Multigene Sequence Analysis of Aster Yellows Phytoplasma Associated with Primrose Yellows
Abstract
Primula acaulis (L.) Hill. plants showing stunting, leaf-yellowing and virescence were first discovered in the Czech Republic. Polymerase chain reactions with subsequent restriction fragment length polymorphism analyses and sequencing enabled classification of the detected phytoplasmas into the aster yellows group, ribosomal subgroup 16SrI-B, tufI-B, rpI-B, groELIB-III and SecY-IB subgroups. Phylogeny of the 16S rRNA gene sequences as well as sequence analysis of several chromosomal regions, such as the 16S-23S ribosomal operon, ribosomal proteins, spc ribosomal protein operon, genes for elongation factor EF-Tu, molecular chaperonin large subunit GroEL, immunodominant membrane protein, ribosome recycling factor, urydilate kinase, ATP- and Zn2+-dependent proteases not only confirmed its affiliation with the ‘Candidatus Phytoplasma asteris’ species but also enabled its detailed molecular characterization. The less researched regions of phytoplasma genome (amp, adk, hflB, pyrH-frr genes) could be valuable as additional markers for phytoplasma through differentiation especially within the 16SrI-B ribosomal subgroup.
Introduction
Phytoplasmas are bacteria lacking a cell wall that inhabit the phloem of plants as well as tissues of their insect vectors. They affect many plant species including vegetables, fruit trees, conifers and ornamental plants worldwide. Severe devastation in crops, yield losses, decreasing of fruits quality and death of plants is often the final result of these infections (Lee et al. 2000). There are more than thirty groups and 100 subgroups of phytoplasmas known today based on restriction fragment length polymorphism (RFLP) analysis of the 16S rRNA gene (Lee et al. 2010a). Aster yellows (AY) phytoplasmas comprise the most diverse and widespread group (16SrI). The strains in the AY phytoplasma group share ≥97% identity in their 16S rDNA sequences (Lee and Davis 2000). Within the AY group, 20 subgroups (16SrI-A to 16SrI-Y) were identified (Fránová et al. 2014), and doubtless the list could be extended with further discoveries (Tseng et al. 2014; Naderali et al. 2015). The ‘Candidatus Phytoplasma asteris’ concept takes in all known subgroups within the 16SrI group, where the subgroup 16SrI-B is the largest and most diverse strain cluster. The gene 16S rRNA is present in all prokaryotes, but it is not sufficiently variable for distinguishing more discerningly among phytoplasmas of subgroup 16SrI-B. For this reason, more variable regions of the phytoplasma genome are being sought. The 16SrI group has been differentiated using the tuf gene, the ribosomal protein operon (rp), the 16S-23S rRNA intergenic spacer region (Marcone et al. 2000; Botti and Bertaccini 2003), the secY gene (Lee et al. 2006) and the groEL gene (Mitrović et al. 2011).
Primrose (Primula acaulis (L.) Hill.) is a species of flowering plant in the family Primulaceae native to western and southern Europe (from the Faroe Islands and Norway south to Portugal and east to Germany, Ukraine, the Crimea and the Balkans), north-western Africa (Algeria) and south-western Asia (eastern Turkey to Iran). Primrose is widely planted as an ornamental garden and potted plant and grown commercially as one of the earliest spring flowers available in a variety of bright colours. Phytoplasmas infecting primrose plants have been recorded only in European countries: France (primula red [PrR] and primula orange [PrO; the EU phytoplasma collection, http://www.ipwgnet.org/doc/phyto_collection/collection-august2010.pdf; items 113c and 113e, respectively], Germany [primula virescence (PRIVA, PRIVB, PRIVC; Schneider et al. 1993) and primula yellows (PY; Keane et al. 1996; Vibio et al. 1996)], Italy [yellows of primrose (IprY; Marcone et al. 2000)], and the UK [primula green yellows (PrG; Mitrović et al. 2011) and primula blue yellows (PrB; Makarova et al. 2012) strains). All strains belong to the 16SrI ribosomal group, except PrB that is a member of 16SrII-B.
We report here the multigene analysis of the ‘Ca. P. asteris’ strain associated with primrose yellows disease in the Czech Republic. Genome sequences from primrose phytoplasma were compared with others in GenBank to identify genes with higher nucleotide variability suitable as additional marker genes for phytoplasma differentiation at 16SrI-B subgroup level.
Materials and Methods
Symptoms, electron microscopy and DNA extraction
In October 2012, two P. acaulis plants with stunting, leaf-yellowing, necrosis and flower virescence (Fig. 1a) were observed in a private garden in South Bohemia. Infected plants neither produced seeds nor survived through the following winter.

