SARS-CoV-2 infection correlates with male benign prostatic hyperplasia deterioration
Abstract
Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) affects extra-respiratory systems, with small-scale studies showing worsened male lower urinary tract symptoms (LUTS) after coronavirus disease 2019 (COVID-19). This study explores the correlation between SARS-CoV-2 infection and male benign prostatic hyperplasia (BPH) complications using large-scale real world data.
Materials and methods
All male patients attending the public healthcare system in Hong Kong receiving alpha-blocker monotherapy for LUTS from 2021 to 2022 were included in this study. Patients with and without positive polymerase chain reaction (PCR) test for SARS-CoV-2 are selected as the exposure group and control group, respectively. Baseline characteristics are retrieved, with propensity score matching performed to ensure balance of covariates between the two groups. BPH complications were then compared and subgroup analyses were performed.
Results
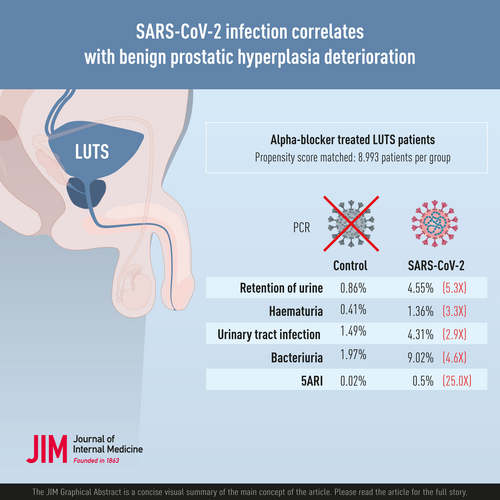
After propensity score matching, 17,986 patients were included for analysis, among which half had PCR-confirmed SARS-CoV-2 infection (n = 8993). When compared to controls, the SARS-CoV-2 group demonstrated statistically significant higher incidence of retention of urine (4.55% vs. 0.86%, p < 0.001), haematuria (1.36% vs. 0.41%, p < 0.001), clinical urinary tract infection (UTI) (4.31% vs. 1.49%, p < 0.001), culture-proven bacteriuria (9.02% vs. 1.97%, p < 0.001) and addition of 5ARI (0.50% vs. 0.02%, p < 0.001). Subgroup analysis demonstrated similar differences across different age groups. There are no statistically significance differences in incidence of retention, haematuria, or addition of 5ARI across different COVID-19 severities.
Conclusions
SARS-CoV-2 infection is associated with increased incidence of urinary retention, haematuria, UTI and the addition of combination therapy in the short term, regardless of COVID-19 severity. This is the largest study demonstrating the detrimental urological effects of SARS-CoV-2 infection.
Graphical Abstract
Introduction
The coronavirus disease 2019 (COVID-19) pandemic was declared a global emergency by the World Health Organization [1] and has affected an unprecedented 600 million cases globally as of January 2023 [2]. The causative organism of COVID-19, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), can also affect many extra-respiratory systems [3]. The underlying pathophysiology involves the entry of SARS-CoV-2 by engaging angiotensin-converting enzyme 2 (ACE2) as an entry receptor [4] in the presence of the co-expression of surface protease TMPRSS2 [5]. ACE2 and TMPRSS2 co-expression in many organ systems explain the extensive systematic effect of SARS-CoV-2.
Prostate epithelial cells are also known to co-express ACE2 and TMPRSS2 [6], which suggests that the male lower urinary tract is a target of SARS-CoV-2. Apart from the ACE2 pathway, the latest systematic review has also revealed other pathophysiological pathways through which the prostate is affected by SARS-CoV-2, including the androgen-receptor dependent TMPRSS2 expression, inflammation cascade and the SARS-CoV-2 associated metabolic dysregulation [7]. Metabolic syndrome and related disorders such as diabetes mellitus are known risk factors of lower urinary tract symptoms (LUTS) [8] and are speculated to affect the prostate gland by increasing sympathetic tone, inducing insulin-mediated prostate growth and causing chronic inflammation. SARS-CoV-2 infection has been observed to cause new-onset metabolic complications, including hyperglycaemia, ketoacidosis and diabetes [9]. It is entirely possible that the SARS-CoV-2 related metabolic dysregulations could exacerbate LUTS.
