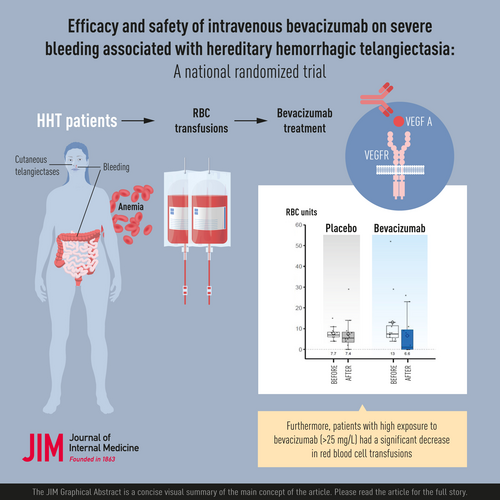
Efficacy and safety of intravenous bevacizumab on severe bleeding associated with hemorrhagic hereditary telangiectasia: A national, randomized multicenter trial
Linked to: H. Al-Samkari et al. J Intern Med 2023; https://doi.org/10.1111/joim.13713
Abstract
Background
Bevacizumab—a humanized monoclonal antibody—has been widely used to treat patients with hereditary hemorrhagic telangiectasia (HHT), but no randomized trial has yet been conducted.
Methods
This study is a double-blind multicenter randomized phase 2 trial with a 1:1 active-treatment-to-placebo ratio. We included patients over the age of 18 with a confirmed diagnosis and the need for at least four red blood cell (RBC) units transfused in the 3 months before study enrollment. Bevacizumab was administered at a dose of 5 mg/kg every 14 days with a total of six injections. The primary efficacy criterion was a decrease of at least 50% in the cumulative number of RBC units transfused in a 3-month period before and after treatment.
Results
A total of 24 patients (12 in each group) were included and randomized at 4 different centers. In intention-to-treat analysis, 63.6% of patients (7/11) in the bevacizumab group versus 33.3% of patients (4/12) in the placebo group decreased the number of blood transfusions by at least 50% (p = 0.22). Hemoglobin levels significantly improved at 6 months in the bevacizumab versus placebo group (p = 0.02). The pharmacokinetics study revealed that patients with high exposure to bevacizumab had a significant decrease in RBC transfusions (p = 0.03). Fifty-nine adverse events were observed, 34 in the placebo arm versus 25 in the bevacizumab arm.
Conclusion
Though the present trial was underpowered, patients with HHT receiving bevacizumab required numerically fewer red blood cell transfusions than those receiving placebo, particularly those with high exposure.
Graphical Abstract
Introduction
Hereditary hemorrhagic telangiectasia (HHT) (OMIM#187300) is a genetic capillary vascular disorder characterized by recurrent epistaxis, cutaneous and mucosal telangiectases, and visceral arteriovenous malformations that affect many organs, including the lungs, gastrointestinal tract, liver, and brain. Diagnosis is based on the Curaçao criteria (recurrent epistaxis, telangiectasia, family history, and visceral lesions) and is considered definite if at least three criteria are fulfilled [1]. Anemia is a frequent complication related to repeated nasal or digestive hemorrhages [2] and can be severe, requiring iron supplementation and sometimes repeated red blood cell (RBC) transfusions. The impact on quality of life can be substantial [3]. No local treatment has successfully attained long-lasting efficacy, and there are considerable side effects of local surgery, including nasal septum perforation and worsening epistaxis.
In most cases, HHT is associated with heterozygous mutations of the ACVRL1 [4] or ENG [5] genes, which, respectively, encode a bone morphogenetic protein receptor, activin receptor-like kinase 1, and a co-receptor called endoglin. In addition, mutations in SMAD4—which are responsible for juvenile polyposis/HHT overlap syndrome—have been described [6]. All the products of these genes regulate the same bone morphogenetic protein 9/10 (BMP) signaling pathway and vascular quiescence [7, 8]. BMP9 activates the endothelial cell expression of vascular endothelial growth factor receptor 1 (VEGFR1)—a high-affinity non-signaling receptor that downregulates the pro-angiogenic action of VEGF through its signaling receptor VEGFR2—and represses the endothelial expression of angiopoietin-2 (ANGPT2), another pro-angiogenic growth factor [9, 10]. There is strong evidence that VEGF/VEGFR2 signaling is a key contributor to the pathogenic process of HHT [11].
Since 2010, anti-angiogenic treatment using bevacizumab—a humanized monoclonal antibody that selectively binds to and neutralizes the biologic activity of human VEGF—has been tested on HHT patients [12-14]. A phase 2 non-randomized open-labeled trial highlighted the efficacy of bevacizumab, not only on liver lesions but also on nosebleeds, which were considerably reduced, thereby significantly improving the patients’ quality of life [15]. Since then, many case reports, surveys, and case series [16-25] showing dramatic improvements in HHT bleeding following bevacizumab treatment have been published. However, to date, no placebo-controlled trial has been performed.
