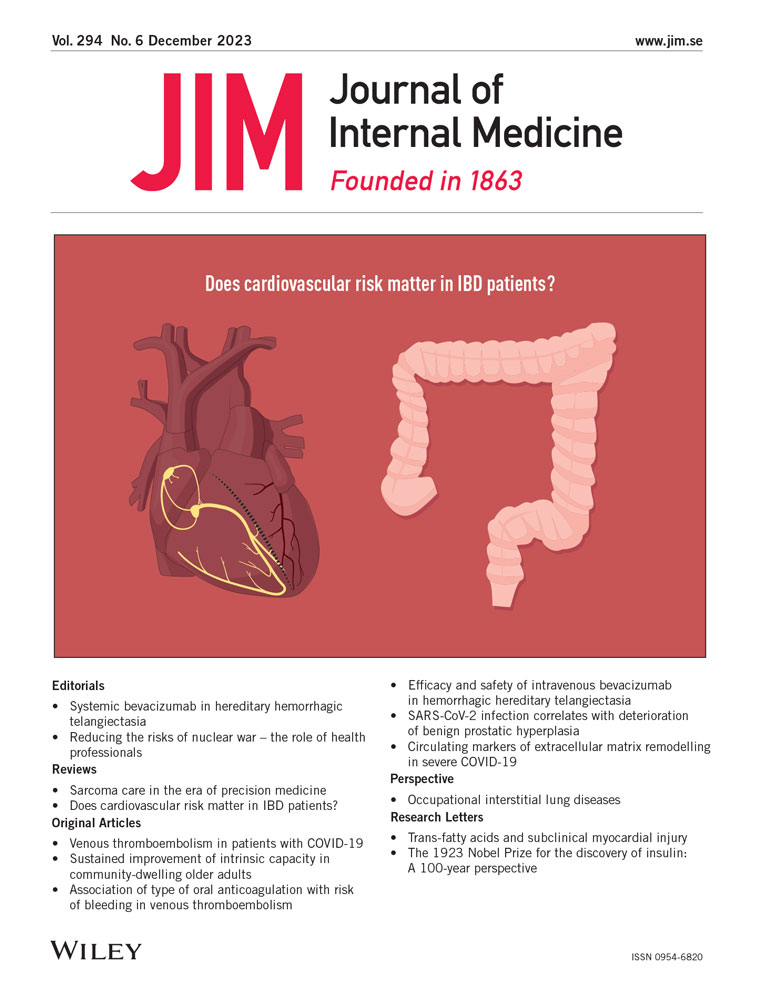
Occupational interstitial lung diseases
Abstract
Millions of workers are exposed to substances known to cause occupational interstitial lung diseases (ILDs), particularly in developing countries. However, the burden of the disease is likely to be underestimated due to under-recognition, under-reporting or both. The diagnosis of occupational ILD requires a high level of suspicion and a thorough occupational history, as occupational and non-occupational ILDs may be clinically, functionally and radiologically indistinguishable, leading to delayed diagnosis and inappropriate management. A potential occupational aetiology should always be considered in the differential diagnosis of ILD, as removal from the workplace exposure, with or without treatment, is a key therapeutic intervention and may lead to significant improvement. In this article, we provide an overview of the ‘traditional’ inorganic dust-related ILDs but also address idiopathic pulmonary fibrosis and the immunologically mediated chronic beryllium disease, sarcoidosis and hypersensitivity pneumonitis, with emphasis on the importance of surveillance and prevention for reducing the burden of these conditions. To this end, health-care professionals should be specifically trained about the importance of occupational exposures as a potential cause of ILD.
Introduction
Interstitial lung disease (ILD) is a large and highly heterogeneous group of conditions characterized by various degrees of inflammation and fibrosis of the lung parenchyma [1]. Epidemiological studies report that occupational ILDs, defined as potentially reversible diseases that result from occupational exposure to dust, fumes, smoke and biological agents, account for 10%–20% of all ILD cases [2]. Common examples of occupational ILDs include the pneumoconioses caused by exposure to crystalline silica (silicosis), asbestos (asbestosis) and coal dust (coal worker's pneumoconiosis; CWP) [3], although the list of occupational exposures that may cause ILD continues to grow, emphasizing the need for ongoing vigilance and effective preventive measures.
