumatic cortical impact device (AmScien Instruments, USA) with the following parameters: a pressure of 10 Kpa, the depth of 1.0 mm, and an impact duration time of 70 mseconds30 The craniotomy then was closed with standard suture material immediately after TBI. Animals were anesthetized and sacrificed for biological studies at the indicated time points. Each injured cortex (the center: the impact site of the ipsilateral cortex, the range: 2 × 2 × 2 mm3) and blood were harvested. Sham-operated animals only underwent the surgical procedure except for cortical impact injury. To minimize the variance in operation, drug administration and CCI modeling were performed by the same skilled operators.31
2.3 Drug administration
Melatonin (N- acetyl- 5- methoxytryptamine, Mel) was purchased from Sigma-Aldrich (St. Louis, MO, USA), dissolved in DMSO (the final concentration: 0.5%), and then diluted by saline. Melatonin (10 mg/kg, once daily) was administered by intraperitoneal (i.p.) injection 1 hour after TBI and once a day till sacrifice. The dosage of melatonin and the dosing time (9:00-9:30 am) were chosen based on previous studies23, 32 and have been proven to be effective. The vehicle (0.5% DMSO in saline) was administered to the mice 1 hour after TBI and once daily until sacrifice.
Liproxstatin-1 (Lip-1), a potent and specific inhibitor of ferroptosis, was purchased from TargetMol. It was dissolved in DMSO (the final concentration: 0.5%) and then diluted by saline. Lip-1 (10 mg/kg, once daily) was also administered by intraperitoneal injection 1 hour after TBI and once a day until sacrifice. The dose of Lip-1 was chosen based on a previous study.33 The vehicle (0.5% DMSO in saline) was administered to the mice 1 hour after TBI and once a day until sacrifice.
Luzindole (Luz), an antagonist of MT1 and MT2, was obtained from Sigma-Aldrich (St. Louis, MO, USA). It was dissolved in 2.5% v/v ethanol/saline and then administered as 30 mg/kg (i.p.) at 30 minutes before TBI. 4-P-PDOT (4-phenyl-2-propionamidotetralin), a selective MT2 receptor antagonist, was obtained from Sigma-Aldrich (St. Louis, MO, USA). It was dissolved in 2.5% v/v ethanol/saline and then administered as 10 mg/kg (i.p.) at 30 min before TBI. The doses of Luz and 4P-PDOT were chosen based on a previous study.34
2.4 Experimental design
There were six parts to the experimental design of this study.
Part 1: To investigate the time pattern of changes in the expression of ferroptosis-related proteins and cellular iron accumulation after TBI, animals were randomly divided into eight groups (n = 6) according to the time points after TBI: sham, 6 hours, 12 hours, 1 day, 2 days, 3 days, 7 days, and 14 days. Western blot and Perl's blue staining were then performed.
Part 2: To determine the effect of melatonin on iron metabolism dysfunction, neuronal damage, behavioral deficits, and lesion volume after TBI, animals were divided into 4 groups (n = 6-8): sham, vehicle + TBI, Mel + TBI, and Lip-1 + TBI (positive control). Vehicle or drugs were injected i.p. 1 hour after TBI and once a day until sacrifice. Western blot, real-time PCR, iron assay, Perls’ blue, Nissl and Fluoro-Jade B (FJB) staining, ELISA, immunofluorescence, wire-grip test, Morris Water Maze test, open field test, and lesion volume were evaluated at indicated time points following TBI.
Part 3: To exploit whether the effect of melatonin is dependent on its receptors following TBI, the time course of melatonin receptors (MT1 and MT2) expression was first evaluated. Mice were then divided into 5 groups (n = 6): sham, vehicle + TBI, melatonin + TBI, melatonin + TBI +Luzindole, and melatonin + TBI +4-P-PDOT. The Western blot for MT1, MT2, Cox2, 4HNE, and the content of GSH were evaluated.
Part 4: To study the putative role of neuronal Fth in TBI-induced iron metabolism dysfunction and ferroptosis, mice were divided into 3 groups (n = 3-6): sham + Fthflox/flox (control), sham + Fth-KO, TBI + Fth-KO. Western blot analysis, real-time PCR, iron assay, - Perls’ blue, Nissl, and FJB staining were evaluated at the indicated time points after TBI.
Part 5: To further ascertain whether loss of neuronal Fth affects melatonin's effects on TBI-induced ferroptosis, we measured several putative biomarkers of ferroptosis. Mice were divided into two groups (n = 3-6): vehicle + TBI + Fth-KO, and melatonin + TBI + Fth-KO.
Part 6: To exploit the effects of melatonin on mechanical scratch injury in vitro, the HT-22 cell line was subjected to mechanical scratch injury and transfected with or without Fth siRNA (siFth). The cells were divided into five groups (n = 6): control, siFth alone, siFth + scratch injury, siFth + melatonin + scratch injury, and scratch injury alone. After treatments, cells were used for protein extraction, cellular ROS detection, and BODIRY 581/591 C11 staining.
2.5 Western blot analysis
Western blot analysis was conducted as published previously.31 The ipsilateral cortical sample was isolated, fully homogenized in Western blot analysis buffer (Beyotime Biotechnology, Shanghai, China). The homogenates were then centrifuged at 12 000 g for 20 minutes at 4°C, and the supernatants were preserved as protein samples at −80°C for later use. Protein concentrations were determined using NanoDrop 2000C (Thermo Scientific, USA). The samples were subjected to 10% or 12% SDS-PAGE and then transferred to polyvinylidene fluoride membranes (Millipore, Boston, MA, USA) on a semidry electrotransferred unit (Bio-Rad, USA). The membranes were blocked in 5% nonfat milk in TBST for 2 hours at room temperature and incubated with the primary antibodies (see Table S2 for details) against xCT, Tfr1, Ferritin Heavy Chain (Fth), Ftl, Ferroportin 1 (Fpn), 4HNE, Nox2, Cox2, Melatonin Receptor 1A (MT1), and Melatonin Receptor 1B (MT2) overnight at 4°C. Membranes were washed with TBST three times after the overnight incubation and then incubated with the secondary antibody at room temperature for 1.5 hours. The protein density was captured using an ECL chemiluminescence system (ChemiScope, Shanghai, China). Western blots were quantified using ImageJ analysis software (NIH) and were normalized to Gapdh.
2.6 Quantitative real-time PCR
The mRNA levels of the ferroptosis-related genes were determined using real-time quantitative PCR. Briefly, the total RNA was isolated with TRIzol reagent (Invitrogen) according to the manufacturer's protocol. After determining the total RNA concentration using a NanoDrop 2000C (Thermo Scientific, USA), reverse transcription to cDNA was carried out using PrimeScript™ RT Master Mix (Takara Bio, Shiga, Japan). The real-time PCR thermal cycler (LightCycler, Roche Applied Science, Indianapolis, IN, USA) was used to conduct quantitative real-time PCR. Real-time PCR of all samples was done in triplicate using the SYBR Green master mix (Roche Applied Science). The mRNA levels were normalized to Gapdh to internal control. The relative expression levels of genes were quantified using the comparative △Ct method, as previously published.35 The primers are presented in Table S3.