The main objective was thus to evaluate the efficacy of intravenous bevacizumab in decreasing the number of RBC transfusions in HHT patients with severe bleeding.
Methods
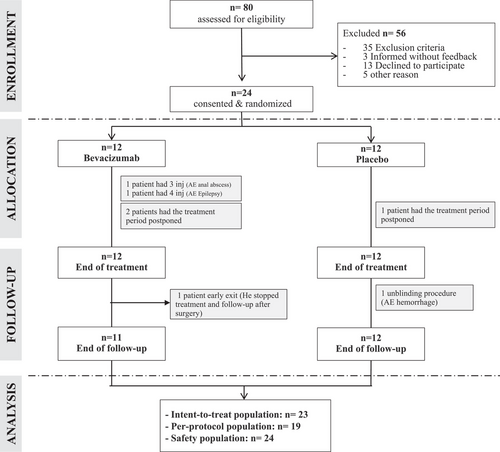
Study design (Fig. 1)
This study was prospective, multicenter, comparative, randomized (ratio 1:1), and carried out in a double-blind setting. It was approved by the local research ethics committee and by the French Medical Products Agency (ANSM). The trial was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. Oral and written informed consents were obtained from all patients in accordance with national regulations. Although this trial was initially classified as phase 3 (as stated in the protocol information available in the Supporting Information section), it has been reclassified as a randomized phase 2 trial given numerous design elements, including the small sample size, modest power, and limited preliminary data used to estimate the detectable difference between the two groups.
Participants
This study enrolled patients over the age of 18 years, with clinically confirmed HHT and RBC transfusions with the need for at least four units of RBC transfused in 3 months before study enrollment, related to epistaxis or digestive bleeding.
Patients with thrombosis in 6 months prior to inclusion, anticoagulant treatment, severe peripheral arterial disease with ulcerations, recent surgery or therapeutic endoscopy, or active infection and/or fever were excluded.
Interventions
Patients received bevacizumab intravenously at a dose of 5 mg/kg/injection every 14 days for a total of 6 injections (60 min of infusion). Bevacizumab was manufactured by the company Roche and commercialized as Avastin 25 mg/mL. The comparative treatment was the solvent (0.9% of sodium chloride) used to dilute Avastin. Diluted solutions of bevacizumab or placebo were prepared, on the day of administration, by the hospital pharmacy at the hospital center in which the patients were managed. The solutions were administered during hospitalization in a suitable department (ENT, internal medicine, gastroenterology, or other).
Primary outcome
The percentages of successful patient outcomes in both groups were compared. Patient outcomes were considered successful if the number of RBC units transfused decreased by at least 50% between the 3-month period before treatment and the period from the third to sixth months after the beginning of treatment.
The mean volume of one unit of RBCs is 284 ± 28 mL, with a mean hemoglobin value of 55.1 ± 7.4 g and a mean hematocrit of 59.2% ± 3.1%.
Secondary outcome
The Epistaxis Severity Score (ESS), quality of life (SF-36) score, and hemoglobin and ferritin levels were measured before treatment (M0) and at 3 and 6 months after the beginning of treatment. Epistaxis (mean monthly epistaxis duration and frequency [number/month]) was assessed based on the monitoring of epistaxis grids completed by the patient. For patients with externalized digestive bleeding before treatment, the presence of gastrointestinal bleeding was evaluated by video capsule endoscopy before and 6 months after starting treatment.
To assess the influence of interindividual variability in bevacizumab pharmacokinetics, serum concentrations were measured before each subsequent injection (residual concentration) and 2 h after the first injection using a validated ELISA technique [26]. Individual exposure to bevacizumab was assessed using a population pharmacokinetic approach, with Monolix (MonolixSuite version 2021R1: Lixoft SAS, 2022). A two-compartment model with first-order elimination rate gave the best results and was used to estimate bevacizumab area under the concentration versus time curve (AUC) until evaluation times, as well as to estimate potential missing bevacizumab serum concentrations at day 14.
Safety
All adverse events (AEs) were coded using the Medical Dictionary for Regulatory Activities (MedDRA) and graded according to the Common Terminology Criteria for AE (CTCAE) classification. A safety committee and a specific independent monitoring committee were set up. The safety committee was composed of four investigators—all HHT specialists—in each of the four centers in charge of patients in the study, along with the methodologist. A representative of the sponsor's safety department was also invited. They met by conference call regularly, about twice a year depending on how the trial was progressing. All AEs collected in the electronic Case Report Forms (eCRF) were discussed and their causality graduated (not related; doubtful; possible; likely; very likely).
Sample size calculation
Regarding the objective and published case reports, we assumed that 80% of patients in the treatment group would show a decrease in the number of RBC units needed by at least 50% versus 20% in the placebo group. A total of 24 patients (12 per group) would make it possible to reach a power of 81% according to Fisher's exact test (alpha = 5%, two-sided).
Randomization
The randomization process was centralized. There was a single list for all centers with blocks of 4 patients up to 24. The randomization list was generated using SAS by the Pôle Santé Publique at the Hospices Civils de Lyon. Patients included were allocated a randomization arm using the Interactive Web Response System (IWRS). The software Ennov Clinical version 7.1 (Clinsight) was used for the data management of this study.