Although millions of workers are exposed to substances known to cause occupational ILD, the disease burden is likely underappreciated due to under-recognition, under-reporting and difficulties in attributing causation, particularly for exposures that are only rarely associated with ILD. In addition, assigning a specific occupational cause of ILD may be limited to cases awarded compensation that typically requires fulfilment of strict and specific legal and medical criteria. Varied latency periods contribute to an underestimation of the true disease prevalence and delayed or improper patient management.
The purpose of this article is to review the clinical approach to occupational ILDs and discuss possible avenues for early recognition and prevention. While focusing on the ‘traditional’ inorganic dust-related diseases, we also address idiopathic pulmonary fibrosis (IPF), the immunologically mediated chronic beryllium disease (CBD), sarcoidosis and hypersensitivity pneumonitis (HP) as well as selected conditions secondary to non-conventional or recently described exposures. ILDs that occur secondary to exposures in non-occupational settings are not included in the present article.
Epidemiology and defining features
The available epidemiological data on occupational ILDs are limited and frequently biased due to lack of standardized diagnostic criteria, limitations of available data sources (e.g. death certificates), and wide variation of regional practices in surveillance of exposed individuals and compensation rules (Table 1) [16]. The long latency period for some causative agents and potential confounding exposures (e.g. smoking) can challenge further causal assumptions. Some exposures (e.g. asbestos) may not be limited to the workplace, and disease can occur through other means [17]. Overall, the true incidence of occupational ILDs is likely underestimated particularly in low- and middle-income countries where reporting systems may be poor, control of workplace exposure less likely to be rigorous, and claims for recognition as work-related disease infrequent. The incidence and prevalence of occupational ILD ranged from 1.6% of all ILDs in an Australian and New Zealand registry to 10.4% based on a county chart review in New Mexico, with this large variability likely driven by variable local exposures [4, 18].
| Study (first author, publication year) | Disease | Time frame | Region | Incidence (/100,000/year) |
|---|---|---|---|---|
| Population based studies | ||||
| Coultas, 1994 [4] | Occupational and environmental ILD | 1988–1990 | New Mexico, United States | I: Men: 6.2 Women: 0.8 P: Men: 20.6/100,000 Women: 0.6/100,000 |
| McDonald, 2005 [5] | Pneumoconiosis | 1992–2001 | United Kingdom | I: 0.6–1 |
| Barber, 2017 [6] | Occupational HP | 1996–2015 | United Kingdom | I: 0.14 |
| Fenclovà, 2009 [7] | Occupational HP | 1992–2005 | Czech Republic | I: 0–0.2 |
| Death certificates | ||||
| Rosenman, 2003 [8] | Silicosis | 1987–1996 | Michigan state | I: 3600–7300/year in the United States |
| Carder, 2017 [9] | All pneumoconiosis | 1996–2014 | United Kingdom | I: 1.9 |
| Carder, 2017[9] | Asbestosis | 1996–2014 | United Kingdom | I: 1.3 |
| Global burden of disease analyses, ASIR/100,000/year | ||||
| Shi, 2020 [10] | Pneumoconiosis | 2017 |
Overall 0.75 Men 1.45 Women 0.15 |
|
| Silicosis | I: 0.3 | |||
| Asbestosis | I: 0.12 | |||
| CWP | I: 0.19 | |||
| Pneumoconiosis | East Asia | I: 1.66 | ||
| Oceania | I: 1.45 | |||
| North America | I: 0.62 | |||
| Western Europe | I: 0.14 | |||
| Li, 2022 [11] | 2019 | China | I: 6.7 | |
| Wang, 2021 [12] | CWP | 2019 | Global | I: 0.09 |
| Proportion of silicosis among screened workforce | ||||
| Tjoe, 2003 [13] | Quartz dust exposed | 1998 | The Netherlands | P: 10.2% |
| Akgun, 2008 [14] | Denim sand blasters | 1991–2006 | Turkey | P: 53% |
| Nandi, 2021 [15] | Sandstone mine workers | NA | India | P: 52% |
- Abbreviations: ASIR, age-standardized incidence rate; CWP, coal worker's pneumoconiosis; HP, hypersensitivity pneumonitis; I, incidence; ILD, interstitial lung disease; P, prevalence.