Ultrathin sections from flower stalks of diseased and asymptomatic control primrose plants were examined using standard techniques and a JEM 1010 transmission electron microscopy (TEM).
Leaf midribs and flowers with stems of diseased P. acaulis plants were subjected to chloroform/phenol DNA extraction followed by isopropanol precipitation. The following, previously identified phytoplasmas were employed as positive controls: aster yellows (AY; ribosomal subgroup 16SrI-B, host: Catharanthus roseus L. Don), clover phyllody (CPh; 16SrI-C, host: Echinacea purpurea (L.) Moench.), clover yellow edge (16SrIII-B, host: E. purpurea) and apple proliferation (16SrX-A, host: Malus domestica L. cv. Idared) (Přibylová et al. 2001; Fránová et al. 2009, 2013a,b). DNA isolated from leaf midribs of one healthy-looking primrose and a no-template control were employed as negative controls in all PCR assays.
Phytoplasma identification by 16S-23S rRNA operon analyses
The primer pair P1/P7 (Deng and Hiruki 1991; Schneider et al. 1995) was used to prime the amplification of a 1.8-kbp product encompassing the 16S ribosomal RNA (rRNA) gene, the intergenic spacer region (ISR) and the start of the 23S rRNA gene region of the phytoplasma genome. The polymerase chain reaction (PCR) products were diluted 1 : 29 with sterile distilled water prior to nested amplification using the general R16F2n/R2 (Lee et al. 1993; Gundersen and Lee 1996) and group-specific R16(I) F1/R1, R16(III) F2/R1 (Lee et al. 1994) and R16(X) F1/R1 (Lee et al. 1995) primer pairs.
Approximately 100 ng of DNA from each R16F2n/R2 amplicon was separately digested with 2.5 U of HhaI, KpnI, MseI and RsaI restriction enzymes for ≥16 h according to the manufacturer's instructions (New England Biolabs, Beverly, MA, USA).
Classification of identified phytoplasma was additionally checked using iPhyClassifier interactive online tool for in silico RFLP analyses (Zhao et al. 2009).
Amplification of the 23S rRNA gene was performed using the P23S5F3/A23S3R3 primer pair (approximately 2.7 kb of PCR product) as described by Guo et al. (2000). To obtain a nearly full sequence of the 23S rRNA gene, both the 16-23S ISR and 23S rRNA gene sequences were amplified by a direct PCR assay using primer P3 (Smart et al. 1996) and new primers named AY23S_2529rev (5′–TGGTTCGGTCCTCCATTTAG-3′) as well as AY23S_2510forw (5′–TGGTTCGGTCCTCCATTTAG-3′) and AY23S_4005rev (5′–AGTCAAACTGCCCACCAAAC-3′) amplifying 1012 bp and 1495 bp, respectively. PCR conditions for amplification with primers P3/AY23S_2529rev were as described by Schaff et al. (1992). For primers AY23S_2510forw/AY23S_4005rev, they were as follows: a denaturation step of 2 min at 94°C followed by 35 cycles consisting of 1 min at 94°C, 2 min at 55°C and 2 min at 72°C, then a final extension step of 10 min at 72°C.
The evolutionary history was inferred using the maximum-likelihood method based on the Tamura 3-parameter model (Tamura 1992). Initial trees for the heuristic search were obtained automatically by applying neighbour-joining and BioNJ algorithms to a matrix of pairwise distances estimated using the maximum composite likelihood (MCL) approach and then selecting the topology with superior log likelihood value. A discrete gamma distribution was used to model evolutionary rate differences among sites [five categories (+G, parameter = 0.6658)]. Evolutionary analyses were conducted in MEGA6 (Tamura et al. 2013).
Phytoplasma multigene sequence analyses
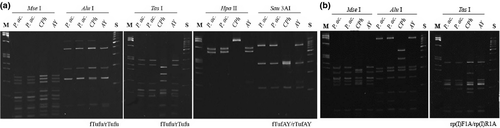
Primrose samples and controls were screened using Tuf1f/r (1.1 kbp) primers by direct PCR, followed by fTufu/rTufu (840 bp) and fTufAy/rTufAy (950 bp) in nested PCR to amplify the portion of the tuf gene encoding for the elongation factor EF-Tu (Schneider et al. 1997). RFLP analyses of the fTufu/rTufu and the fTufAy/rTufAy amplicons were then performed using the AluI, MseI and TasI restriction enzymes for the former and HpaII and Sau3AI for the latter.