2.7 Assay for GSH/GSSG and MDA
Cortical GSH content was measured using a GSH and GSSG assay kit (#S0053) as per the manufacturer's instructions (Beyotime Biotechnology, Shanghai, China). The content was measured on a colorimetric microplate reader (Thermo Scientific Multiskan FC, OD = 593 nm). GSH content was expressed as μmol/g, and the GSH content of the test samples was calculated as: Total Glutathione-GSSG × 2. Cortical malondialdehyde (MDA) content was measured using a lipid peroxidation MDA assay kit (#S0131S) following the manufacturer's instructions (Beyotime Biotechnology, Shanghai, China). In brief, the ipsilateral cortex was weighed and homogenized in cold PBS on ice. Tissue lysates were then centrifuged at 10 000-12 000 g at 4°C for 10 minutes to harvest the supernatant. After 200 μL MDA solution added to each sample or standard (0.1 mL), the mixture was heating at 100°C for 15 minutes and then centrifuged at 1000 g for 10 minutes to harvest the supernatant. The supernatant (200 μL) was colorimetrically quantified (Thermo Scientific Multiskan FC, OD = 532 nm). MDA levels were expressed as μmol/mg.36
2.8 Nonheme iron content determination
The level of ferrous and ferric irons in the serum and ipsilateral cortex was assessed using an iron assay kit (Colorimetric) (#ab83366, Abcam) following the manufacturer's instructions. In brief, blood samples were harvested and centrifuged at 1000 g for 15 minutes to obtain serum. For tissue samples, cortical tissue samples were weighed and homogenized in iron assay buffer using a Dounce homogenizer sitting on ice, after washing in cold PBS. The homogenate was then centrifuged at 16 000 g for 10 minutes at 4°C, and the supernatant was harvested. For Fe2+ assay, 5 μL assay buffer was added to each sample. For total iron (Fe2+ + Fe3+) assay, 5 μL iron reducer was added to each sample or standard well. The mixture was incubated at 37°C for 30 minutes, and the output was promptly measured on a colorimetric microplate reader (Thermo Scientific Multiskan FC, OD = 593 nm). Fe3+ content of the test samples can be calculated as Fe3+ = Total iron − Fe2+.
2.9 Perls’ Prussian blue staining
Perls’ Prussian blue staining was used to detect cellular iron accumulation, as described previously.4 Briefly, after washing with PBST three times, brain sections were covered with the solution of 0.3% Triton X-100 in PBS for 5 minutes at room temperature. Subsequently, the sections were incubated in Perls’ solution (5% potassium ferrocyanide [Yeasen Biotech]/5% hydrochloric acid) for 30 minutes, followed by washes in PBST for three times. The solution of 0.3% hydrogen peroxide in methanol was then used to blocked endogenous peroxidase activity for 15 minutes, followed by washes in PBST three times. Signals were developed by incubation for 15 minutes in 3,3-diaminobenzidine (DAB), and hematoxylin was used for counterstaining.
2.10 Nissl staining
For Nissl staining, brain sections were fixed in formaldehyde and washed in PBST for three times. Then, the sections were incubated with a Nissl staining solution (Beyotime Biotechnology, Shanghai, China) for 10 min at 50°C. Shrunken and/or contained vacuoles, and darker in the stained nuclei were observed in the cell bodies of injured neurons, compared with normal neurons. Five random interest regions were selected for quantification under the microscope (Nikon TE300; Nikon) to observe the positively stained cells surrounding injury areas.
2.11 Fluoro-Jade B (FJB) staining
Frozen whole brains were cryosectioned, and coronal sections (12 μm) were created starting from bregma to the posterior of the brain, as previously described.37 The sections were then fixed in 4% paraformaldehyde for 1 hour. Fluoro-Jade B (FJB) staining was conducted according to the specification of the manufacturer (#AG310, Millipore, Germany). In brief, coronal brain sections were immersed in a basic alcohol solution (70% ethanol and 1% sodium hydroxide) for 5 minutes. They were then rinsed for 2 minutes in 70% ethanol and washed for 2 minutes in distilled water. Subsequently, tissues were immersed in a potassium permanganate solution (0.06%) for 10 minutes. Following two minutes of water rinse, the slides were then incubated for 30 minutes in a solution of Fluoro-Jade B (0.0004%). Images were captured using a microscope (Nikon TE300, Japan). The number of Fluoro-Jade B-positive cells was measured and quantified from at least three tissue sections per mouse.
2.12 Immunohistochemical and immunofluorescence staining
Brains were frozen and cut into coronal slices (12 μm), after being fixed with 4% paraformaldehyde for 24 hours. Brain sections were rehydrated in PBS for 10 minutes, subsequently, covered with blocking buffer (0.3% Triton X-100 and 10% BSA in PBS) for 1 hour. These sections were then incubated with a mixture of corresponding primary antibodies (see Table S2 for details): 4HNE, Ferritin Heavy Chain (Fth), NeuN, GFAP, and Iba-1 overnight at 4°C. After washing with PBS, the sections were incubated with secondary antibodies (Beyotime Biotechnology, Shanghai, China) for 1 hour at room temperature. For immunofluorescence staining, to visualize the overall density of the total cells, slides were mounted with 4, 6-diamidino-2-phenylindole (DAPI) for nuclear staining (Beyotime Biotechnology) and then observed under a fluorescence microscope.38
2.13 Behavioral analysis
A wire-grip test was conducted, as previously described.31, 39 Briefly, an upside-down lid was held at the height of 45 cm, and a 5-point scale was used to assess the motor function. One test included three trials and the average value was regarded as the final score each day. To evaluate the effect of melatonin and Lip-1 on memory performance of mice after TBI, the Morris Water Maze (MWM) test was carried out on days 9-16 post-TBI, as previously described.31 Briefly, mice were trained in the MWM on days 3 before TBI (three trials per day). During each trial, each animal was permitted 90 seconds to reach the submerged platform and remained on it for 15 seconds. If a mouse failed to reach the platform within 90 seconds, it was guided to the platform and allowed to stay for 15 seconds. Swimming escape latency (the time to reach the visible platform) was automatically recorded and analyzed with a video/compute system (a tracking device and software).
An open field test was conducted to explore the presence of anxiety or disinhibition in CCI injured mice at 16 days after TBI. The open field test was performed, as previously described.40 Before each behavioral session, the apparatus was cleaned by the solution of 70% ethanol. Animals were removed from their home cage and placed in an open field test arena (30 cm × 30 cm × 30 cm). Behaviors and parameters were recorded and analyzed by an automated behavioral tracking system (#JLBehv-OFG-4, Shanghai Jiliang Software Technology, Shanghai, China). Ambulatory activity (the total distance travelled in centimeter) was recorded for 20 minutes and analyzed. The following parameters were calculated: the total distance, distance moved in the center, and time spent in the center of the open field arena.