Pneumoconioses
Asbestos, silica and coal are the most common causative dusts inducing pneumoconiosis, whereas diseases caused by beryllium and hard metals are much less common. The Global Burden of Disease study identified more than 60,000 incident cases of pneumoconiosis in 2017; 39% of those were silicosis, 25% CWP and 16% asbestosis [10]. Pneumoconioses, though initially described in countries with a high sociodemographic index (SDI), are now seen mostly in developing countries and low SDI regions. In India, over 3 million workers are exposed to silica dust, with 8.5 million more working in construction [19, 20]. Another study from India found that the prevalence of radiological silicosis and progressive massive fibrosis (PMF) was 52% and 7.5%, respectively, among the 529 sandstone mineworkers screened [15]. In a Turkish study, 153 young denim sandblasters reported frequent symptoms, including dyspnea (52%), chest pain (46%) and chronic cough (19%). Based on chest X-rays, 77 (53%) were diagnosed with silicosis, with those diagnosed having a longer exposure duration and lower lung volumes compared to their counterparts [14]. The incidence of CWP has been declining in many regions of the world but is still increasing in some African regions, Southeast Asia and Oceania [10]. Similarly, cases of rapidly progressive CWP continue to occur among coal miners [21]. In the long term, the incidence of asbestosis is also anticipated to decrease in countries where asbestos is no longer used for construction and manufacturing. However, due to the long latency period between exposure and disease, most regions will continue to report a large number of new cases before a drop in incidence can be expected [10].
Over the last two decades, reports from various countries around the world have suggested a concerning increase in silicosis caused by manufacturing and processing of artificial stone, which is largely used for the production of kitchen or bathroom countertops [22]. Artificial stone is composed of more than 90% quartz combined with polymer resins and pigments, which are then heat cured. Cutting of artificial stone produces particularly high levels of respirable crystalline silica dusts. Compared to silicosis developed in traditional silica exposure settings, this form of disease affects younger workers following shorter latency periods – due to either the higher intensity of exposure or increased toxicity of the dust, or both – and rapid progression to advanced lung disease [23-25]. In a recent study from the Bay of Cádiz (southern Spain), one third of patients with artificial stone silicosis progressed from simple silicosis to PMF after a mean of only 4 years of follow-up and despite being removed from continued exposure [26]. The true incidence and prevalence of this new form of silicosis remain unknown but are likely to continue to rise if practices are not changed. In this regard, ventilation systems and wet blade cutting methods are effective measures to reduce dust generation when working on artificial stone [27]. CWP (or ‘coal pneumoconiosis’) is caused by long-term (typically over ≥20 years) inhalation of dust from high-carbon coal (anthracite and bituminous) and rarely graphite. Many cases will arise from co-exposures and radiologically have features of other diseases (e.g. silicosis in coal miners) [30]. Although pure coal pneumoconiosis is increasingly uncommon, severe and rapidly progressive cases of the disease continue to occur. Notably, affected miners are significantly younger than other miners with CWP, implicating recent mining conditions [21].
An estimated 21,500 people died from pneumoconiosis in 2016 [28], and over 12,900 deaths were due to silicosis in 2019 [29]. With its large workforce, China is particularly impacted by pneumoconiosis, accounting for 44% of deaths globally [1]. Reassuringly, there seems to be an overall drop in mortality rates, although stagnant and increasing mortality rates are still reported in women and in low- and middle-income countries [29].