A nested PCR using the primer pair rpF1/rpR1 (Lim and Sears 1992) followed by rp(I)F1A/rp(I)R1A (Lee et al. 2004) was used to amplify a phytoplasma DNA segment of the ribosomal protein operon which encompassed the genes rps19, rpl22 and rps3. The products were digested with AluI, MseI and TasI restriction enzymes.
Two primer pairs – groelAYF1/groelAYR1 (2.2 kbp) and groelAYend/qns1 (1.35 kbp) – were applied according to Šeruga Musić et al. (2014) to amplify the portion of the groES gene and the genomic region encompassing groEL and amp genes. The groES encodes chaperonin small subunit GroES (cpn10), while groEL and amp encode, respectively, chaperonin large subunit GroEL (cpn60) and antigenic membrane protein. For RFLP classification, DNA was amplified with AYgroelF/AYgroelR (1.4 kbp) primers and digested with TruI and AluI according to Mitrović et al. (2011). Nevertheless, conditions for amplification were modified as follows: a denaturation step of 2 min at 94°C followed by 35 cycles consisting of 1 min at 94°C, 1 min at 50°C and 2 min at 72°C, then a final extension step of 10 min at 72°C.
For finer characterization, a direct PCR using the primer pair BB88F1/BB88R1 (740 bp, Gundersen et al. 1996) was used to amplify phytoplasma DNA segments of the portion of the ribosome recycling factor (frr) and urydilate kinase (pyrH). ATP- and Zn2+-dependent proteases (hflB) gene was amplified using primers AY19p/m (1.150 bp, Schaff et al. 1992). Both amplicons were digested with AluI and MseI restriction enzymes. To obtain the full sequence of the hflB gene, DNA from symptomatic plants was amplified by a direct PCR assay using primer AY19p and a new primer named hflBprebegin (5′-GAATGTGTGGTCGAATAG-3′) under conditions as described by Schaff et al. (1992). AY19p/hflBprebegin primers amplify complete hflB and parts of neighbour genes (2.300 bp).
Ribosomal protein L15 (rpl15), protein translocase (secY), adenylate kinase (adk) and methionine aminopeptidase (map) genes (spc ribosomal protein operon) were PCR amplified with L15F1/MapR1 primers (2840 bp) in the first PCR amplification followed by group-specific nested PCR with primers L15F1A-a/MapR1A-a (2790 bp), L15F-646-a/MapR1A(I) (2180 bp) and L15F-806-a/MapR1A(I) (2030 bp) (Lee et al. 2010b).
All the PCR amplifications products (6 μl) and all the digested products were analysed by electrophoresis through 1% agarose and 8% acrylamide gels in 1 × TBE buffer, respectively. The DNA was stained with GelRed nucleic acid stain (Biotium, Inc., Hayward, CA, USA) and visualized under UV light at 312 nm using a transilluminator.
PCR amplicons (without non-specific bands) from one primrose plant (designated 14/2012) were purified using the QIAquick PCR Purification kit (QIAGEN, Hilden, Germany) and bidirectionally sequenced using a BIG DYE sequencing terminator kit (PE Biosystems, Warrington, UK) and ABI PRISM 310 sequencer (PE Applied Biosystems, Foster City, CA, USA). The sequences were compared with others in GenBank by BLASTn 2.2.16 service (http://www.ncbi.nlm.nih.gov/BLAST/Blast.cgi).
Results
Electron microscopy
Plant cells of flower stems partially or completely filled with spherical, ovoid or pleomorphic particles (average size 259 × 346 nm) were observed in cross sections of mature and immature phloem sieve tube elements of affected plants under JEM 1010 TEM (Fig. 1b). These particles were found in infected plants but not in asymptomatic ones. No other micro-organisms, such as bacteria, viruses or virus-induced structures, were observed.
Molecular identification of ‘Ca. P. asteris’ (16SrI-B)
When the primer pair P1/P7 was used in direct PCR assays, visible amplification products of approximately 1.8 kbp were obtained from both of the examined diseased primroses. In nested PCR with phytoplasma universal primers R16F2n/R2 and primers specific for the 16SrI phytoplasma group R16(I) F1/R1, products of the expected sizes of 1.2 and 1.1 kbp, respectively, were also obtained in symptomatic plants. No product was amplified using DNA from symptomatic primroses with primers specific for phytoplasmas belonging either to the 16SrIII [primers R16(III) F2/R1] or to 16SrX [primers R16(X) F1/R1] ribosomal groups.