It is therefore of clinical interest to investigate and delineate how the prostatic involvement of SARS-CoV-2 would consequently affect male LUTS associated with benign prostatic hypertrophy (BPH). BPH affects the quality of life of patients, and it can also lead to complications such as retention of urine (ROU), urinary tract infection (UTI), haematuria and bladder stone formation. The prevalence of BPH and LUTS increases with age and is one of the commonest urological conditions affecting ageing men. Classical literature has noted that the prevalence of BPH is more than 80% in patients older than the age of 70 [10]. Incidentally, male patients of advanced age are also more significantly affected by COVID-19 [11]. Our hypothesis is that male patients infected with SARS-CoV-2 are more likely to have BPH complications when compared to patients not infected with SARS-CoV-2.
Current literature only contains small-scale case series and observational studies investigating the clinical correlation between COVID-19 and male LUTS, albeit with interesting results. Five small-scale studies on male COVID-19 patients that included up to 356 subjects observed statistically significant increase of LUTS scores, such as the International Prostate Symptom Score (IPSS) or the National Institute of Health-Chronic Prostatitis Symptom Index (NIH-CPSI) after COVID-19 compared to that before COVID-19 [12-16]. Apart from symptom score, bladder diary data also demonstrated worsening LUTS in COVID-19 patients [15]. This study explores the correlation between SARS-CoV-2 infection and BPH outcomes and hopes to tackle the current research gap through large-scale real world data.
Methodology
Study design
This is a retrospective cohort study comparing the incidences of urological outcomes including BPH complications, as well as medical treatment regimen change in male patients on baseline alpha blocker monotherapy for LUTS who are infected by SARS-CoV-2, compared to the control group with no SARS-CoV-2 infection. Data of the study is retrieved from the territory-wide electronic patient record database, Clinical Data Analysis and Reporting System (CDARS), of the Hong Kong Hospital Authority. The Hospital Authority is the sole publicly funded healthcare provider for the population of 7.4 million in Hong Kong, covering up to 94% of all secondary and tertiary healthcare in the territory [17]. CDARS contains clinical information including patient demographics, survival, hospitalization information, out-patient clinic attendance and information, diagnoses based on the International Classification of Diseases, Ninth Revision (ICD-9), procedural records, laboratory results and medication prescription records. The coding process was conducted by clinicians and administrative staff within daily clinical practice not involved in the study process. The Hong Kong Hospital Authority also has internal auditing processes to ensure good practices in medical record management, with ICD-9 coding reviewed by delegated officers. Numerous investigators have employed CDARS to conduct territory-wide observation studies [18-21], highlighting a reliable coding accuracy ranging from 85% to 99%.
Data extraction from all patients within the public healthcare system in Hong Kong was performed via CDARS. Patients fulfilling the inclusion and exclusion criteria were identified in CDARS and assigned an anonymous identifier, with their relevant clinical information extracted and analysed. Information regarding the outcome of interest was extracted. Relevant concomitant medications usage (including alpha blockers, 5-alpha reductase inhibitors, steroids, antivirals, interferon) were also retrieved for the analysis of LUTS control by identifying any alteration of medical therapy, and for inclusion as factors to stratify COVID-19 severity. Propensity score matching was performed to balance co-variates in order to minimize confounding.
The study was conducted in accordance with the Declaration of Helsinki. Ethical approval was obtained before the commensal of the study, with approval granted by the Joint Chinese University of Hong Kong-New Territories East Cluster Clinical Research Ethics Committee (Reference Number 2022.319).
Participants
All patients on monotherapy with long acting alpha-1 adrenoreceptor blockers (AARB) recommended for LUTS treatment [22] (Terazosin, Doxazosin, Alfuzosin, Tamsulosin) between 1 January 2021 and 31 December 2021 are included. Silodosin is not included in the Hong Kong Hospital Authority drug formulary and therefore is not included, as no Silodosin was dispensed in the public setting. Exclusion criteria include all patients with past history of prostate cancer or urolithiasis in the last 5 years. Patients with prostate cancer are not included as prostate malignancy and the associated treatments affect LUTS and may alter the pathophysiology of SARS-CoV-2 on the prostate compared to BPH patients. Patients with stone disease are excluded in order to prevent the inclusion of patients on alpha-1 blockers for the purpose of medical expulsive therapy. Patients with recent history of ROU within 1 year are also excluded.