2.14 Lesion volume assessment
To determine whether melatonin alleviates tissue damage caused by TBI, we evaluated lesion volume in the ipsilateral cortical hemisphere at 16 days following TBI. Briefly, the brains were fixed in 4% paraformaldehyde for 24 hours and then cut into serial coronal sections (30 μm) with a cryostat. A random sampling scheme was used to yield five total sections, by estimating every tenth section from rostral to caudal. Sections were then stained with hematoxylin and eosin before being calculated for injury volume (the area of tissue loss) using NIH ImageJ software in a blinded fashion, as previously described.41 Data were presented as the volume percentage of the lesion versus the contralateral hemisphere.41
2.15 In vitro TBI and transfection
In vitro TBI was performed using a well-established model of mechanical scratch injury.42, 43 HT-22 cells were cultured in Dulbecco's modified Eagle's medium (DMEM, Gibco) with 10% fetal bovine serum (Gibco), 100 μg/mL streptomycin (Invitrogen), and 100 U/mL penicillin (Invitrogen).44 To exploit the effects of melatonin on mechanical scratch injury in vitro, HT-22 cells subjected to mechanical scratch injury were scratched by a 200-μl yellow gunshot, as previously described.42, 43 HT-22 cell line was transfected with or without Fth siRNA for 24 hours before subjected to mechanical scratch injury. The si-m-Fth mimic, inhibitor, and their negative controls were obtained from RiboBio (Guangzhou, China). Transfections were performed as per the manufacturer's instructions (RiboBio, Guangzhou, China). Melatonin was then added 3 hours before the scratch injury. Cell viability was assessed using the cell counting kit-8 (CCK-8) kit as per manufacturer's instructions (Beyotime Biotechnology, Shanghai, China). Finally, cells were used for protein extraction, cellular reactive oxygen species (ROS) detection, and BODIRY 581/591 C11 staining. The ROS generation by the cells was measured with a cellular ROS detection assay kit (#S0033S, Beyotime Biotechnology, Shanghai, China). Harvested cells were washed with serum-free culture medium (1:1000) and incubated with 2ʹ,7ʹ-dichlorofluore scindiacetate (DCFH-DA) at 37°C for 20 minutes. The DCF fluorescence distribution of 4 × 105 cells was recorded every 10 minutes by a fluorospectrophotometer at an excitation wavelength of 488 nm and an emission wavelength of 525 nm.45 To detect lipid peroxidation (Lipid ROS), cells were incubated with working solution of BODIRY 581/591 C11 (10 μmol/L).46 The fluorescence was undergoing a shift from red to green fluorescence emission when lipid peroxidation increased.
2.16 Statistical analysis
Data are presented as mean ± SEM. Statistical analyses were performed using GraphPad Prism 8 Software (San Diego, CA, USA). A two-tailed unpaired Student's t test was performed, for experiments with only two groups. For comparisons involving more than two groups, statistical analysis was evaluated using a one-way or two-way ANOVA, followed by Tukey's post hoc test. All P values were two-sided, and statistical significance was set at P < .05.
3 RESULTS
3.1 Melatonin attenuates TBI-induced ferroptosis
Accumulating evidence demonstrates that ferroptosis contributes to neuronal death induced by TBI.4, 7 First, we determined the time course of ferroptosis-related protein expression after TBI. As the biomarkers of ferroptosis, the expression of xCT (a sodium-independent cystine-glutamate antiporter, Slc7a11), Fth, Tfr1, Fpn, Ftl, Nox2, Cox2 (a key enzyme in prostaglandin biosynthesis), and 4HNE (a noxious by-product of lipid peroxidation) was analyzed. As shown in Figure S2, the expression of xCT, Cox2, Tfr1, Nox2, and Fpn markedly increased and reached a peak from 12 to 24 hours post-TBI, then gradually decreased afterward, approaching or slightly below normal. Interestingly, we found a remarkable upregulation of Fth, Ftl, and 4HNE at 3 days post-TBI and had a slight decline from 3 to 14 days.
To ascertain whether melatonin could rescue TBI-induced ferroptosis, Western blot for ferroptosis-related proteins was performed at the respective peak expression (eg, 1 and 3 days). We found that melatonin administration inhibited TBI-induced upregulation of xCT, Cox2, Tfr1, Fpn, and Nox2 protein expression at 1 day after TBI (Figure 1A). Likewise, melatonin treatment prevented the expression of Fth, Ftl, and 4HNE proteins at 3 days following TBI (Figure 1B). Similar results were obtained with an inhibitor of ferroptosis, liproxstatin-1 (Lip-1) (Figure 1A,B). In addition, immunohistochemical staining showed that treatment with melatonin and Lip-1 reduced the number of 4HNE-positive cells in neurons at 7 days after TBI, compared with the vehicle group (Figure 1C,D), suggesting melatonin's neuroprotection against TBI-induced lipid peroxidation.
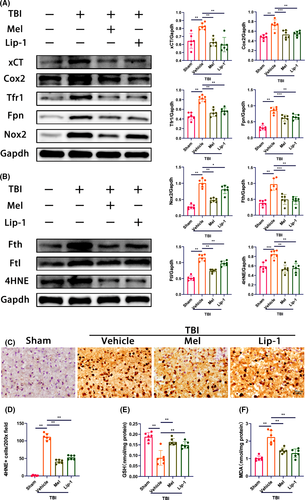
Next, we measured several other putative biomarkers of ferroptosis, including mRNA of Slc7a11, Ptgs2, Tfr1, Fpn, Fth, and Ftl, along with GSH and lipid peroxidation‒derived MDA levels. Consistent with the results of the Western blot analysis, we found the mRNA levels of Slc7a11, Ptgs2, Tfr1, Fpn, Fth, and Ftl were upregulated at 1 day after TBI (Figure S3). However, both melatonin and Lip-1 treatment reversed the above changes. GSH plays an important role in ferroptosis, and reduced GSH levels can trigger ferroptosis. As expected, both melatonin and Lip-1 inhibited TBI-induced the decrease of cortical GSH levels (Figure 1E). Given that MDA is the ferroptosis end-product, we measured and found that melatonin and Lip-1 inhibited TBI-induced the upregulation of MDA content in the injured cortex at 3 days following TBI (Figure 1F). Taken together, these data suggest that TBI resulted in the time course of changes in the expression of ferroptosis-related proteins, as well as the changes of several putative biomarkers of ferroptosis in the ipsilateral cortex, but treating with melatonin potently rescued TBI-induced ferroptosis.
3.2 Melatonin rescues iron accumulation and neuronal damage in the ipsilateral cortex after TBI
To further study the putative role of melatonin in iron accumulation and neuronal damage following TBI, nonheme iron content, Perls’ blue, Nissl, and FJB staining were conducted. The level of Fe2+ reflects the iron overload that can generate highly reactive hydroxyl radicals through the Fenton reaction.47 We found although no significant increase in serum Fe2+ level was observed in the vehicle TBI group, the levels of Fe3+, and total iron in serum were significantly increased on days 1 and 3 after TBI, relative to the sham group (Figure S4A,B). Importantly, a significant increase in Fe2+, Fe3+, and total iron content was observed in the injured cortex at 3 days after TBI (Figure 2A). Whereas, melatonin or Lip-1 inhibited TBI-induced the upregulation of iron content mentioned above in both serum at 1 day (Figure S4) and ipsilateral cortex at 3 days (Figure 2A). Perls’ blue staining showed that the number of iron-positive cells was significantly increased at 7 days after TBI and continued to increase gradually afterward (Figure S4C). Importantly, 7 days after TBI, fewer iron-positive cells were observed in melatonin or Lip-1-treated group than the vehicle-treated group (Figure 2B). Given TBI-induced the iron overload was accompanied by the upregulation of Fth expression,14 and neurons are rich in Fth chains in brain tissue,17 we then observed the expression of Fth in neurons following TBI using immunofluorescence staining. We found Fth-positive neuron percentage was increased in the vehicle TBI group relative to the sham group, whereas melatonin and Lip-1 treatment significantly reduced the percentage of Fth-positive neurons, compared with the vehicle TBI group, respectively (Figure 2C,D).