Primrose samples individually analysed using RFLP of R16F2n/R2 amplicons showed the same restriction profiles with HhaI, KpnI, MseI and RsaI enzymes and indicated the presence of a phytoplasma with profile identical to the AY phytoplasma control, ribosomal subgroup 16SrI-B. This phytoplasma classification was additionally confirmed by in silico restriction analyses (Zhao et al. 2009) and revealed patterns characteristic of a 16SrI-B member – even for those enzymes not used in the real restriction experiments (results not shown).
A nearly full sequence of 23S rDNA was amplified from symptomatic plants by P23S5F3/A23S3R3, P3/AY23S_2529rev and AY23S_2510forw/AY23S_4005rev primers. The resulting sequence of 4469 bp obtained from genome of the phytoplasma-infecting primrose plant contained sequences of the 16S rRNA gene, the 16S-23S ISR and nearly complete 23S rRNA gene. The sequence was deposited in the GenBank database (accession number KJ494340). In detail, the partial 16S rDNA (1459 bp) as well as the complete sequence of the 16S-23S ISR (247 bp) of the genome of the phytoplasma-infecting primrose in the Czech Republic revealed 100% identity with the corresponding regions of ‘Silene vulgaris’ yellows phytoplasma strain SIL, a member of the 16SrI-B ribosomal subgroup (AC: HQ589186). A partial 23S rDNA (2764 bp) showed the highest nucleotide identity with that of onion yellows phytoplasma OY-M (AC: AP006628) (Table 1).
| Gene/Accession number | Related phytoplasma strain/host/origin | Identity (%) (bp identical/bp compared) |
|---|---|---|
| 16S-23S rRNA operon | ||
| AP006628 | Onion yellows phytoplasma OY-M/Chrysanthemum coronarium/Japan | 99.84 (4462/4469) |
| 16S rRNAa | ||
| HQ589186 | ′Silene vulgaris′yellows phytoplasma strain SIL/Silene vulgaris/Italy | 100.00 (1459/1459) |
| 16S-23S spacer region | ||
| HQ589186 | ′Silene vulgaris′yellows phytoplasma strain SIL/Silene vulgaris/Italy | 100.00 (247/247) |
| 23S rRNAa | ||
| AP006628 | Onion yellows phytoplasma OY-M/Chrysanthemum coronarium/Japan | 99.86 (2760/2764) |
| tuf genea | ||
| AB588121 | Porcelain vine witches′-broom phytoplasma/Ampelopsis brevipedunculata/South Korea | 100.00 (1021/1021) |
| AP006628 | Onion yellows phytoplasma OY-M/Chrysanthemum coronarium/Japan | 100.00 (1021/1021) |
| DQ402361 | Aster yellows phytoplasma strain WAY/watercress/Hawaii | 100.00 (1021/1021) |
| AJ271312 | Aster yellows phytoplasma isolate DEV/Delphinium hybrid/Germany | 100.00 (1021/1021) |
| rps19-rpl22-rps3 genes | ||
| DQ406588 | Aster yellows phytoplasma strain WAY/watercress/Hawaii | 100.00 (1109/1109) |
| rps19 genea | Too short sequence (length 10 bp) | |
| rpl22 gene | ||
| DQ406588 | Aster yellows phytoplasma strain WAY/watercress/Hawaii | 100.00 (390/390) |
| AY264855 | Aster yellows phytoplasma strain AVUT/aster/Germany | 100.00 (390/390) |
| AY264854 | Primrose virescence phytoplasma strain PRIVC/Primula sp./Germany | 100.00 (390/390) |
| rps3 gene | 33 phytoplasma strains | 100.00 (706/706) |
| cpn10-cpn60-amp genes | ||
| AB124811 | Phytoplasma sp. AYBG/Conodon dactylon/Thailand | 99.81 (3073/3079) |
| cpn10 genea | 17 phytoplasma strains | 100.00 (39/39) |
| cpn60 gene | 11 phytoplasma strains | 100.00 (1610/1610) |
| amp gene | ||
| KF686807 | ‘Brasica napus'phytoplasma isolate Brassica-8/Brassica napus/Croatia | 99.29 (697/702) |
| rplO-secY-adk-map genes | ||
| AP006628 | Onion yellows phytoplasma OY-M/Chrysanthemum coronarium/Japan | 99.47 (2440/2453) |
| rplO genea | ||
| AP006628 | Onion yellows phytoplasma OY-M/Chrysanthemum coronarium/Japan | 99.45 (182/183) |
| GQ351284 | Coorg black pepper yellows phytoplasma/Piper nigrum/India | 99.45 (182/183) |
| HQ285919 | Azalea little leaf phytoplasma/azalea/China | 99.45 (182/183) |
| secY gene | ||
| KF573433 | Lettuce yellows phytoplasma strain LetY-TX7 clone 3/lettuce/Texas | 100.00 (1242/1242) |
| KC354610 | Carrot yellows phytoplasma isolate ca2006/9/carrot/Serbia | 100.00 (1242/1242) |
| AY803167 | Aster yellows phytoplasma strain AV2192/Callistephus chinensis/Germany | 100.00 (1242/1242) |
| adk gene | ||
| AP006628 | Onion yellows phytoplasma OY-M/Chrysanthemum coronarium/Japan | 99.54 (651/654) |