Polymerase chain reaction (PCR) test results for SARS-CoV-2 are extracted from CDARS, with patients diagnosed with SARS-CoV-2 included in the patient group. Patients with no positive PCR test results for SARS-CoV-2 are included in the control group. Several reasons exist to justify the selection of patients with no positive PCR test results, instead of patients with negative PCR test results, as controls. First, during the height of the COVID-19 pandemic, the public healthcare system in Hong Kong has adopted a very stringent SARS-CoV-2 screening and testing policy. All in-patients will receive SARS-CoV-2 screening prior to admission, either with PCR test for elective admissions, or rapid antigen test (RAT) initially for emergency admissions. Day service patients are also advised to obtain a negative SARS-CoV-2 result with RAT within 72 h prior to their appointments, with temperature check and symptom screening in all clinics before consultations, where patients with high suspicion for COVID-19 will be tested [23]. Positive RAT will be subsequently confirmed with PCR test. Therefore, the lack of PCR positivity in Hospital Authority service attendees is a surrogate for lack of COVID-19 given the aforementioned stringent SARS-CoV-2 screening policy. Second, RAT is frequently used as the first line screening tool, and patients with negative RAT would not otherwise receive a PCR test unless clinically suspicious. Therefore, the majority of SARS-CoV-2 negative patients would not have received a PCR test. It is postulated that the patients who received a PCR test and subsequently tested negative likely represent a subgroup of SARS-CoV-2 negative patients with different clinical characteristics (e.g. patients with respiratory symptoms hence PCR tested) than the rest of the group. Therefore, bias will be introduced if PCR negative patients are chosen as controls. Admittedly, not all COVID-19 patients have a record of positive PCR test within the Hospital Authority and CDARS, as records of PCR tests by other institutions are not available under CDARS. By using patients with no positive PCR test as controls, although a loss of statistical power is expected due to the possibility of untested SARS-CoV-2 positive patients contaminating the group, it would also represent a more conservative analysis as the potential contamination would render the two groups more similar.
Study outcome
Primary outcomes include the incidences of BPH complications, namely, ROU, haematuria, UTI, bacteriuria, as well as the addition of 5-alpha reductase inhibitor for combination therapy of LUTS. Information on ROU, haematuria and UTI are based on ICD-9 coding extracted from CDARS (Table S1). Bacteriuria on the other hand is based on positive urine culture results on CDARS. Both UTI and bacteriuria are selected as outcomes as the two are differently defined within CDARS, with the former more subjective with emphasis on symptom elicited by clinicians, and the latter more objective based on microbiological investigation results. Subgroup analyses are also performed for the above outcomes, with stratification by age and COVID-19 severity.
Statistical methods
Key co-morbidities of the included patients are extracted with the corresponding ICD-9 codes from CDARS (Table S1) and selected as co-variates. Propensity scores of each patient with confounding co-variates were calculated with a logistic regression model. Propensity score matching with a narrow caliper of 0.008 and 1:1 match ratio is performed to adjust and balance potential confounding co-variates, including age, diabetes mellitus, hypertension, dyslipidemia, obesity, ischemic heart disease, stroke, smoking and related diseases, alcohol-related diseases, admission duration and follow-up duration. All primary outcomes are compared using chi-squared test. Subgroup analysis is performed based on age, the 5-tier severity classification of COVID-19 by the NIH [24] and local guideline [25] and other medication use. COVID-19 severity classes are defined based on medication use (e.g. steroid, antiviral and interferon), intervention (e.g. oxygen therapy and extracorporeal membrane oxygenation), admission and ICU care. Post hoc analysis was performed where indicated with Bonferroni correction. SPSS Statistics (version 25.0. IBM Corp) and RStudio (build 576. PBC: Rstudio Team) are used for the data analyses in this study.