Patients in the French HHT network were informed during a standard follow-up consultation in an HHT center or an ENT or Gastroenterology department. All French HHT centers were involved in patient selection, but treatments were centralized in four hospitals across the country.
When inclusion was validated, the patient was randomized, and a therapeutic unit was allocated. The treatment was then dispensed by the pharmacy of the Hospital Center. Neither the patient nor the investigator was aware of the nature of the treatment administered.
Statistical analysis
At the end of the study, the data analysis was carried out by the Hospices Civils de Lyon, who had the randomization codes. The intention-to-treat (ITT) population consisted of all randomized patients who started treatment. The per-protocol (PP) population comprised all patients who had received at least five infusions and had completed the final visit. Moreover, to be considered in this population, patients had to respect the infusion protocol (14 days between infusions, over a duration of 70 days [±14 days]). Patients undergoing nasal or gastrointestinal surgery during the study period were not considered part of this population. All time-related computations were performed with days as the time unit (e.g., 3 months = 84 days), for homogeneity. All the initial characteristics of the patients were summarized by means of descriptive statistics (number, mean, standard deviation, median, minimum, and maximum for the quantitative variables, and numbers and percentages for the qualitative variables).
Initial characteristics were compared between groups with a Mann–Whitney exact test for the quantitative variables (due to sample size) and Fisher's exact test for the qualitative variables. The percentage of improved patients was calculated in each group and was compared using Fisher's exact test.
To analyze the influence of individual exposure to bevacizumab on the outcomes, patients were categorized as “not exposed” (placebo patients), “below median” (bevacizumab concentrations at day 14 below or equal to median value), and “above median” (bevacizumab concentrations at day 14 above-median value). Median estimated AUC was redundant as it separated the patients into the same subgroups.
Missing data
The epistaxis criterion was based on grids filled in daily by patients. However, if 1 day was missing, the value was replaced by an average of the four values before and the four values after the missing value. This strategy was applied up to 10 missing values over 3 months (i.e., about 10%). If more than 10 days but less than 30 days (included) were missing, a daily average was computed from the data available (from the 3-month period evaluated) and was multiplied by the number of days in the reporting period, to obtain the overall estimated duration.
The bevacizumab serum concentration value on day 14 was missing in one patient and was estimated using the pharmacokinetic model and his/her individual parameters. The analysis was also performed after removing this patient.
If a patient was lost to follow-up or refused to communicate his/her nosebleed grids or had more than 30 days missing on the grids, the outcome of the patient in question was not considered.
Results
Patient characteristics before treatment ()
Twenty-four patients were included and received a randomized treatment at 4 different centers from September 27, 2017, to November 19, 2019–12 in the bevacizumab group and 12 in the placebo group. The baseline characteristics are summarized in Table 1. One patient (bevacizumab group) withdrew from the study prematurely and is therefore missing in the analysis of the primary endpoint (Fig. 1). Twenty-three patients constitute the ITT population (11 in the bevacizumab group and 12 in the placebo group). Five patients did not complete the full theoretical treatment scheme and were not kept in the PP population (19 patients). One patient underwent an unblinding procedure during the follow-up period due to a particularly severe nosebleed and was found to be in the placebo group.
| Variable | Modality | All | Placebo group n (%) | Bevacizumab group n (%) | p |
|---|---|---|---|---|---|
| Number | n | 24 | 12 | 12 | |
| Age (years) | Median (min–max) | 60 (42–80) | 68 (42–75) | 59.5 (50–80) | 0.50 |
| Mean (SD) | 61.21 (10.81) | 62.5 (12.89) | 59.92 (8.64) | ||
| Females | n (%) | 10 (41.7) | 6 (50) | 4 (33.3) | 0.68 |
| Mutated gene | n (%) | 0.64 | |||
| ALK1 | 17 (70.8) | 8 (66.7) | 9 (75) | ||
| ENG | 5 (20.8) | 2 (16.7) | 3 (25) | ||
| Ongoing | 2 (8.3) | 2 (16.7) | 0 | ||
| Parameters on inclusion | |||||
| Nasal surgery | n (%) | 19 (79.2) | 11 (91.7) | 8 (66.7) | 0.32 |
| Nasal septum perforation | n (%) | 8 (34.8) | 5 (45.5) | 3 (25) | 0.40 |
| Digestive bleeding | |||||
| No | n (%) | 14 (58.3) | 4 (33.3) | 10 (83.3) | |
| Yes | n (%) | 10 (41.7) | 8 (66.7) | 2 (16.7) | 0.04 |
| Hemoglobin level | Mean ± SD | 86.09 (14.66) | 84 (13.6) | 88 (15.91) | 0.32 |
| (g/L) | Median (min–max) | 91 (54–104) | 86 (54–104) | 94.5 (56–104) | |
| Ferritin level (ng/mL) | Mean ± SD | 66.96 (117.89) | 61.82 (92.97) | 71.67 (141.02) | 0.50 |
| Median (min–max) | 25 (4–515) | 15 (5–302) | 30.5 (4–515) | ||
| Systolic blood pressure (mmHg) | Mean ± SD | 125.25 (19.54) | 123.25 (23.86) | 127.25 (14.84) | 0.88 |
| Median (min–max) | 127.5 (85–158) | 127.5 (85–158) | 125.5 (103–149) | ||
| Diastolic blood pressure (mmHg) | Mean ± SD | 68 (12.63) | 66.83 (13) | 69.17 (12.71) | 0.60 |
| Median (min–max) | 64.5 (47–91) | 62.5 (48–88) | 69.5 (47–91) |
- Note: p is the value for the exact Mann–Whitney test or Fisher's exact test.