| map genea | ||
| GU004341 | Maize bushy stunt phytoplasma strain MBS/maize/Mexico | 99.72 (357/358) |
| GU004337 | Paulownia witches’-broom phytoplasma strain PaWB/paulownia/Taiwan | 99.72 (357/358) |
| pyrH – frr genesa | ||
| AP006628 | Onion yellows phytoplasma OY-M/Chrysanthemum coronarium/Japan | 100.00 (692/692) |
| hflB gene | ||
| CP000061 | Aster yellows witches’-broom phytoplasma AYWB/Lactuca sativa/Ohio | 91.07 (1927/2116) |
- a Partial sequence of analysed gene(s) is available.
After the maximum likelihood-based phylogenetic analysis on 16S rDNA sequence, the tree with the highest log likelihood (−3054.0515) was selected. There were a total of 1207 positions in the final data set (n = 31) after gaps and missing data removal. This phylogenetic tree is in agreement with the results of RFLP analyses indicating a close relationship of ‘Primula acaulis’ yellows phytoplasma with several strains of 16SrI ribosomal group (Fig. 2).

Primrose phytoplasma multigene characterization
Gene for elongation factor EF-Tu
The tuf gene was amplified in direct (primers Tuf1f/r) and nested PCR (fTufu/rTufu; fTufAY/rTufAY). In both PCR assays, visible amplification products of the expected sizes were obtained only from symptomatic plants. The restriction patterns were indistinguishable from the reference strain of AY phytoplasma (Fig. 3a). A partial tuf gene sequence from primrose phytoplasma (1021 bp; AC: KJ494341) was entirely identical to those of members of the tufI-B subgroup: porcelain vine witches’ broom phytoplasma (AC: AB588121), OY-M, AY phytoplasma strain WAY (AC: DQ402361), and AY phytoplasma strain DEV (AC: AJ271312) (Table 1).
Ribosomal protein rps19, rpl22 and rps3 genes
PCR assays using the rpF1/rpR1 primer pair obtained no amplicons of expected length 1240 bp from any of those primrose plants examined, while nested PCRs with rp(I)F1A/rp(I)R1A resulted in fragments of the expected lengths of 1210 bp in symptomatic plants but not in the negative controls. The restriction patterns of the products from primrose phytoplasmas were in agreement with AY (Fig. 3b). The obtained sequence (1109 bp; AC: KJ494343) contained rps19, rpl22 and rps3 genes and was deposited in GenBank. The complete rpl22 (390 bp) gene sequence was identical to those of AY phytoplasma strain WAY (AC: DQ406588), AY phytoplasma strain AVUT (AC: AY264855) and primrose virescence phytoplasma strain PRIVC (AC: AY264854), a member of the rpI-B ribosomal protein subgroup. A partial sequence of rps3 (706 bp) was identical to those of 33 records available in GenBank originating from phytoplasmas belonging in particular to the rpI-B subgroup infecting various plants worldwide.
Molecular chaperonin subunits (GroEL, GroES) and antigenic membrane protein
Amplicons from primroses’ and from AY and CPh controls’ nucleic acid extracts obtained with groelAYF1/groelAYR1, groelAYend/qns1 primers were of the expected size. PCR/RFLP procedure conducted according to Mitrović et al. (2011) with AYgroelF,R/TruI, AluI primer/enzyme combination resulted in bands of appropriate size together with several non-specific products (data not shown), while modified PCR conditions minimalized the presence of non-specific bands. Phytoplasmas from primroses and AY controls yielded then an identical RFLP profile (Fig. 4) as previously described for members of groELIB-III subgroup (Mitrović et al. 2011). The sequence of cpn10, cpn60 and amp genes (3079 bp) was deposited in the GenBank database (AC: KJ494342). Partial cpn10 (39 bp) and complete cpn60 (1610 bp) gene sequences were completely identical to those of 17 and 11 phytoplasma strains, respectively, available in GenBank (mostly members of the 16SrI-B ribosomal subgroup). The amp gene sequence (702 bp) was 99.29% identical with that of ‘Brassica napus’ phytoplasma (AC: KF686807). Nucleotide substitutions at five positions (2325: C→G, 2326: A→T, 2332: T→C, 2503: G→T, 2546: T→G) introduced four amino acid (AA) changes in the immunodominant membrane protein Amp (V to Q, I to T, G to V and D to E at the positions 107, 109, 166 and 180, respectively).