Results
Study cohort
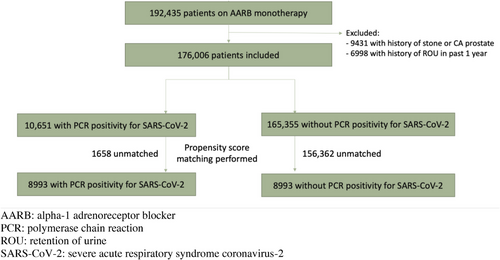
A total of 192,435 male patients were prescribed AARB monotherapy in 2021 in the public healthcare system of Hong Kong. After applying the exclusion criteria, 176,006 patients were included. PCR test results for SARS-CoV-2 were retrieved, with 10,651 patients having positive PCR test for SARS-CoV-2, and 165,355 patients with no positive PCR test for SARS-CoV-2. Propensity score matching was performed, and 17,986 patients were included in the final analysis, with 8993 patients in each group (Fig. 1). Imbalances in the pre-determined co-variates exist between the two groups prior to propensity score matching, which was subsequently accounted for after propensity score matching, evident by the standardized mean difference of <0.1 [26]. Table 1 shows the co-variates before and after propensity score matching, demonstrating similar incidence of baseline characteristics in the two groups after matching. All patients received medical care under the Hospital Authority in the period of January–May 2022, with at least one admission or out-patient follow-up.
| Pre-PSM | Control (n = 165,355) | SARS-CoV-2 (n = 10,651) | SMD |
|---|---|---|---|
| Age [mean (SD)] | 72.02 (11.28) | 75.91 (11.91) | 0.335 |
| Diabetes mellitus [n (%)] | 51,723 (31.28) | 3936 (36.95) | 0.120 |
| Hypertension [n (%)] | 98,774 (59.73) | 6738 (63.26) | 0.073 |
| Dyslipidemia [n (%)] | 8931 (5.40) | 648 (6.08) | 0.029 |
| Obesity [n (%)] | 1586 (0.96) | 94 (0.88) | 0.008 |
| Ischaemic heart disease [n (%)] | 6077 (3.68) | 617 (5.79) | 0.100 |
| Stroke [n (%)] | 15,643 (9.46) | 2031 (19.07) | 0.277 |
| Smoking and related diseases [n (%)] | 2577 (1.56) | 400 (3.76) | 0.137 |
| Alcohol-related diseases [n (%)] | 295 (0.18) | 31 (0.29) | 0.023 |
| Admission duration [mean (SD)] | 1.36 (29.79) | 13.19 (173.42) | 0.095 |
| Follow-up duration [mean (SD)] | 109.76 (36.06) | 52.01 (29.83) | 1.745 |
| Post PSM | Control (n = 8993) | SARS-CoV-2 (n = 8993) | SMD |
|---|---|---|---|
| Age [mean (SD)] | 76.68 (10.79) | 75.59 (11.64) | 0.097 |
| Diabetes mellitus [n (%)] | 3315 (36.86) | 3216 (35.76) | 0.023 |
| Hypertension [n (%)] | 5755 (63.99) | 5659 (62.93) | 0.022 |
| Dyslipidemia [n (%)] | 552 (6.14) | 544 (6.05) | 0.004 |
| Obesity [n (%)] | 52 (0.58) | 77 (0.86) | 0.033 |
| Ischaemic heart disease [n (%)] | 514 (5.72) | 486 (5.40) | 0.014 |
| Stroke [n (%)] | 1528 (16.99) | 1578 (17.55) | 0.015 |
| Smoking and related diseases [n (%)] | 300 (3.34) | 325 (3.61) | 0.015 |
| Alcohol-related diseases [n (%)] | 25 (0.28) | 25 (0.28) | <0.001 |
| Admission duration [mean (SD)] | 6.16 (95.58) | 13.32 (188.53) | 0.048 |
| Follow-up duration [mean (SD)] | 54.16 (34.56) | 55.27 (28.54) | 0.035 |
- Abbreviations: PSM, propensity score matching; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; SD, standard deviation; SMD, standardized mean difference.
Outcomes
The two groups demonstrated statistically significant differences in incidence of primary outcomes, with patients infected with SARS-CoV-2 showing a significantly higher incidence rate in terms of ROU (0.86% vs. 4.55%, p < 0.001), haematuria (0.41% vs. 1.36%, p < 0.001), clinical UTI (1.49% vs. 4.31%, p < 0.001), bacteriuria (1.97% vs. 9.02%, p < 0.001) and addition of 5ARI for combination therapy upon follow-up (0.02% vs. 0.50%, p < 0.001). Table 2 summarizes the respective incidence rates in the two groups. The results demonstrate that SARS-CoV-2 group has a relative risk of 5.31 (4.17–6.76, 95% CI) for ROU, a relative risk of 3.30 (2.29–4.76, 95% CI) for haematuria, a relative risk of 2.90 (2.38–3.52, 95% CI) for clinical UTI and a relative risk of 4.58 (3.90–5.38, 95% CI) for bacteriuria.