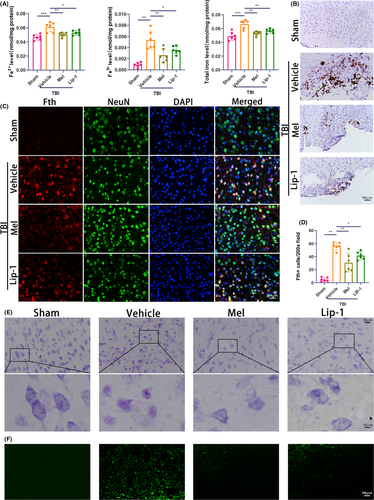
Neuronal damage was visualized using Nissl and fluoro-jade B (FJB) staining. Compared with the sham group (living neurons), the cell bodies of injured neurons exhibited cytoplasmic shrinkage or nuclear pyknosis at 7 days following TBI. Melatonin and Lip-1 treatment attenuated these histopathological changes, as determined according to the mouse cortex (Figure 2E). Brain sections were also stained with FJB to determine neuronal degeneration at 7 days after TBI and found that TBI resulted in an increase in the number of degenerating neurons in the cerebral cortex, but both melatonin and Lip-1 administration obviously reduced the number of degenerating neurons compared with the vehicle group (Figure 2F). These data indicate that melatonin protects against TBI-induced iron accumulation and prevents neuronal damage in the ipsilateral cortex.
3.3 MT2 receptor is likely involved in melatonin's protection against TBI-induced ferroptosis
To elucidate whether the protective effect of melatonin is dependent on its receptors following TBI, we first determined the expression pattern of melatonin receptors (MT1 and MT2) after TBI. As shown in Figure 3A-C, A significant decrease of cortical MT1 and MT2 expression was observed from 12 hours to 14 days, though they were unchanged from sham levels at 6 hours. Our results were consistent with the previous report that both MT1 and MT2 levels were reduced in a time point-dependent manner, although the expression of MT1 and MT2 was evaluated during the acute (6 and 24 hours) period post-TBI.29 Furthermore, melatonin significantly ameliorated MT1 and MT2 loss in the brains of melatonin-treated TBI mice at 24 hours (Figure 3D-F).
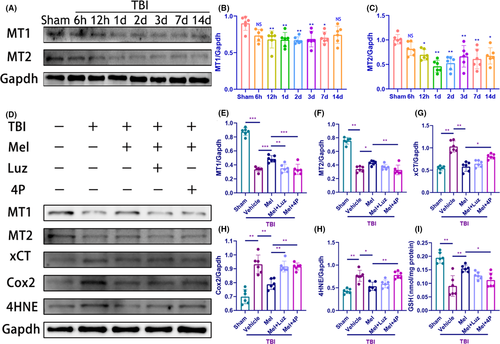
To ascertain whether melatonin's receptors are involved in melatonin's protection against TBI-induced ferroptosis, the effect of melatonin on ferroptosis-related xCT, Cox2, 4HNE, and GSH was investigated when mice were pretreated with Luzindole (30 mg/kg, IP, an antagonist of MT1 and MT2 receptors) or 4P-PDOT (10 mg/kg, IP, a selective MT2 receptor antagonist) before melatonin treatment, respectively (Figure D-J). We found pretreatment with Luzindole and 4P-PDOT both abolished the effect of melatonin on MT1 and Cox2 expression at 24 hours following TBI (Figure 3D,E,H). Notably, pretreatment with 4P-PDOT (10 mg/kg), but not Luzindole (30 mg/kg) abolished the restorative effect of melatonin on MT2 expression and GSH content (Figure 3F,J), as well as the effect of melatonin on xCT and 4HNE (Figure 3G,I). Considering the dose of Luz was chosen based on a previous study about ischemic stroke,34 dosage and model may matter. We added the other dose of Luz (60 mg/kg) and found that pretreatment with Luz (60 mg/kg) could abolish the restorative effect of melatonin on xCT, 4HNE, and MT2 expression (Figure S5A-D), as well as the GSH content (Figure S5E). Taken together, the effect of melatonin was blocked by MT1 and MT2 receptors antagonists. Considering Luzindole has a higher affinity toward the MT2 receptor than the MT1 receptor subtype,48 the data of the present study suggest that the MT2 receptor is the major melatonin receptor subtype likely involved in melatonin's protection against TBI-induced ferroptosis.
3.4 Melatonin decreases TBI-induced brain behavioral deficits and lesion volume in mice
To exploit the effect of melatonin on TBI-induced neurological outcome and lesion volume, we performed behavioral analysis as well as hematoxylin and eosin staining (Figure 4A). Regarding the wire-grip test, TBI resulted in a significant decline in motor performance on days 1-8 compared with the sham group, but treatment with melatonin and Lip-1 ameliorated the motor performance on days 2-6, as compared with the vehicle-treated TBI group (Figure 4B). In the MWM test, animals that find the platform more quickly are regarded as having better learning and memory function.38 We observed that TBI caused an increased latency on days 10-15 compared with the sham group, but both the melatonin and Lip-1-treated TBI mice exhibited significantly shorter escape latency than the vehicle-treated TBI mice (Figure 4C).
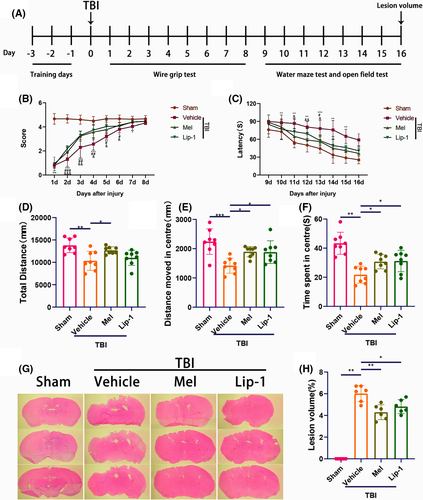
An open field test was then conducted to explore the presence of anxiety or disinhibition on days 16 after TBI. Animals in the vehicle TBI group travelled markedly shorter total distance (Figure 4D) than that in the sham group. In parallel, significantly shorter distance moved (Figure 4E) and less time spent in the center of the open field arena (Figure 4F) were observed in the vehicle-treated TBI group than that in the sham group. Melatonin treatment significantly reversed the parameters as mentioned above, but Lip-1 treatment could not statistically reverse the downregulation of the total distance caused by TBI (Figure 4D-F). Hematoxylin and eosin staining showed that lesion volumes were significantly smaller at 16 days after TBI in melatonin and Lip-1-treated TBI groups than in the vehicle-treated TBI group, respectively (Figure 4G). Taken together, the results provide the evidence of improved motor, memory, and anxiety outcomes as well as lesion volume following TBI in melatonin-treated mice.