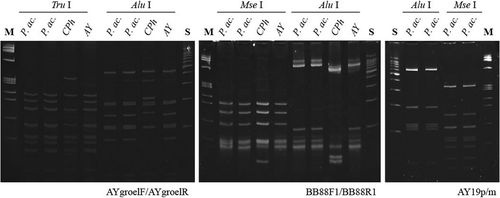
Ribosome recycling factor and urydilate kinase genes
The partial sequence of the ribosome recycling factor and urydilate kinase genes was amplified using primers BB88F1/R1. The restriction patterns from primrose samples obtained by the BB88F1/R1/AluI, MseI PCR/RFLP procedure were identical to those of AY but distinct from CPh (Fig. 4). The obtained sequence of 692 bp was deposited in GenBank (AC: KJ561217) and revealed 100% identity with those of OY-M. In addition, the nucleotide sequences of only AY witches’ broom phytoplasma AYWB (AC: CP000061, host: Lactuca sativa; Ohio, 16SrI-A), phytoplasma sp. DIV (AC: AY077837, host: Diplotaxis; Spain, 16SrI-B) and ‘Populus nigra’ phytoplasma clones 3B1, 2B9 and 1B4 (AC: JN029841, JN029840, JN029839, host: Populus nigra; Croatia, 16SrI-P) were found to be 92 to 99% identical with primrose phytoplasma.
ATP- and Zn2+-dependent proteases (hflB gene)
Fragments of expected length were obtained with primers AY19p/m only in symptomatic primroses but not in the AY and CPh controls. RFLP analyses of AY19p/m amplicons with AluI and MseI is demonstrated in Fig. 4. Amplicons of expected length (2.3 kbp) were obtained with primer pair AY19p/hflBprebegin from symptomatic primrose plants but not from the negative controls or the AY or CPh controls. The complete hflB gene sequence (2106 bp; AC: KJ580955) revealed the highest identity with those of AYWB, the reference strain of the 16SrI-A subgroup (cover 100%). Three segments of the reference strain were compared with the sequence presented here and identities of 91, 90 and 78% were calculated in the segments 209 263–211 371 bp, 418 730–420 835 bp and 204 820–205 181 bp, respectively. Similarly, sequence identities ranging from 99 to 74% was found in four matches with those of OY-M, the reference strain of the 16SrI-B subgroup. However, the query cover was 44% (position 465 311 to 465 484: 99% identity; 458 444 to 458 623: 93%; 404 877 to 405 452 and 819 862 to 820 370: 74%). The HflB protein from the primrose phytoplasma was similar to those of Chrysanthemum coronarium (AC: GAK73856, 88% identity) and AYWB (AC: WP011412469, 87%). The majority of nucleotide substitutions introduced 82 and 91 AA changes for the HflB protein of C. coronarium and AYWB, respectively.
Ribosomal protein spc operon
The ribosomal protein spc operon, containing rpl15, secY, adk and map genes, was amplified by L15F1/MapR1, L15F1A-a/MapR1A-a, L15F-646-a/MapR1A(I) and L15F-806-a/MapR1A(I) primer pairs. Symptomatic primrose plant samples exhibited a PCR band of expected size in all reactions. The obtained sequence of 2453 bp was deposited in the GenBank database (AC: KJ494344). A partial rpl15 gene sequence (183 bp) was found to be 99.45% identical with sequences from Coorg black pepper yellows (AC: GQ351284), azalea little leaf (AC: HQ285919) and OY-M phytoplasmas. Relative to AC: KJ494344, there is identical synonymous substitution in the single nucleotide at position 60 (A→G). The Czech phytoplasma from P. acaulis was found to be 100% identical in the secY gene (1242 bp) with three phytoplasma strains belonging to the 16SrI ribosomal group and infecting lettuce (AC: KF573433), carrot (AC: KC354610) and China aster (AC: AY803167), a member of the SecY-IB subgroup (Lee et al. 2006). The complete adk gene sequence (654 bp) revealed the highest identity (99.54%) to those of OY-M. Three nucleotide substitutions were recognized at the positions 1591 (T→G), 1599 (A→T) and 1955 (G→A), the first two of which introduced AA substitution from S to I (polar to non-polar) and F to I (both non-polar). A partial map gene sequence (358 bp) from the characterized primrose phytoplasma was nearly identical (99.72%) to those of maize bushy stunt (AC: GU004341) and paulownia witches’ broom (AC: GU004337) phytoplasma strains. Substitutions at positions 2135 (A→G) and 2153 (T→C) for phytoplasma strains from maize and paulownia, respectively, did not introduce substitution in the Map protein sequence. In Table 1, data on host and origin (North America, Asia, Europe and Oceania) of the most related phytoplasmas to the ‘Primula acaulis’ phytoplasma are shown.