| Variable | Modality | All | Placebo group | Bevacizumab group | p |
|---|---|---|---|---|---|
| Hemoglobin level (g/L) | |||||
| At inclusion |
n Median (min–max) Mean (std) |
23 91 (54–104) 86.09 (14.66) |
11 86 (54–104) 84 (13.6) |
12 94.5 (56–104) 88 (15.91) |
0.32 |
| At 3 months (D84) |
n Median (min–max) Mean (std) |
21 97 (66–144) 98.86 (17.02) |
11 96 (66–115) 94.27 (15.42) |
10 99 (87–144) 103.9 (18.04) |
0.40 |
| At 6 months (D168) |
n Median (min–max) Mean (std) |
23 95 (60–116) 92.22 (15.23) |
12 85 (60–105) 85.25 (13.57) |
11 101 (78–116) 99.82 (13.62) |
0.02 |
| Ferritin level (ng/mL) | |||||
| At inclusion |
n Median (min–max) Mean (std) |
23 25 (4–515) 66.96 (117.89) |
11 15 (5–302) 61.82 (92.97) |
12 30.5 (4–515) 71.67 (141.02) |
0.50 |
| At 3 months (D84) |
n Median (min–max) Mean (std) |
20 31.5 (8–497) 85.8 (126.46) |
10 32.5 (8–330) 89.7 (109.64) |
10 31 (10–497) 81.9 (147.32) |
0.85 |
| At 6 months (D168) |
n Median (min–max) Mean (std) |
22 27.5 (5.9–603) 85.95 (141.67) |
11 32 (5.9–240) 66.35 (83.89) |
11 23 (9–603) 105.55 (185.11) |
0.49 |
| Epistaxis total duration (min) | |||||
| Before inclusion (D84–D0) | n | 21 | 11 | 10 | 0.20 |
| Median (min–max) | 679 (103–6294) | 505.5 (103–4401) | 928.35 (139–6294) | ||
| Mean (std) | 1324.14 (1561.95) | 1058.4 (1333.73) | 1616.46 (1806.59) | ||
| During treatment (D0–D84) | n | 21 | 11 | 10 | 0.22 |
| Median (min–max) | 724 (105–4172) | 360 (122–2870) | 1043.5 (105–4172) | ||
| Mean (std) | 1129.55 (1091.58) | 895.32 (996.52) | 1387.2 (1184.78) | ||
| After treatment (D84–D168) | n | 21 | 11 | 10 | 0.39 |
| Median (min–max) | 619 (84–3489) | 212.2 (84–2954) | 640.5 (97–3489) | ||
| Mean (std) | 782.32 (923.56) | 659.84 (882.01) | 917.06 (996.28) | ||
| Epistaxis total number (n) | |||||
| Before inclusion (D84–D0) | n | 21 | 11 | 10 | 0.56 |
| Median (min–max) | 90 (26–265) | 57 (27–265) | 115.95 (26–218) | ||
| Mean (std) | 107.09 (77.03) | 100.15 (90.77) | 114.72 (62.53) | ||
| During treatment (D0–D84) | n | 21 | 11 | 10 | 0.60 |
| Median (min–max) | 78 (17–246) | 60 (26–246) | 99.5 (17–176) | ||
| Mean (std) | 99.49 (65.37) | 96.65 (78.17) | 102.6 (51.83) | ||
| After treatment (D84–D168) | n | 21 | 11 | 10 | 0.55 |
| Median (min–max) | 46 (12–240) | 42 (12–240) | 64.5 (21–125) | ||
| Mean (std) | 73.09 (58.67) | 75.42 (75.18) | 70.53 (36.8) | ||
| ESS | |||||
| At inclusion (D0) | n | 22 | 12 | 10 | 0.96 |
| Median (min–max) | 7.37 (0.72–10) | 7.37 (4.24–10) | 7.39 (0.72–9.09) | ||
| Mean (std) | 6.89 (2.26) | 7.12 (1.83) | 6.61 (2.76) | ||
| At 3 months (D84) | n | 20 | 10 | 10 | 0.78 |
| Median (min–max) | 5.31 (2.43–8.08) | 5.25 (3.04–8.08) | 5.4 (2.43–7.68) | ||
| Mean (std) | 5.33 (1.66) | 5.31 (1.73) | 5.35 (1.69) | ||
| At 6 months (D168) | n | 20 | 10 | 10 | 0.48 |