3.5 Fth-KO mice develop mildly impaired iron homeostasis in cortex under basal conditions
Given that Fth is expressed predominantly in the neurons (Figure S6A), we generated a neuron-specific Fth conditional knockout mouse (Fth-KO) by crossing Fthflox/flox mice with Map2-Cre transgenic mice and then injecting with tamoxifen (Figure S1A,B). Fthflox/flox mice (homozygous for the floxed allege and expressing Fth in the neurons) were used as controls. Loss of neuronal Fth expression under basal conditions was confirmed using Western blot analysis, real-time PCR, and Fth immunofluorescence. Lower expression of Fth mRNA and protein from cortex was observed in Fth-KO mice, compared with Fthflox/flox mice (Figure 5A-C). We also looked at the neuronal level of Fth by immunofluorescence. Brain sections from tamoxifen-treated Fth-KO mice and control mice were stained with the anti-Fth antibody. As shown in Figure 5D, cortical neurons from tamoxifen-treated Fth-KO mice had a reduction of Fth immunogenicity. Importantly, virtually rare Fth-positive cells were observed in neurons of Fth-KO mice. Interestingly, the loss of Fth in neurons did not lead to a compensatory increase in Ftl expression (Figure 6A), as observed in the liver19 and heart.21
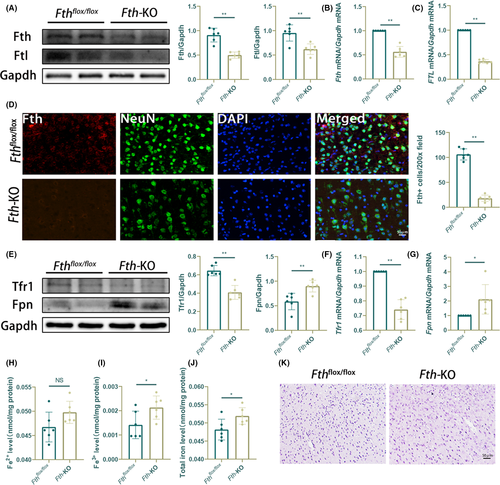

Next, we examined the putative role of neuronal Fth in iron metabolism and redox homeostasis. We measured the expression of the known iron-trafficking proteins Tfr1 and Fpn under physiological conditions. Impaired cortical iron metabolism in Fth-KO mice was reflected by a significant decrease of Tfr1 expression and the increase of Fpn expression at the mRNA and protein levels in the cortex of Fth-KO mice (Figure 5E-G), as compared to the control mice. Strikingly, although an increasing trend in cortical Fe2+ level was observed in Fth-KO mice, there was a significant increase of the total cortical iron and Fe3+ level in the Fth-KO group compared with the control group (Figure 5H-J). Notably, Perls’ blue staining showed that relatively few positive cells were observed in the Fth-KO and control groups, under physiological conditions (Figure 5K). To evaluate the health status of the remaining cortical neurons of Fth-KO mice, we assessed their reactivity to 4HNE and found no significant difference in cortical 4HNE positivity between Fth-KO mice and wild-type mice (Figure S6B). Besides, Fluoro-Jade B reactivity of cortical neurons of the Fth-KO group was not markedly increased compared with that of control mice, under physiological conditions (Figure S6C). These results suggest that Fth-KO mice are viable and fertile but have altered iron metabolism, providing the evidence that neuronal ferritin's iron-storage function plays an important role in maintaining cortical iron homeostasis basal conditions.
3.6 Fth-KO mice are susceptible to TBI-induced ferroptosis
To further study the putative role of neuronal Fth in TBI-induced ferroptosis, we first examined the role of neuronal Fth in TBI-induced cortical iron overload. Compared with control mice, Fth-KO mice developed more severe iron overload in the injured cortex and serum following TBI (Figure 6A-C, Figure S6D). The higher level of cortical nonheme iron in Fth-KO mice was supported by more intense Perls’ blue staining of brain sections (Figure 6D), and reflected by no significant changes of Tfr1 expression and a marked decrease of Fpn expression in the ipsilateral cortex of Fth-KO mice (Figure 6E-G).
We then measured the mRNA levels of Slc7a11 and Ptgs2 (an in vivo biomarker for ferroptosis)49 in Fth-KO mice and control with TBI. A significant increase of cortical Slc7a11 mRNA and no significant change of Ptgs2 mRNA in Fth-KO mice were observed at 1 day after TBI compared with control mice (Figure 6H,I). Western blot analysis showed a significant increase of xCT, and Cox2 expression following TBI was observed in the Fth-KO group, compared with Fthflox/flox group (Figure 6E). Given that reduced GSH levels can trigger ferroptosis,50 we observed the decreased cortical GSH level at 1 day after TBI in Fth-KO mice, relative to Fthflox/flox mice (Figure 6J). Considering MDA is the ferroptosis end-product, we measured and found a significant increase in cortical MDA levels in Fth-KO mice at 3 days following TBI (Figure 6K). In addition, we used immunohistochemistry to measure lipid peroxidation-derived 4HNE and found an increased number of 4HNE-positive cells in the injured cortex of Fth-KO mice after TBI, indicating increased levels of lipid peroxidation (Figure 6L). Neuronal damage was visualized using FJB staining. The number of FJB-positive neurons was significantly increased in KO mice on the injured side following TBI compared with control mice (Figure 6M), suggesting that Fth deficiency results in exacerbated neuronal damage after TBI. Taken together, these results indicate that due to their increased susceptibility to iron accumulation, Fth-KO mice are more vulnerable to TBI-induced ferroptosis, providing the evidence that loss of neuronal Fth contributes to TBI-induced ferroptosis.
3.7 Neuroprotection by melatonin following TBI is largely abolished in Fth-KO mice
Considering melatonin's effect depends on its receptors, and melatonin attenuates TBI-induced ferroptosis in wild-type mice mentioned above, we then checked melatonin receptors in Fth-KO mice subjected to TBI or not. There was no significant difference in MT1 and MT2 expression between control and KO mice subjected to TBI or not (Figure S7A). In addition, melatonin treatment did not reverse the expression of MT1 and MT2 in the Fth-KO group following TBI, indicating that Fth-KO did not affect melatonin's effects on its receptors after injury (Figure S7B). These results indicate that Fth-KO did not affect the expression of melatonin receptors and melatonin's effects on its receptors.
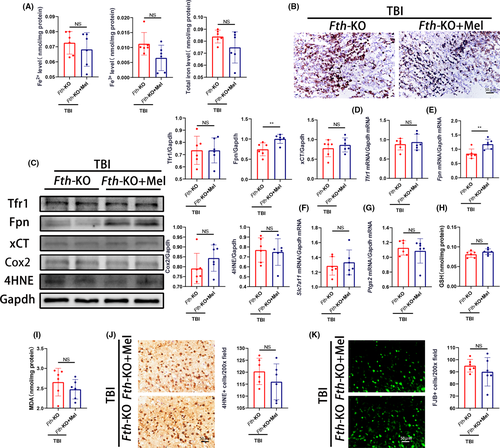
To further exploit whether loss of neuronal Fth affects melatonin's effects on TBI-induced ferroptosis, we measured several putative biomarkers of ferroptosis. We found that treating Fth-KO mice with melatonin (10 mg/kg/day) had no effect on cortical nonheme iron levels compared with non-treated Fth-KO mice (Figure 7A), and supported by Perls’ blue staining (Figure 7B). Strikingly, although there was no significant difference in the level of Tfr1 mRNA and protein expression between melatonin-treated and non-treated Fth-KO mice, a significant increase in the level of Fpn mRNA and protein expression was observed in melatonin-treated Fth-KO mice (Figure C-E). Of note, treating Fth-KO mice with melatonin failed to significantly change the expression of mRNA (Slc7a11, Ptgs2) as well as proteins (xCT, Cox2, 4HNE) compared with non-treated Fth-KO mice (Figure 7C,F,G). In addition, treating Fth-KO mice with melatonin did not statistically decrease the levels of GSH (Figure 7H) and MDA (Figure 7I), as well as the number of both 4HNE (Figure 7J) and FJB-positive cells (Figure 7K). These results suggest that melatonin did not statistically decrease TBI-induced ferroptosis and neuronal degeneration in Fth-KO mice subjected to TBI.