Discussion
We demonstrate here for the first time deep multigene sequence analysis of ‘Ca. P. asteris’ (16SrI-B ribosomal subgroup) associated with primrose yellows as well as the presence of phytoplasma-like bodies in diseased primrose plants. In view of the fact that nucleotide sequences of several chromosomal regions (16S rRNA, 16S-23S rRNA ITS and 23S rRNA), as well as the genes tuf; rps19, rpl22, rps3; cpn10, cpn60, amp; pyrH, frr; rpl15, secY, adk, map and hflB were amplified and sequenced (altogether 14 929 bp), it seems that this phytoplasma strain is hitherto one of the best characterized among members of the 16SrI-B ribosomal subgroup by this approach.
Most of the chromosomal region and gene sequences were identical (100%) to those of 16SrI-B ribosomal subgroup members. However, nucleotide sequences of 23Sr RNA, rpl15, map, amp, adk and hflB genes of primrose phytoplasma from the Czech Republic showed differences (Table 1). Substitutions in single-nucleotide positions (SNPs) introduced changes in Amp and Adk proteins. In agreement with previously reported data for ‘Ca. P. asteris’ infecting oilseed rape in Croatia (Šeruga Musić et al. 2014), nucleotide substitutions at five positions of amp gene introduced four AA substitutions also in primrose phytoplasma. At the positions 107 and 109 in the Amp protein sequence, changes from non-polar to polar (V to Q and I to T, respectively) might have an impact on the protein structure and function, thus affecting the insect vector transmissibility and specificity. Additional two substitutions in protein sequence G to V and D to E (position 166 and 180, respectively) do not lead to polarity change. These results are consistent with previous reports that the central hydrophilic region (amino acid positions 33 to 203) of the amp gene is variable, thus suggesting that the Amp protein plays a biologically important role in the phytoplasma–host interaction (Barbara et al. 2002; Kakizawa et al. 2006).
The highest variability was revealed in the sequence of the hflB gene. In that gene, more than 80 and 90 AA substitutions were recognized in the HflB protein, in comparison with the protein sequences of Chrysanthemum coronarium and AYWB phytoplasmas, respectively, the most similar phytoplasma proteins available in GenBank. HflB genes encode membrane-associated ATP- and Zn2+-dependent proteases that are conserved among bacteria and are involved in membrane-associated processes such as protein secretion and membrane protein assembly as well as adaptations to nutritional conditions and osmotic stress (Bai et al. 2006). AY19p/m primer pair was designed on the basis of partial sequences of cloned AY MLO DNA fragment AY19 by Schaff et al. (1992). Relative to the complete sequence of AYWB phytoplasma genome, the AY19p primer is located at position from 34 to 51nt (from 5′end: ATG) of the upstream gene for a conserved hypothetical protein (ABC65305.1). This primer is specific for amplification of the partial sequence of the hflB gene copy, which is located on AYWB phytoplasma genome at position from 209 263 to 211 371 nt. AY19 m primer is located at position from 1024 nt to 1041 nt in this hflB gene copy (relative to the 5′end: ATG). To amplify the whole sequence of hflB gene, the primer hflBprebegin was designed at position 827 to 844 bp relative to the 5′end (from ATG) of a downstream gene for a hypothetical protein (ABC65303.1). In phytoplasma genomes, between six and 24 copies of hflB gene have been identified (Oshima et al. 2004; Bai et al. 2006; Arashida et al. 2008; Tran-Nguyen et al. 2008). The AYWB chromosome contains three copies of hflB gene located in potential mobile units (PMU) and also single copies of hflB homologues, which are clearly different in sequences from the PMU genes (Bai et al. 2006). In the present study, the hflB gene sequence from primrose phytoplasma revealed identity with those located at three different positions of AYWB phytoplasma genome.