| Median (min–max) | 4.51 (1.23–10) | 5.25 (1.23–10) | 4.51 (2.43–6.09) | ||
| Mean (std) | 4.51 (1.23–10) | 5.25 (1.23–10) | 4.51 (2.43–6.09) | ||
| SF36 score: Evolution of underscores between inclusion and 6 months | |||||
| Physical functioning | n | 22 | 12 | 10 | 0.78 |
| Median (min–max) | 5 (−25 to 65) | 5 (−10 to 65) | 2.5 (−25 to 40) | ||
| Mean (std) | 9.09 (20.45) | 10.83 (21.62) | 7 (19.89) | ||
| Role—physical | n | 19 | 11 | 8 | 0.03 |
| Median (min–max) | 0 (−75 to 100) | 0 (0 to 100) | 0 (−75 to 50) | ||
| Mean (std) | 13.16 (41.97) | 29.55 (40.03) | −9.38 (35.2) | ||
| Bodily pain | n | 21 | 11 | 10 | 0.33 |
| Median (min–max) | −9 (−22 to 38) | 0 (−11 to 21) | −9.5 (−22 to 38) | ||
| Mean (std) | −3.14 (15.22) | −0.91 (11.38) | −5.6 (18.92) | ||
| General health | n | 22 | 12 | 10 | 0.71 |
| Median (min–max) | 5 (−25 to 45) | 2.5 (−25 to 45) | 10.63 (−14.5 to 16.25) | ||
| Mean (std) | 5.31 (17.32) | 5.17 (21.58) | 5.48 (11.42) | ||
| Vitality | n | 21 | 11 | 10 | 0.62 |
| Median (min–max) | 0 (−20 to 30) | 0 (−15 to 30) | 12.5 (−20 to 25) | ||
| Mean (std) | 3.1 (14.7) | 1.82 (12.1) | 4.5 (17.71) | ||
| Social functioning | n | 21 | 11 | 10 | 0.32 |
| Median (min–max) | 0 (−25 to 75) | 0 (−12.5 to 75) | 12.5 (−25 to 25) | ||
| Mean (std) | 6.55 (20.77) | 5.68 (24.6) | 7.5 (16.87) | ||
| Role—emotional | n | 21 | 11 | 10 | 0.74 |
| Median (min–max) | 0 (−100 to 100) | 0 (−100 to 66.67) | 0 (−100 to 100) | ||
| Mean (std) | −7.94 (48.2) | −6.06 (49.03) | −10 (49.81) | ||
| Mental health | n | 21 | 11 | 10 | 0.79 |
| Median (min–max) | 0 (−32 to 24) | 0 (−32 to 20) | 0 (−20 to 24) | ||
| Mean (std) | −0.38 (13.5) | −0.73 (14.73) | 0 (12.79) | ||
- Note: p is the value for the exact Mann–Whitney test or Fisher's exact test.
- Abbreviation: ESS, Epistaxis Severity Score.
| Variable | Modality | Alln (%) | Placebo groupn (%) | Bevacizumab groupn (%) | p-Value |
|---|---|---|---|---|---|
| Patients with at least one AE | No | 2 (8.3) | 1 (8.3) | 1 (8.3) | 1.00 |
| Yes | 22 (91.7) | 11 (91.7) | 11 (91.7) | ||
| Number of AE | Patients (n) | 22 | 11 | 11 | 0.42 |
| Events (n) | 107 | 61 | 46 | ||
| Mean (std) | 4.86 (2.88) | 5.55 (3.53) | 4.18 (1.99) | ||
| Median (min–max) | 4.5 (1–11) | 6 (1–11) | 3 (2–8) | ||
| Related to the treatment | No | 48 (44.9) | 27 (44.3) | 21 (45.7) | 1.00 |
| Yes | 59 (55.1) | 34 (55.7) | 25 (54.3) | ||
| Severe AE | |||||
| Patients with at least 1 SAE | No | 16 (66.7) | 9 (75) | 7 (58.3) | 0.67 |
| Yes | 8 (33.3) | 3 (25) | 5 (41.7) | ||
| Number of SAE | Patients (n) | 8 | 3 | 5 | 0.82 |
| Events (n) | 13 | 4 | 9 | ||
| Mean (std) | 1.63 (1.06) | 1.33 (0.58) | 1.8 (1.3) | ||
| Median (min–max) | 1 (1–4) | 1 (1–2) | 1 (1–4) |
- Note:p is the value for the exact Mann–Whitney test or Fisher's exact test.
- Abbreviations: AE, adverse event; SAE, severe adverse event.