3.8 Melatonin cannot rescue mechanical scratch injury-induced ferroptosis in HT-22 cell line transfected with Fth siRNA
To further confirm the aforementioned effects of melatonin following TBI in Fth-KO mice, the HT-22 cell line was transfected with or without Fth siRNA (siFth) and then subjected to mechanical scratch injury in vitro. Knockdown of Fth by siRNA decreased the protein level of Fth as indicated by Western blotting. Lower expression of Fth and Ftl was observed in siFth alone group, compared with the control group (Figure 8A). CCK-8 assay was used to assess cell survival rate for choosing the time course of the damage and the dose of melatonin. The results showed that the general trend of cell survival rate was decreased and reached the bottom with 62% at 24 hours after injury, then increased gradually afterward (Figure S8A). In addition, a smooth increase with the increment of the dose reached a peak at 10 μmol/L dose (P < .01, compared with control, Figure S8B). Therefore, the cells were treated for 24 hours after scratch injury, and an appropriate dose of melatonin treatment was chosen: 10 μmol/L.
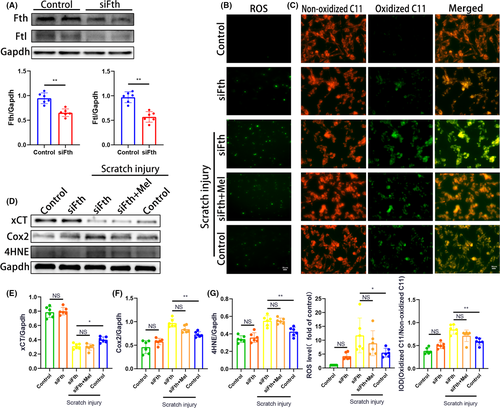
Given lipid peroxidation is a hallmark of ferroptosis, we assessed the cytosolic ROS using 2’7’-dichlorodihydrofluorescien diacetate (DCF) and BODIRY 581/591 C11 as convenient proxies. As shown in Figure 8B, the fluorescence intensity increased significantly in cells of siFth + scratch injury group, compared with the control + scratch injury group, indicating that mechanical scratch injury-induced the upregulation of ROS production was enhanced by siFth in HT-22 cells. Importantly, treating siFth-transfected HT-22 cells with melatonin failed to significantly reduce the upregulation of ROS production following scratch injury, compared with the non-treated siFth group (Figure 8B). In addition, siFth alone could not significantly increase the ROS production relative to the control group under physiological conditions. Resistance to ferroptosis correlated with suppression of BODIRY-C11 oxidation. Similar to the above ROS results, we found mechanical scratch injury significantly promoted lipid peroxide formation with red fluorescence of the BODIPY shifted to green fluorescence, indicating that scratch cell injury rises to lipid peroxidation (Figure 8C). The upregulation of lipid peroxidation following scratch injury was facilitated by siFth in HT-22 cells. Importantly, treating siFth-transfected HT-22 cells with melatonin failed to significantly reduce BODIRY-C11 oxidation following scratch injury, compared with the non-treated siFth group (Figure 8C).
We then measured several putative biomarkers of ferroptosis, including xCT, Cox2, and 4HNE expression. As expected, a significant decrease of xCT expression along with a significant increase of Cox-2 and 4HNE expression were observed in siFth-transfected HT-22 cells subjected to scratch injury, compared with the control + scratch injury group (Figure 8D-G). Importantly, treating siFth-transfected HT-22 cells with melatonin failed to significantly change the expression of xCT, Cox2, and 4HNE following scratch injury, compared with the non-treated siFth group (Figure 8D-G). In addition, there was no significant difference in the expression of the proteins mentioned above between siFth alone and control groups under physiological conditions.
Considering melatonin's effect depends on its receptors, we then checked melatonin receptors in HT-22 cells. Immunofluorescence staining showed that both MT1 and MT2 expressed in HT-22 cells subjected to mechanical scratch injury or not (Figure S8C). There was no significant difference in MT1 and MT2 expression between control and siFth groups subjected to scratch injury or not (Figure S8D), suggesting that siFth did not affect the expression of melatonin receptors. In addition, melatonin treatment did not reverse the expression of MT1 and MT2 in siFth group following scratch injury, indicating that siFth did not affect melatonin's effects on its receptors after injury (Figure S8E).
These results suggest that HT-22 cells transfected with siFth are susceptible to mechanical scratch injury-induced lipid peroxidation and ferroptosis, while melatonin did not statistically attenuate ferroptosis after injury and siFth did not affect the expression of melatonin receptors.
4 DISCUSSION
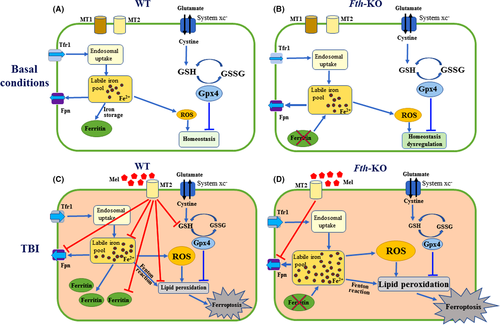
To the best of our knowledge, this is the first study to elucidate a novel protective role of melatonin and the ferroptosis inhibitor Lip-1 against TBI-induced ferroptosis. Here, we provide evidence that melatonin produces cerebroprotection in a mouse TBI model, at least partly by inhibiting neuronal Fth mediated ferroptosis, as shown schematically in Figure 9. Based on this model, loss of ferritin H in neurons increases ROS production, resulting in mildly impaired iron and facilitating the susceptibility to TBI-induced ferroptosis. Importantly, we found the neuroprotection by melatonin following TBI was mostly lost in Fth-KO mice.
