Occurrence of Passalora Leaf Spot Caused by Passalora ampelopsidis on Virginia Creeper in Korea
Abstract
A previously unknown leaf spot disease was observed on Virginia creeper (Parthenocissus quinquefolia) vines in Seoul, Korea. Affected plants exhibited leaf spots and discoloration, resulting in leaf blight and premature defoliation. The causal agent of the disease was identified as Passalora ampelopsidis by comparing morphological characteristics and analyzing the internal transcribed spacer region of the rDNA. Pathogenicity tests were conducted twice with the same results, fulfilling Koch's postulates. Passalora leaf spot on Virginia creeper vines was first recorded in the United States in 1878. Recent reports of Passalora leaf spots on Virginia creeper vines in 2005 in Germany, in 2012 in China and now in Korea indicate the prevalence of the disease.
Introduction
Virginia creeper or five-leaved ivy [Parthenocissus quinquefolia (L.) Planch.], belonging to the family Vitaceae, is a woody vine native to North America. Similar to its relative Boston ivy [P. tricuspidata (Siebold & Zucc.) Planch.], the Virginia creeper vine adheres to surfaces by disks and, therefore, is used as a bioshader on buildings with masonry walls (Ip et al. 2010). Several studies have examined the use of Virginia creeper as a wall plant and traffic noise barrier in Korea (Lee and Sim 1994; Jung et al. 2000). Although grown extensively as an ornamental plant, Virginia creeper is also considered a harmful weed affecting the production of fruit tree orchards and Fraser fir plantations in the United States. A number of studies have investigated methods for controlling Virginia creeper (Tworkoski et al. 1988; Tworkoski and Young 1990; Richardson et al. 2009).
In August 2011, a previously unknown leaf spot disease was observed for the first time on Virginia creeper vines in Seoul, Korea. Nearly 100% of the mature leaves and more than half of young leaves were affected, greatly reducing the aesthetic value of the vine. No previous records of Virginia creeper disease in Korea exist (The Korean Society of Plant Pathology 2009). To the best of our knowledge, this is the first report examining the causal agent of the disease affecting Virginia creeper using mycological characterization, molecular analysis and pathogenicity tests.
Materials and Methods
The fungus associated with leaf spots was removed from the lesions and mounted on a glass slide in a few drops of distilled water. The morphological characteristics of the fungal structures in fresh samples were examined by bright field- and differential interference contrast (DIC)-light microscopy, using an Olympus BX51 microscope (Olympus, Tokyo, Japan) for measurements and a Zeiss AX10 microscope equipped with AxioCam MRc5 (Carl Zeiss, Göttingen, Germany) for imaging. Thirty measurements were taken at 100, 200, 400 and 1000× magnification.
To isolate the pathogen associated with the leaf spots, affected leaves were collected. Small sections of leaf tissue exhibiting abundant sporulation were cut and, using a fine forceps, floated on a drop of sterilized water on a glass slide. Conidia dispersal in water was visualized using a dissecting microscope. A loop of spore suspension was streaked onto 2% water agar plates containing 200 mg/l streptomycin sulphate. Subsequent to 1–2 days of incubation at 20°C, germinated conidia forming small colonies were transferred individually onto potato dextrose agar (PDA; Difco Laboratories, Sparks, MD, USA) plates for subsequent studies.
To determine fungus pathogenicity, the culture was incubated on V8 juice agar at 26°C under near UV light conditions for 10 days to obtain conidia. Conidia were suspended in water containing a trace amount of Tween 80 and adjusted to 2 × 103 conidia/ml. Next, the conidia suspension was sprayed onto leaves of five potted Virginia creeper vines until run-off occurred. Five control plants were sprayed with sterilized water. The plants were covered with plastic bags to maintain 100% relative humidity for 48 h and then transferred to a greenhouse (26–32°C). Symptom development was examined every 24 h for 10 days. Pathogenicity tests were conducted twice.