| Control (n = 8993) | SARS-CoV-2 (n = 8993) | p-Value | |
|---|---|---|---|
| ROU [n (%)] | 77 (0.86) | 409 (4.55) | <0.001 |
| Haematuria [n (%)] | 37 (0.41) | 122 (1.36) | <0.001 |
| Clinical UTI [n (%)] | 134 (1.49) | 388 (4.31) | <0.001 |
| Bacteriuria [n (%)] | 177 (1.97) | 811 (9.02) | <0.001 |
| Addition of 5ARI for combination therapy [n (%)] | 2 (0.02) | 45 (0.50) | <0.001 |
- Abbreviations: 5ARI, 5-alpha reductase inhibitor; ROU, retention of urine; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; UTI, urinary tract infection.
Subgroup analysis stratified by age showed that the statistically significant higher incidence of BPH complications and outcomes in SARS-CoV-2 patients can be consistently observed across the majority of the age groups, with the exception of younger age groups. Table 3 summarizes these findings, with ROU and bacteriuria demonstrating statistically significant differences in all age groups older than 50, and clinical UTI and 5ARI addition in all age groups older than 60. It is also noted that the incidences of outcomes of interest are also higher in more advanced age groups.
| Age >90 (n = 2003) | Age 80–89 (n = 5515) | Age 70–79 (n = 5509) | Age 60–69 (n = 3623) | Age 50–59 (n = 1076) | Age <50 (n = 260) | ||
|---|---|---|---|---|---|---|---|
| ROU | SARS-CoV-2 [n (%)] | 56 (5.94) | 143 (5.27) | 134 (4.97) | 62 (3.36) | 10 (1.66) | 4 (2.09) |
| Control [n (%)] | 20 (1.89) | 33 (1.18) | 13 (0.50) | 9 (0.51) | 1 (0.21) | 0 (0.00) | |
| p-Value | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p = 0.019 | p = 0.226 | |
| Haematuria | SARS-CoV-2 [n (%)] | 18 (1.91) | 49 (1.80) | 44 (1.63) | 7 (0.38) | 4 (0.66) | 0 (0.00) |
| Control [n (%)] | 11 (1.04) | 12 (0.43) | 9 (0.32) | 4 (0.23) | 1 (0.21) | 0 (0.00) | |
| p-Value | p = 0.103 | p < 0.001 | p < 0.001 | p = 0.399 | p = 0.278 | N/A | |
| Clinical UTI | SARS-CoV-2 [n (%)] | 74 (7.85) | 159 (5.85) | 106 (3.93) | 35 (1.90) | 11 (1.83) | 3 (1.57) |
| Control [n (%)] | 37 (3.49) | 69 (2.47) | 16 (0.57) | 9 (0.51) | 3 (0.63) | 0 (0.00) | |
| p-Value | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p = 0.086 | p = 0.295 | |
| Bacteriuria | SARS-CoV-2 [n (%)] | 153 (16.22) | 319 (11.75) | 220 (8.16) | 90 (4.88) | 22 (3.65) | 7 (3.66) |
| Control [n (%)] | 40 (3.77) | 74 (2.64) | 35 (1.24) | 24 (1.35) | 3 (0.63) | 1 (1.45) | |
| p-Value | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p = 0.001 | p = 0.361 | |
| Addition of 5ARI for combination therapy | SARS-CoV-2 [n (%)] | 9 (0.95) | 16 (0.59) | 11 (0.41) | 7 (0.38) | 2 (0.33) | 0 (0.00) |
| Control [n (%)] | 0 (0.00) | 1 (0.04) | 1 (0.04) | 0 (0.00) | 0 (0.00) | 0 (0.00) | |
| p-Value | p = 0.001 | p < 0.001 | p = 0.003 | p = 0.009 | p = 0.209 | N/A |
- Abbreviations: 5ARI, 5-alpha reductase inhibitor; BPH, benign prostate hyperplasia; N/A, not applicable; ROU, retention of urine; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; UTI, urinary tract infection.