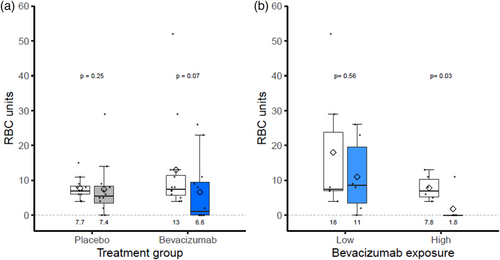
Primary outcome (Fig. 2A)
In the ITT analysis, 63.6% (7/11) of patients in the bevacizumab group versus 33.3% (4/12) of patients in the placebo group had a number of RBC units transfused that decreased by at least 50% between the 3-month period before treatment and the period from the third to sixth months after the beginning of treatment (p = 0.22).
The mean number of RBC units transfused before and after treatment was 12.83 (SD = 14.07), decreasing to 7.18 (SD = 9.53) in the bevacizumab group (−44%) and 7.67 (SD = 3.03), decreasing to 7.42 (SD = 7.84) in the placebo group (−4%) (p = 0.17) (Table S1).
Secondary outcome (Table 2)
Median and mean hemoglobin levels were significantly higher at 6 months after the beginning of the treatment in the bevacizumab versus the placebo group (mean hemoglobin levels were 84 and 85 g/L, respectively, at inclusion and at 6 months in the placebo group versus 88 and 100 g/L in the bevacizumab group, p = 0.02). A decrease in the average duration of epistaxis was observed in both groups. The decrease in the mean duration of epistaxis was greater in the bevacizumab group (−233.1 min; SD = 294.4) than in the placebo group (−132.9 min; SD = 168.6) (p = 0.43). Evolution in the ESS between inclusion and the end of the study was in favor of the bevacizumab group: −2.63 (SD = 1.35) versus −1.57 (SD = 2.27) in the placebo group, although this difference is not significant (p = 0.12).
Regarding the quality of life, limitations due to physical activity significantly improved in the bevacizumab group between inclusion and 6 months after the beginning of treatment (p = 0.03) (Table S2).
Ten patients with digestive bleeding before treatment were included, and nine had a video capsule endoscopy. Of these 10 patients, 8 were randomized in the placebo group and 2 in the bevacizumab group (p = 0.04). For these two patients, a decrease >50% in the number of RBC units transfused was observed (29 vs. 2 and 4 vs. 0 RBC units before and after treatment, respectively). Of the eight patients in the placebo group, one was in the success group (4 vs. 0 RBC unit) (p = 0.07).
Bevacizumab pharmacokinetics
Of the 12 patients who received bevacizumab, the median elimination half-lives and residual concentrations of bevacizumab at day 14, before the 2nd injection, were 16.4 (13.1–23.3) days and 24.8 (15.5–38.0) mg/L, respectively. The decreases in RBC units transfused and in epistaxis after treatment were not related to day 14 bevacizumab concentrations. However, the five patients with above-median exposure to bevacizumab (day 14 concentration higher than the median value) had a greater decrease in RBC units transfused than patients with below-median exposure (Fig. 2B, p = 0.03). Additionally, there was a significant association between bevacizumab exposure (none, below-median, or above-median) and the absence of RBC units transfused (Fisher's exact test p = 0.04). The comparison while removing the patient with missing data shows a p-value of 0.06.
Other information
We compared the number of iron infusions and the dose (mg) of iron administered in the 3-month period before, during, and after treatment in each group and did not observe significant differences.
Adverse effects (Table 3)
Of the 24 patients included, 22 experienced AEs. The total number of AEs was 107, including 13 serious AEs in 8 patients. Of all the AEs, 59 were considered related to the trial treatment, 34 in the placebo arm versus 25 in the bevacizumab arm. Of the 25 AEs observed in the bevacizumab group, we observed asthenia (n = 5), infections (n = 6), headaches (n = 3), paresthesia (n = 1), epilepsy (n = 1), high blood pressure (n = 2), nausea (n = 2), arthralgia and neck pain (n = 3), proteinuria (n = 1), and lupus nephritis (n = 1). Of these, six were considered severe and possibly, probably, or certainly related to the treatment: Three infections related to three different germs (Staphylococcus epidermidis, Staphylococcus hominis, and Staphylococcus aureus bacteremia) were observed in the same patient who had a central line catheter as a probable portal of entry, and one patient presented a perianal abscess. The progression of these complications was favorable after removing the central line catheter and initiating antibiotic treatment for the first patient, and after surgery and antibiotic treatment for the second patient. One patient presented an episode of epilepsy with favorable progression on an adapted treatment, and lupus glomerulonephritis was observed in a patient who had previously experienced this disease before treatment.
There was no relationship between concentration and the number of serious AEs; there appeared to be an increase in the number of AEs with concentration at day 14, but this relationship was nonsignificant.