Based on RFLP analyses, low and high degrees of polymorphism in the pyrH, frr (primers BB88F1/R1) and hflB (primers AY19p/m) genes, respectively, had been recognized in AY 16SrI-B phytoplasma strains by Botti and Bertaccini (2003). Nevertheless, the restriction profiles from identical amplicons of primrose phytoplasma, AY and CPh control strains did not correspond to any of those previously demonstrated. Specificity of AY19p/m primers only for some AY phytoplasma (Schaff et al. 1992; Botti and Bertaccini 2003) was confirmed; primers amplified DNA fragments only from diseased primroses, but not from the AY and CPh controls. RFLP analyses of AY19p/m amplicons revealed unique patterns not corresponding to any of the five AluI profiles (A, B, C, D and E) demonstrated for 25 phytoplasma strains of the 16SrI-B ribosomal subgroup (for comparison see Botti and Bertaccini 2003). We show here, for the first time, RFLP patterns obtained with AY19p/m amplicons and MseI.
The first phytoplasmas to be described in Primula sp. were associated with flower virescence. Strains PRIVA, PRIVB and PRIVC had been examined by RFLP analysis of 16S rDNA and all three were assigned to group I (Schneider et al. 1993). PRIVA, PRIVB and PRIVC together with IprY were classified into subgroups 16SrI-L/B tuf I-B, 16SrI-A tuf I-A and 16SrI-B tuf I-B, respectively (Marcone et al. 2000). Analysis of the ribosomal protein operon classified PRIVA, PRIVB and PRIVC strains into the rpI-B, rpI-A and rpI-B subgroups, respectively (Lee et al. 2004). PY was assigned into the 16SrI-B AY phytoplasma ribosomal subgroup using monoclonal antibodies (Keane et al. 1996) as well by PCR/RFLP assays (Vibio et al. 1996).
Nucleotide sequences from five phytoplasma strains infecting primrose (PRIVA, PRIVC, PrB, PrG, PrR) are available in the GenBank database. Comparison of the primrose phytoplasma sequences revealed identical gene sequences of the Czech ‘Primula acaulis’ yellows phytoplasma with those of the tuf (PRIVA, AC: AJ271320, subgroup: tufI-B), cpn60 (PRIVA and PrG, AC: AB599705 and AC: AB599696, respectively) and rps19, rpl22 and rps3 (PRIVC, AC: AY264854, subgroup: rpI-B) genes. Sequence identity of 16S rRNA between the Czech strain with those of members of the 16SrI-B ribosomal subgroup phytoplasma strain PrG (AC: HM590623) and PRIVC (AC: AY265210) was found to be 99.86 and 99.73%, respectively. Sequence identity of 99.92% in the secY gene was found between the Czech isolate and the PRIVC strain (AC: AY803176).
The findings presented here show that phytoplasmas of the 16SrI-B subgroup naturally infect and induce virescence and yellowing in P. acaulis. The characterized phytoplasma is the first one detected in P. acaulis within the Czech Republic. Moreover, the presence of phytoplasmas of the 16SrIII and 16SrX ribosomal groups was excluded by means of nested PCR using group-specific primers. Based on RFLP and sequence analysis of 16S rDNA, rpS19-S3 operon, as well as tuf and groEL genes, the Czech P. acaulis phytoplasma strain was assigned as a member of the 16SrI-B ribosomal subgroup, ribosomal protein rpI-B, tufI-B and groELIB-III subgroups. The comparison of secY gene sequence with those available in GenBank allowed classification of the phytoplasma into SecY-IB subgroup. RFLP analyses of phytoplasma segments of the pyrH, frr and hflB genes showed genetic variability; however, as unique, previously unreported patterns were obtained after digestion of BB88F1/R1 and AY19p/m amplicons with both MseI and AluI endonucleases. In addition, significant SNPs were found in the amp gene encoding a specific protein crucial for insect transmission specificity, the adk gene important for maintaining energy charge and regulating phospholipid synthesis, as well as the hflB gene encoding membrane-associated ATP- and Zn2+–dependent proteases. Therefore, these genes could be subjects for further research as possible additional markers for phytoplasma through differentiation especially within the 16SrI-B ribosomal subgroup.
Acknowledgements
The work was supported by institutional support RVO: 60077344. The authors are deeply indebted to Mrs J. Rakouská for her technical assistance.