Most of the studies evaluated the effects of melatonin on cellular, histopathological and behavioral outcomes following TBI, including but are not limited to: markers of oxidative stress, inflammatory cytokines, edema, lesion size, and cognitive impairment.51 Although melatonin exerts multiple protective effects on treating TBI, including anti-apoptotic, anti-oxidative, and anti-inflammatory properties,24 there is no information regarding the effects of melatonin on ferroptosis after TBI. As a novel form of cell death, ferroptosis plays an important role in various pathological conditions, including TBI. Zhang et al (2013) demonstrated that deferoxamine (DFO, an iron chelator) attenuated iron overload-induced toxicity in rats with TBI.52 Subsequently, Xie et al (2019) also demonstrated that Fer-1 (an anti-ferroptosis agent) treatment reduced iron deposition and attenuated behavioral outcomes following TBI.4 Similarly, baicalein (a 12/15-lipoxygenase inhibitor) attenuated ferroptosis signaling and cognitive outcome after TBI.7 Together, the previous reports indicated that ferroptosis is involved in the pathogenesis of TBI. However, the underlying mechanisms of ferroptosis following TBI and the anti-ferroptosis actions of melatonin have still not been clarified. Our current study demonstrated TBI induced the time-course changes of ferroptosis-related protein expression from 1 hour to 14 days in the ipsilateral cortex, but both melatonin and Lip-1 (an inhibitor of ferroptosis) administration significantly attenuated TBI-induced ferroptosis, neuronal damage, lesion volume, and behavioral deficits. Considering the half-life of melatonin is short,53 the animals received melatonin (10 mg/kg, i.p.) 1 hour after TBI and once a day until sacrifice in this study. The effects of melatonin are observable both after a single administration (at 1 day) and after repeated administration (eg, at two or more days). Future studies should also address the other treatment duration of melatonin (eg, 0, 2, and 4 hours post-TBI), as described previously.22, 51
One of the typical characteristics of ferroptosis is lipid ROS accumulation. Measuring lipid peroxidation is necessary for determining whether ferroptosis occurs in specific contexts. Numerous studies have suggested that endogenous glutathione (GSH) exerts a neuroprotective effect against TBI. Cortical GSH content is significantly reduced following TBI in rats54 and mice.55 A naturally occurring genetic variation in the expression of glutathione-S-transferase-4, a GSH-dependent enzyme that reduces 4HNE, affects the extent of neurodegeneration following TBI.56 Importantly, as a sodium-independent cystine-glutamate antiporter, system xc_ (xCT) and its key component Slc7a11 are also responsible for the generation of intracellular GSH against oxidative stress. Inhibition of xc_ depletes GSH levels and impairs glutathione peroxidase 4 (Gpx4) activity, thereby increasing lipid peroxidation. Certain membrane lipids have been found to be oxidized during ferroptosis. Our previous report proved that genetically deleting Slc7a11 facilitates the onset of ferroptosis specifically under high-iron conditions, although it was not sufficient to induce ferroptosis in basal conditions.57 Withal, conditional deletion of Gpx4 in forebrain neurons promotes cognitive impairment and neurodegeneration.58 Ptgs2, also known as Cox2, is a key enzyme in prostaglandin biosynthesis and acts both as a peroxidase and as a dioxygenase.59 Intriguingly, in our current study, we presented the novel finding that TBI resulted in the downregulation of GSH levels, the upregulation of xCT, Cox2, 4HNE, and MDA levels, as well as neuronal damage and neurobehavioral abnormalities. Interestingly, both melatonin and Lip-1 reversed the changes mentioned above. In addition, we found the expression of MT1 and MT2 reduced in a time-dependent fashion after TBI, while melatonin inhibited the MT1 and MT2 deficiency following TBI. However, there is no report about how melatonin treatment prevents a decrease in MT1 and MT2 expression after insults. More research work needs to address this in the future. Importantly, pretreatment with 4P-PDOT and Luzindole abolished the effect of melatonin on ferroptosis-related Cox2 expression following TBI, indicating that this effect of melatonin was blocked by MT1 and MT2 receptors antagonists. Notably, pretreatment with 4P-PDOT (10 mg/kg) and Luzindole (60 mg/kg, but not 30 mg/kg) significantly abolished the effect of melatonin on MT2, xCT, and 4HNE expression, as well as TBI-induced GSH decease. We speculate that the dosage of luzindole matters or heterodimerization of melatonin receptors may affect the agonist response of luzindole.60 Given Luzindole has a higher affinity toward the MT2 receptor than the MT1 receptor subtype,48 the data of the present study suggest that the MT2 receptor is the major melatonin receptor subtype likely involved in this phenomenon. We propose melatonin as a pharmacological and therapeutic agent to inhibit TBI-induced ferroptosis, likely dependent on the MT2 receptors. Considering melatonin receptors are G-protein-coupled receptors (GPCRs), the signaling cascade downstream of G proteins involves ERK1/2 activation by MT1 mediated though the Gi/o subfamily, while ERK1/2 activation by MT2 is dependent on the cooperative activation of Gi/o and Gq/11 proteins.61 Moreover, inhibition of MAPK/ERK activation is correlated with anti-ferroptotic mechanisms in a renal ischemia/reperfusion injury (IRI) model.62 Additional studies are necessary to elucidate whether Gi and Gq signaling modulates intercellular iron and prevents ferroptosis in our experimental TBI model. To our knowledge, this study is the first study directly investigating the effects of melatonin on ferroptosis after TBI. These findings indicate that a strategy using melatonin aimed at attenuating lipid peroxidation may be a viable approach to mitigate ferroptosis and the subsequent long term physical, cognitive, and emotional deficiencies caused by TBI.
Another characteristic of ferroptosis is iron accumulation. Iron homeostasis appears to play an important role in the pathobiology of TBI.63 As a crucial micromineral required for all living beings, iron serves as a co-factor in heme and the iron-sulfur cluster (eg, nonheme iron enzymes). However, abnormal iron deposition in specific regions of the brain is directly involved in the poor cognitive outcome, since nonheme bound iron accumulates in deep gray matter areas of the brain and may contribute to secondary pathological injury following TBI.64 Microbleeds, which can be detected by Perls’ Prussian blue iron staining, reveal an abnormal accumulation of iron that is toxic to brain-specific cells (including neurons, astrocytes, and microglia).65 We found that the number of Perls’ positive cells elevated at 7 days following TBI and continued to increase gradually afterward, but fewer iron-positive cells were observed in both melatonin and Lip-1–treated groups than the vehicle-treated group. In addition, we found although the levels of nonheme bound Fe3+ were elevated in both serum and the ipsilateral cortex following TBI, the accumulation of redox-active ferrous iron (Fe2+) was only observed in the ipsilateral cortex, which is considered more problematic.66 Importantly, melatonin and Lip-1 inhibited TBI-induced the upregulation of Fe2+, Fe3+, and total iron content in the injured cortex, along with Fe3+ in serum. The higher level of cortical nonheme iron in Fth-KO mice was supported by more intense Perls’ blue staining of brain sections. These findings underscore the importance of maintaining iron homeostasis in the brain.
In mammals, the uptake, transport, and storage of iron are tightly coordinated by various proteins and pathways to maintain iron homeostasis at both the cellular and systemic levels. In the central nervous system (CNS), cells, including neurons, have evolved highly regulated mechanisms for controlling cellular iron levels, including the iron-storage protein ferritin, iron importer transferrin receptor 1 (Tfr1), and iron exporter ferroportin (Fpn).67 Ferritin has regarded as the endpoint protein to store and remove excess iron to reduce cellular damage and stress. In contrast, autophagic degradation of ferritin mediated by the specific cargo receptor NCOA4 has been demonstrated to accumulate labile iron and promote ferroptosis.68 Dysregulation of iron metabolism in brain following TBI can result in the accumulation of redox-active ferrous iron in various brain cells. This is possibly due to alterations in the expression/function of regulatory proteins such as ferroportin and ferritin, which fail to export iron from cells and thereby increase the labile iron pool. As a key iron-handling protein in the brain, ferritin is elevated in the serum of patients with TBI as well as the human contused brain, indicating that ferritin might be involved in the pathophysiological process and could be a therapeutic target for patients with TBI.13, 14 Ferritin levels can also be an interesting postmortem biomarker to estimate wound age after TBI and the survival time of traumatic fatalities.69 As the main form of intracellular iron storage, ferritin is composed of heavy (Fth, also known as Fth1) and light (Ftl) chain subunits. Fth has ferroxidase activity, converting Fe2+ to Fe3+, whereas Ftl takes up and maintains iron content by nucleation. Although ferritin plays a vital role in iron metabolism by storing excess cellular iron, its precise function in the brain and whether it involves melatonin's neuroprotection remain unexplored. Considering a straight knockout of Fth gene provokes embryonic lethality in mice,15 we and others previously tried to generate cardiac or liver-specific Fth conditional knockout mice.19, 21 The present results extend these studies to the brain under physiological and pathological conditions. In the CNS, Fth is mainly in neurons, whereas Ftl is enriched in microglia cells.17, 18 To characterize the functional role of ferritin H (Fth) in the brain, we generated neuron-specific Fth conditional knockout mice (Fth-KO) in the present study. We found that although the free iron pool (Fe2+) is not dramatically altered in the cortex of Fth-KO mice, both Fe3+ and the total nonheme iron levels increase under physiological condition. As a result, the accumulated iron inactivates the iron-responsive element (IRE)-binding capacity of iron regulatory proteins (IRPs), which leads to an increase in the stability of Fpn mRNA and suppress the translation of Tfr1 mRNA.21 This is mainly because both Tfr1 and Fpn are post-transcriptionally regulated by the IRP/IRE system through the IREs at the 3’UTR of the Trf1 gene and 5’UTR of Fpn gene in an iron-dependent manner.70 Upon Fth gene knockout, iron cannot be redeposited into ferritin and leaves the tissue as observed for cortex. Our data provide clear evidence for the requirement of ferritin H in iron storage and homeostasis under physiological conditions. When the Fth gene is knocked out in neurons, free iron in these cells cannot be redeposited and stored in ferritin even with the normal physiological conditions, thereby resulting in mildly impaired iron homeostasis. Moreover, the above phenotype is severely exacerbated with TBI, leading to brain dysfunctions, suggesting Fth-KO mice with TBI are more susceptible to ferroptosis-involved brain injury, relative to the wild-type group. Mechanistically, the loss of neuronal Fth in Fth-KO mice promotes Slc7a11-dependent ferroptosis following TBI, consisting of loss of GSH, and the increase of xCT, Cox2, and 4HNE, thereby leading to exacerbated neuronal damage after TBI. In addition, the importance of ferritin is highlighted by the fact that phenotype changes such as induction of free Fe2+ and total iron following TBI were severely increased in Fth-KO mice compared with wild-type mice, which are supported by Perls’ blue staining, and reflected by a significant no significant changes of Tfr1 and a marked decrease of Fpn expression in Fth-KO mice. The high redox potential of iron may underlie its toxicity, as excessive Fe2+ results in an accumulation of toxic ROS and lipid peroxidation, leading to ferroptosis.