Discussion
This is the first randomized trial evaluating the efficacy of bevacizumab in HHT. This vascular disease is not actually that rare [27], but we focused on HHT patients with very severe anemia resulting in a significant number of RBC transfusions, representing less than 5% of the HHT patients in our cohort. Anemia related to nose and digestive bleeds is a major life-threatening complication in HHT. Our results on efficacy are very encouraging, as 63.6% of patients in the bevacizumab group decreased the number of RBC transfusions by at least 50% versus only 33.3% of patients in the placebo group, even though the main outcome was not achieved. However, we found that 80% of patients with high bevacizumab concentrations on day 14 decreased the number of RBC transfusions they needed by at least 50%. Patients sufficiently exposed to bevacizumab, unlike others, had a significant decrease in RBC transfusions. This result could lead to the hypothesis that there is a bevacizumab concentration threshold for efficacy and thus justify further studies on the relationship between concentration and efficacy for bevacizumab in HHT. As with treatment response, but in cancer, below-median day 14 bevacizumab serum concentrations were associated with lower overall and progression-free survival than above-median patients [28].
Interestingly, yet unsurprisingly, the increase in hemoglobin levels was significantly higher in the bevacizumab group than in the placebo group. This confirms data from case reports published in the literature [24, 29]. However, epistaxis duration and frequency seemed to improve in both groups, as well as the ESS—which takes frequency, duration, and RBC transfusions into account. Similar results were observed in other studies on HHT [30, 31]. This score is subjective, and we hypothesized that these results could be attributed to a Hawthorne effect related to participation in research and the resulting knowledge of being studied, as well as the possible impact on behavior [32, 33].
Gastrointestinal bleeding develops in approximately 20%–30% of patients with HHT [34] and can be the cause of extremely life-threatening anemia. Endoscopic treatment is difficult due to the large number of lesions. Apart from treatments using somatostatin [35, 36]—which are being evaluated in HHT (NCT02874326)—no treatment is effective, and treatments are symptomatic. Bevacizumab is currently used widely in digestive bleeding with remarkable efficacy, as published in many cases [2, 18, 19, 29, 37]. In the present study, 10 patients had digestive bleeding but eight of them were randomized in the placebo group, thereby preventing any comparative analysis. Despite the very small number of patients treated for GI bleeding in this study, the response was spectacular, as neither of the two patients had further transfusions after the start of treatment. These results are consistent with those reported in the literature and reinforce what is already known about the rapid and significant efficacy of bevacizumab against GI bleeding in HHT. An alternative explanation of the improvements in digestive bleeding is that the bevacizumab also reduced low-grade hemolysis that was contributing to anemia, as described by Thielemans et al. [38]. Most of the patients in the placebo group were treated after the end of the study with bevacizumab, and those data are currently being analyzed.
The safety assessment showed that bevacizumab was well tolerated, and that the adverse effects were low and easily manageable in this study, similar to what has already been published on HHT [15, 18, 24]. The number of AEs was similar in both groups. In the literature, bevacizumab use is associated with significant rates of systemic hypertension [39]. In the present study, two patients had easily manageable systemic hypertension (8%). Other adverse effects were mainly headaches, asthenia, and arthralgia during treatment with spontaneously favorable progression. Two patients had infections in the bevacizumab group, possibly related to the treatment, and had a favorable outcome. Even though the infections occurred during the treatment, the types of infection (on the central line catheter and a perianal abscess) are not rare. Reassuringly, a recent meta-analysis in cancer patients has shown that the use of bevacizumab was not associated with a significantly higher risk of infections [40]. No side effects regarding bleeding or clotting were observed.
This study had several limitations. First, though the difference between the placebo and the treatment group is substantial, it was not enough to reach significance as the study was powered to detect 60% of difference between groups. For comparison, the power of Fisher's exact test for detecting the difference between 63.6% and 33.3% with 23 patients is 26%. A study that demonstrates a significant difference between 63.6% and 33.3% would have required 48 patients instead of 12 patients per group. Although this trial was initially classified as phase 3 (as stated in the protocol information available in the Supporting Information section), it was reclassified as a randomized phase 2 trial given numerous design elements, including the small sample size and limited preliminary data used to estimate the detectable difference between the two groups. The variability in epistaxis duration and bleeding in HHT is considerable, even within the same patient, and this has probably been underestimated. In research on rare diseases, it has been shown that even though randomized controlled trials are considered the gold standard for reducing bias and demonstrating efficacy, they expose investigators to feasibility challenges and lead them to set very high efficacy criteria to have a realistic sample size. New hybrid designs including randomization and using pragmatic outcomes have been considered as real-world data and real-world evidence [41], and novel methods are needed as clinical trials are generally designed using the classic frequency-based designs ranging from several hundred to several thousand patients to limit type I and type II error rates [42, 43]. Second, it is difficult to conduct a randomized placebo-controlled study when the treatment is available and prescribed off-label because of the many studies reporting that the treatment is effective. This is one of the reasons why some patients did not wish to participate in the study. Third, we chose to include HHT patients with very severe anemia requiring RBC transfusions secondary to bleedings, but without splitting hemorrhages of digestive origin from those of nasal origin, as sometimes there is a combination of both. Though better improvement for anemia of digestive origin than for epistaxis is recognized by clinicians, there is no published evidence. Unfortunately, most patients with GI bleeding in this study were in the placebo group. It is probable that neither of these populations is homogeneous in terms of response to treatment, and both need to be analyzed separately. Finally, the data were collected in four different centers, and results may have been influenced by within-center dependence. However, since 2004, centers in France have been organized on a national level to homogenize patient care, which should limit the center effect.