To confirm identification based on morphological characteristics, genomic DNA was extracted from mycelia grown on PDA plates using the DNeasy Plant Mini Kit (Qiagen Inc., Valencia, CA, USA). The internal transcribed spacer (ITS) region of the rDNA was amplified with primers ITS1 and ITS4 as described by White et al. (1990). The PCR amplicons were purified using the QIAquick PCR purification Kit (Qiagen Inc.) and directly sequenced by Macrogen Sequencing Service (Macrogen, Seoul, Korea). The ITS rDNA sequences of a number of Mycosphaerellaceous fungi were retrieved from GenBank for phylogenetic analysis. The phylogenetic tree was constructed using the neighbour-joining method and Maximum composite likelihood model with MEGA6 (Tamura et al. 2013). The relative robustness of the individual branches was estimated by bootstrapping using 1000 replicates.
Results and Discussion
Leaf spot disease symptoms and damage appeared once more in 2012, reducing plant vigour and diminishing the beauty of the glossy green leaves. The leaf spots were purplish brown to dark brown, with or without chlorotic margins. The spots were circular at first and later angular to irregular, mostly small (<4 mm diameter), often coalesced, and led to leaf blight and premature defoliation (Fig. 1a, b). A Passalora sp. was consistently associated with disease symptoms.

Fruiting was hypogenous, but occasionally also epigenous in heavily damaged tissues. Stromata were substomatal and consisted of a few brown cells. Conidiophores were loosely or densely verticillate (n = 2–18), olivaceous brown to greyish brown, generally darker than the conidia, straight to mildly curved or geniculate, 30–105 × 3.5–6.5 μm, 1–5-septate (Fig. 1c–d) and had thickened, darkened conidial scars (Fig. 1 e–i). Conidia were pale olivaceous to olivaceous brown, obclavate, occasionally fusiform, rounded at the apex, obconically truncate at the base, 3–7-septate, 26–95 × 5–8 μm, guttulate and had small, darkened hila (Fig. 1j). The morphological characteristics of the fungus were consistent with previous descriptions of Passalora ampelopsidis (Peck) U. Braun (Braun and Melnik 1997; Guo 2012). A representative voucher specimen was deposited in the Korea University Herbarium (KUS-F25998). A monoconidial isolate on PDA (Fig. 1k) was deposited in the Korean Agricultural Culture Collection (Accession No. KACC46393).
Pathogenicity tests using cultures on V8 agar (Fig. 1l) revealed that small lesions, identical to those observed in the field, formed on all inoculated leaves after 8 days. The pathogen was re-isolated from lesions on the inoculated leaves, fulfilling Koch's postulates. No symptoms were observed on the control leaves. The pathogenicity test was conducted twice, yielding the same results.
The 495 bp sequence obtained from the molecular analysis was deposited in GenBank (Accession No. KP123844). A GenBank BLAST search of this sequence showed 100% identity with two previously deposited P. ampelopsidis accessions (AF362053, AY293063). Our phylogenetic analysis demonstrated that P. ampelopsidis formed a separate clade distinct from other Passalora species in a neighbour-joining tree (Fig. 2).
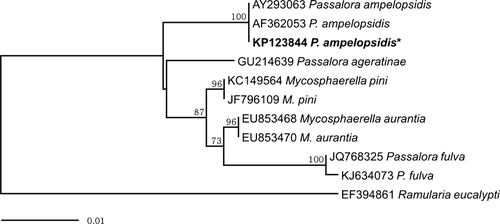
Passalora leaf spot affecting Virginia creeper vines was first recorded in New York, United States in 1878 (Peck 1878). The disease has been reported in other regions of the United States since 1953 (Chupp 1953; Farr and Rossman 2014), and more recently in Germany (Ale-Agha et al. 2005) and China (Guo 2012). To the best of our knowledge, this is the first report of Passalora leaf spot on Virginia creeper vines caused by P. ampelopsidis in Korea. Based on our field observations in Korea, this disease becomes more prevalent after the monsoon season (usually from the end of June to the middle of July), when both humidity and temperature are high, diminishing the beauty of the glossy green leaves. Importantly, the strains isolated in this study can be used as a potential biocontrol agent for managing problematic Virginia creeper vines in the United States.
Acknowledgements
This work was partly supported by the BK21 Plus program (2013) funded by the National Research Foundation of Korea (NRF). Additional support was provided by a grant from the Regional Subgenebank Support Program of Rural Development Administration, Republic of Korea.