To exploit whether loss of neuronal Fth affects melatonin's protection against TBI-induced ferroptosis, we treated Fth-KO mice with melatonin after TBI and investigated histologic and molecular parameters. We found that treating Fth-KO mice with melatonin did not rescue TBI-induced brain damage such as ferroptosis, neuronal degeneration, supporting the notion that melatonin produces cerebroprotection predominantly via inhibiting neuronal ferritin H-mediated ferroptosis, although its protection might not be solely dependent on this pathway. Similarly, the induction of lipid peroxidation following mechanical scratch injury in vitro was facilitated by Fth siRNA in HT-22 cells, but melatonin treatment failed to significantly prevent ferroptosis in neuronal cell lines transfected with Fth siRNA. Noteworthy, the outcome mentioned above in vivo and in vitro may result from the fact that as a multifunctional agent, melatonin can exert its neuroprotective effects after TBI through multiple mechanisms.4, 24 Although ferroptosis is emerging as an important mechanism of cell death, other death pathways (eg necrosis, apoptosis, necroptosis, autophagy, and neuroinflammation) are simultaneously activated and modulated by TBI and melatonin.4, 24 For instance, we showed elevated expression of Nox2 in a time-dependent fashion after TBI, and melatonin prevented TBI-induced increase of Nox2 expression in wild-type mice, demonstrating melatonin's anti-oxidative stress following TBI. However, a recent study reports that Nox2 activation is critical for the enhanced inflammatory response in microglia/ macrophages and neuronal damage following TBI,71 providing a cellular and molecular mechanism linking neuroinflammation and oxidative stress following TBI. It is noted that autophagy is activated to degrade ferritin (a process known as ferritinophagy), which is mediated by the cargo receptor NCOA4, thereby increasing iron levels and leading to oxidative injury.72 Future research needs to exploit the effects of neuron-specific Fth conditional knockout on other action mechanisms of melatonin such as neuroinflammation and autophagy following TBI. In addition to neurons, we found Fth also seldom expressed in other brain cells such as astrocytes and microglia (Figure S6A). The fate of excess iron in the absence of ferritin H in astrocytes and microglia remains elusive. Additional studies are also necessary to elucidate the effects of Fth on other brain areas during the pathogenesis of TBI.
The brain is vulnerable to the increase of ROS which predominantly manifests as lipid peroxidation. Lipid peroxidation is regarded as the driving force of ferroptosis. Although the role of ferroptosis in the pathophysiological process of TBI has been illustrated, future research is required to investigate whether ferroptosis could serve as an intervention target for TBI. To our knowledge, our study represents the first analysis of Lip-1 administration for ferroptosis inhibition after TBI in vivo. Considering distinct mechanisms of ferroptosis inhibition exist, the action of its inhibitors appears to act through different mechanisms. The characterization of new inhibitors should be accompanied by an evaluation of iron-chelating or antioxidant activity. For example, melatonin exhibits antioxidant activity, which is probably based on their ability to inhibit ferroptosis. In our present study, we found melatonin treatment exhibits iron-chelating and antioxidant activity in wild-type mice following TBI, similar to a specific inhibitor of ferroptosis Lip-1. Thus, our findings underscore the protective role of melatonin in inhibiting ferroptosis, supporting the notion that melatonin is an excellent inhibitor of ferroptosis. The notion is supported by two recent reports documenting that (a) melatonin could be utilized as a pharmacological and therapeutic agent to suppress ferroptosis and platelet activation73; and (b) melatonin attenuates the hypoxic-ischemic brain damage in neonatal rats by suppressing neuronal ferroptosis via the Akt/Nrf2/Gpx4 pathway.74 Importantly, we found that loss of ferritin H in neurons almost abolished the neuroprotection of melatonin against ferroptosis following TBI, further demonstrating the important role of ferritin H in maintaining the protection of melatonin against TBI-induced ferroptosis.
In conclusion, we report that melatonin produces cerebroprotection in a mouse TBI model, via inhibiting neuronal ferritin H-mediated ferroptosis. The conclusion is based on four key findings. First, both melatonin and the ferroptosis inhibitor Lip-1 can rescue the characteristic features of TBI-induced ferroptosis associated with neurological outcome in wild-type mice. Second, the protective effect of melatonin against ferroptosis induced by TBI is mainly dependent on melatonin receptor 1B (MT2). Third, loss of neuronal ferritin increases the susceptibility to ferroptosis via an increase in lipid ROS and iron metabolism dysfunction following TBI. Finally, treating Fth-KO mice or Fth siRNA neuron cell line with melatonin mostly failed to rescue TBI-induced ferroptosis. Finally, Fth-KO or siFth itself did not affect the expression of melatonin receptors and melatonin's effects on its receptors after injury. The present study sheds new light on the understanding of the diverse biological functions of melatonin and provides a path for investigating the anti-ferroptosis actions of melatonin following TBI. Considering the anti-ferroptosis potential of melatonin, it could be a potential therapeutic target for treating TBI.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (81530062, 81971163, 81971800, 31530034, and 31930057), the National Key R&D Program (2018YFA0507800), Suzhou Municipal Science and Technology Bureau (SYS2019027), China Postdoctoral Science Foundation (2020T130583), and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
CONFLICT OF INTEREST
The authors have no competing financial interests to declare.
AUTHOR CONTRIBUTIONS
CL, LT, FW, and TR designed the experiments. TR, HW, QL, YC, YG, XM, GC, CG, CW, ZG, SS, JZ, ZW, TW, MZ, and CL performed the research. XC, JM, LT, FW, and CL provided intellectual contributions throughout the project. FW, XF, and JZ contributed essential reagents or tools. TR, HW, and CL analyzed the data. CL and TR wrote the manuscript. All authors have read and approved the final manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the Supplementary Material of this article.