Patients infected with SARS-CoV-2 were subsequently stratified by COVID-19 severity. There was no statistically significant difference in terms of incidence of ROU (3.98%–6.51%), haematuria (0.57%–3.07%), or addition of 5ARI (0.00%–0.69%) across different severities of COVID-19. However, for clinical UTI and bacteriuria, statistically significant differences in incidence existed across COVID-19 severity. Post hoc analysis demonstrated a statistically significant lower incidence of UTI (3.37%, p < 0.001) or bacteriuria (6.52%, p < 0.001) in patients with the least COVID-19 severity (asymptomatic or pre-symptomatic infection). Table 4 summarizes these findings.
| Asymptomatic or pre-symptomatic infection (n = 5908) | Mild illness (n = 875) | Moderate illness (n = 1871) | Severe illness (n = 176) | Critical illness (n = 163) | p-Value | |
|---|---|---|---|---|---|---|
| ROU [n (%)] | 256 (4.33) | 57 (6.51) | 81 (4.33) | 7 (3.98) | 8 (4.91) | p = 0.066 |
| Haematuria [n (%)] | 82 (1.39) | 15 (1.71) | 19 (1.02) | 1 (0.57) | 5 (3.07) | p = 0.142 |
| Clinical UTI [n (%)] | 199 (3.37) | 54 (6.17) | 120 (6.41) | 7 (3.98) | 8 (4.91) | p < 0.001 |
| Adj. z = −6.11 | Adj. z = 2.85 | Adj. z = 5.02 | Adj. z = −0.22 | Adj. z = 0.38 | ||
| p < 0.001 | p = 0.004 | p < 0.001 | p = 0.826 | p = 0.704 | ||
| Bacteriuria [n (%)] | 385 (6.52) | 100 (11.43) | 268 (14.32) | 31 (17.61) | 27 (16.56) | p < 0.001 |
| Adj. z = −11.46 | Adj. z = 2.62 | Adj. z = 9.00 | Adj. z = 4.02 | Adj. z = 3.39 | ||
| p < 0.001 | p = 0.009 | p < 0.001 | p < 0.001 | p = 0.001 | ||
| Addition of 5ARI for combination therapy [n (%)] | 29 (0.49) | 6 (0.69) | 9 (0.48) | 1 (0.57) | 0 (0.00) | p = 0.833 |
- Abbreviations: 5ARI: 5-alpha reductase inhibitor; Adj. z: adjusted z-score; BPH, benign prostate hyperplasia; ROU, retention of urine; UTI, urinary tract infection.
Considering the potential confounding effects of supportive medical therapy for COVID-19 in this study, additional subgroup analyses based on heparin and steroid use were performed. After exclusion of patients on heparin therapy, a higher incidence of haematuria was still consistently observed in SARS-CoV-2 patients (0.35% vs. 1.44%, p < 0.001). After the exclusion of patients on steroids, higher incidences of UTI (1.29% vs. 3.62%, p < 0.001) and bacteriuria (1.74% vs. 6.52%, p < 0.001) were still observed in SARS-CoV-2 patients.
Discussion
This study is the largest cohort study demonstrating that SARS-CoV-2 infection, in male patients being medically treated for baseline LUTS, is associated with increased incidence of BPH complications in terms of ROU, UTI and haematuria, as well as the addition of combination therapy in the SARS-CoV-2 group. Such outcome follows our hypothesis that male patients infected with SARS-CoV-2 are more likely to have deterioration of LUTS. This association is not without biological plausibility, as the co-expression of ACE2 and TMPRSS2 in the prostate renders it a target for SARS-CoV-2, leading to inflammation and therefore these outcomes of interest. Metabolic dysregulation associated with SARS-CoV-2 infection could have accelerated pathophysiological mechanisms that increase systematic inflammation and oxidative stress, which in turn worsen LUTS. In addition, the concomitant psychological and environmental stress during an infective episode of SARS-CoV-2 could have also contributed to lower urinary tract dysfunction [27, 28]. The results show a strong positive correlation that suggests significant urological manifestation of SARS-CoV-2, with relative risks of BPH complications, including ROU, haematuria, clinical UTI and bacteriuria in COVID-19 patients up to 5.31, 3.30, 2.90 and 4.58, respectively. Given the high infectivity and unprecedented scale of the COVID-19, these urological symptoms and complications represent a significant clinical burden that clinicians and urologists should be aware of.