Conclusions
Because the present trial was underpowered, the primary outcome—comparison of the success rate (50% decrease in RBCs transfused)—did not reach statistical significance (63.6% vs. 33.3%). However, it is clinically relevant to show that the number of RBCs transfused decreased by 44% in the bevacizumab group versus 4% in the placebo group, particularly in those with high exposure. Further drug monitoring studies on the potential therapeutic relationship between the concentration and effect of bevacizumab in HHT are needed through larger studies.
Author contributions
Sophie Dupuis-Girod contributed as the first author to patient recruiting, management, and follow-up of patients, designing the study, detailed data collection and reviewing the cases, and writing the manuscript. She had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. Sophie Rivière contributed as a co-author by recruiting patients (Montpellier Center), management and follow-up of patients, and reviewing the manuscript. Christian Lavigne contributed as a co-author by recruiting patients (Angers Center), management and follow-up of patients, and reviewing the manuscript. Anne-Emmanuelle Fargeton contributed by recruiting the patients (Lyon Center), management and follow-up of patients, participating in the study design, detailed data collection, and reviewing the manuscript. Brigitte Gilbert-Dussardier contributed as a co-author by recruiting patients, management and follow-up of patients, and reviewing the manuscript. Vincent Grobost, Nicolas Saroul, Vanessa Leguy-Seguin, Hélène Maillard, and Shirine Mohamed contributed as co-authors by recruiting patients and reviewing the manuscript. Evelyne Decullier contributed as a co-author by designing the study and carrying out statistical analyses. Adeline Roux contributed as a co-author by managing the data. Lorraine Bernard contributed as a co-author by carrying out statistical analyses. Jean-Christophe Saurin contributed as a co-author by performing digestive endoscopies when necessary, administrating treatments, and reviewing the manuscript. Frédéric Faure, Cesar Cartier, Laurent Laccourreye, and Ruben Hermann contributed as co-authors by performing nose examinations and reviewing the manuscript. Romain Altwegg and Frédéric Oberti contributed as co-authors by performing digestive endoscopies when necessary and reviewing the manuscript. Marjolaine Beaudoin contributed by recruiting the patients (Lyon Center), data collection, and reviewing the manuscript. Carole Dhelens contributed as co-authors by preparing the drug and reviewing the manuscript. Céline Desvignes, Nicolas Azzopardi, and Gilles Paintaud contributed as co-authors by performing pharmacokinetic analyses and reviewing the manuscript. Thierry Chinet contributed as a co-author by recruiting patients (A. Paré Center), management and follow-up of patients, and reviewing the manuscript.
Acknowledgments
Measurements of bevacizumab serum concentrations were carried out within the Pilot Center for Therapeutic Antibodies Monitoring (PiTAM/CePiBAc). PiTAM/CePiBAc is a platform at the LabEx MAbImprove, Grant Agreement ANR-10-LABX-53 (“Investissements d'avenir,” French National Research Agency) and is co-financed by the FEDER European Union program. Europe is committed to the Center region of France through the European Regional Development Fund. We would like to thank all the patients who participated in this study, the French Association of patients (AMRO), and all the members of the French HHT Network, particularly L. Alric, G. Armengol, L. Astudillo, N. Baize, M-F Carette, A. Contis, R. Corre, F. Coudert, J. Dion, S. El Chehadeh, O. Espitia, F. Galtier, J-R Harle, G. Lesur, J. Lesniak, S. Leroy, P. Magro, I. Ollivier, A. Parrot, M.-A. Pistorius, M. Rondeau-Lutz, A.-C. Simon, and D. Ternant for assistance in pharmacokinetic analyses. We would also like to thank the members of the DSMB: Drs E. Buscarini, Y. Donazzolo, and X. Lassartesse. This work was sponsored by the Hospices Civils de Lyon and received a grant from the National Research Program (PHRC 2016) from the French Ministry of Health, which did not influence how the study was designed or conducted, or its analysis and report.
Conflict of interest statement
C. Lavigne has received expertise fees from Sanofi-Genzyme, Boehringer-Ingelheim, and Takeda. V. Leguy-Seguin received honoraria, congress fees, and travel assistance from Shire-Takeda, Amicus, and Sanofi- Genzyme. J.-C. Saurin has received expertise fees from Provepharm, Medtronic. R. Altwegg has received consultant/lecture fees from Abbvie, MSD, Takeda, Amgen, Biogen, Tillots, Pfizer, Janssen, Celltrion, and Ferring. Gilles Paintaud has received grants for his research team from Roche Pharma, Chugai, Pfizer, Novartis, and Sanofi-Genzyme. The other authors have no conflicts of interest to declare.
Funding information
Hospices Civils de Lyon; National Research Program, French Ministry of Health, Grant Number: PHRC2016
Open Research
Data availability statement
Deidentified individual participant data that underlie the results reported will be made available 3 months after publication for a period of 5 years after the publication, upon request by email to the corresponding author.