Subgroup analyses demonstrated the statistically significant higher incidence of BPH complications can be consistently observed across different age groups greater than 50 years of age. The lack of difference in the younger age groups is likely due to these groups’ lower incidence and severity of BPH, a condition associated with advanced age. A notable finding is that incidence rate of these BPH outcomes are higher in more advanced age groups. This is similar to how COVID-19 more severely affects older patients when compared to younger patients, and this phenomenon can be observed urologically now. The natural history of BPH is also known to display longitudinal progression with age with enlarging prostate size and worsening symptoms, which likely also rendered older patients more susceptible to the urological complications of COVID-19 once infected. Data on BPH medical therapy compliance was not available in this study, but a decreased in compliance with advanced age may have also contributed to this finding.
Our study also found no difference in the incidence of urinary retention, haematuria, or addition of 5ARI for combination therapy across different clinical severities of COVID-19. This suggests that even patients with asymptomatic or mild infection, which represents the majority of the COVID-19 population, can still be affected by SARS-CoV-2 urologically and suffer from BPH complications. On the other hand, the lower incidence of UTI and bacteriuria observed in patients with low severity COVID-19 is postulated to be related to a relatively higher risk of UTI in patients with more severe disease due to immunocompromised state as well as possible catheterization. Nevertheless, subgroup analysis by excluding patients with heparin and steroid use demonstrated consistently higher incidence of haematuria and UTI, thus showing that the results of this study are not secondary to the adjunctive therapies for COVID-19.
This study is not without limitations. First the nature of the study being a retrospective cohort study has inevitably introduces bias to the study due to lack of randomization or blinding. Several precautions have been adopted in the study design to minimize bias. Propensity score matching has allowed elimination of statistical imbalance in the many included baseline co-variates, allowing a comparison with minimized confounding. The primary outcomes selected are also objective in nature, thus reducing reporting bias. Other subjective LUTS outcomes such as questionnaire-based symptoms scores (IPSS or NIH-CPSI) were not studied as these information are not included in CDARS. Although the use of existing clinical data is prone to missing information and coding errors, CDARS has nonetheless a high coding accuracy that has been previously verified [18–21]. Second, the follow-up duration of the cohort is relatively short, with a mean of 54–55 days in the two groups. However, we have ensured that all included patients had a subsequent medical consultation after the index COVID-19 diagnosis, thus ensuring any short-term complications in the period should be noticed. Further study with longer follow-up period would be helpful to delineate the long term effects of SARS-CoV-2 in terms of change in LUTS medical therapy and the subsequent need for surgical interventions such as transurethral resection of the prostate. Third, as mentioned in the methodology, the lack of universal PCR testing in this cohort has pressed us to select the control group based on the lack of positive PCR test results for SARS-CoV-2, instead of PCR negativity. Although this has inevitably introduced an uncertain degree of contamination in the control group, we believe this choice reduced bias and produced a conservative analysis that still yielded positive findings as previously explained.
Nevertheless, this study still has merit being the largest cohort study illustrating the urological manifestations of SARS-CoV-2 infection in male patients. Clinicians should be aware of the significantly higher incidence of LUTS complications with COVID-19 in this patient group and understand that these urological manifestations can occur regardless of COVID-19 severity, with similar incidence of ROU, haematuria and medication addition observed even in asymptomatic infection.
Author contributions
Study design; data collection; analysis; manuscript preparation: Alex Qinyang Liu. Study design; manuscript preparation: Peter Ka-Fung, Samuel Chi-Hang Yee. Study design; manuscript preparation; supervision: Chi-Fai Ng, Jeremy Yuen-Chun Teoh.
Conflict of interest statement
There is no conflict of interest to be declared.
Guarantor
Alex Qinyang Liu, the corresponding author, attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding information
There is no funder or sponsor for this study.
Data access, responsibility and analysis
Alex Qinyang Liu had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Open Research
Data availability statement
De-identified data on individual participants and the study protocol will be available after publication up to 36 months, with investigators whose proposed use of the data has been approved by an independent review committee identified for this purpose to achieve aims in an approved and methodologically sound proposal. Proposals should be directed to Alex Qinyang Liu, with data requestors needing to sign a data access agreement.