Chronic beryllium disease
Beryllium is utilized in the aerospace, ceramics, electronics and defence industries, and CBD occurs in 2%–16% of exposed workers [31, 32]. Exposure to beryllium remains a major public health concern, with an estimated 800,000 individuals currently at risk for developing beryllium sensitization (BeS) or CBD [31, 33]. In addition to workers directly exposed to beryllium, many more are likely to be exposed indirectly [34]. The disease is characterized by an accumulation in the lung of activated CD4+ T cells that respond to beryllium as an ‘antigen’ in an MHC class II-dependent manner [35-37]. Genetic susceptibility to CBD is strongly linked to HLA-DPB1 alleles possessing a glutamic acid at the 69th position of the β-chain (βGlu69) [38, 39].
Cobalt-induced hard metal disease
Other metals, including aluminium, cobalt, titanium and zirconium, are associated with granulomatous lung disease. Cobalt-induced hard metal disease (also referred to as giant cell interstitial pneumonitis) has a strong association with βGlu69-containing HLA-DP alleles, similar to CBD [40]. The latency period between exposure and clinically overt disease ranges from months to years, with the development of cough and dyspnea heralding the onset of ILD.
Hypersensitivity pneumonitis
Occupational HP is estimated to account for one fifth of all HP cases [2]. The epidemiology of the disease has been investigated most frequently in farmers, with varying incidence being observed depending on agricultural practices and climate conditions. A high prevalence has been reported in mountain areas and countries with a cold and humid climate [41, 42]. However, with the industrialization of many farming practices and application of preventive measures, the incidence may be falling [43]. Modern occupational HP is the most frequently reported in industrial environments, where solvents, chemicals and metalworking fluids are the main inciting agents [44]. Occupational HP may also occur in workplace outbreaks where multiple workers are affected [45, 46]. For instance, industrial machine-manufacturing workers may be exposed to metalworking fluids, which are heavily colonized by bacteria, such as Pseudomonas pseudoalcaligenes and Curtobacterium, although the metalworking fluid bacterial microbiome is likely to be far more complex [47]. The annual incidence of occupational HP in the United Kingdom is estimated at 1–2 cases per million, with metalworking fluids, farming and chemicals being the most frequent causative exposures [6]. Earlier reports from the Czech Republic showed similarly low incidences [7]. A US-based study reported the 1-year HP prevalence ranging from 1.67 to 2.71 per 100,000 persons, with a 1-year cumulative incidence of 1.28–1.94 per 100,000 among an insured population between 2004 and 2013 [48]. When occupational HP is suspected, industrial hygiene assessment or consultation with occupational respiratory specialists should be sought, given the frequent under-recognition of occupational HP by non-occupational specialists [49].
Sarcoidosis
Sarcoidosis is a systemic disorder characterized by the presence of granulomatous inflammation in affected organs. Disease pathogenesis remains poorly understood but is likely to involve a complex interplay between a triggering antigen (or, most likely, antigens) and the host genetic background leading to an exuberant immune response [50]. Although commonly referred to as a disease of ‘unknown cause’, epidemiological studies have revealed significant associations between multiple occupational exposures and increased risk for sarcoidosis [51, 52]. Using data from seven studies, Blanc et al. estimated that the contribution of occupational exposures to the burden of sarcoidosis was 30% (95% CI, 17–45) [2]. The a case control etiologic study of sarcoidosis study that recruited 706 patients newly diagnosed with sarcoidosis and 706 age-, race- and sex-matched controls found positive associations between sarcoidosis and agricultural employment (odds ratio [OR] 1.46), exposures to insecticides at work (OR 1.52) and work environments with mould/mildew (OR 1.61) [53]. Further analysis of the same dataset revealed that workers for suppliers of building materials, hardware and gardening materials were also at higher risk of sarcoidosis [54]. In addition, firefighters exposed to World Trade Center ‘dust’ from construction and furnishing materials were found to be at significantly increased risk of developing sarcoidosis within the first 4 years after exposure [55], although it is difficult to identify the causative compound/s due to heterogeneity of the exposure. More recently, Beijer et al. found immunoreactivity to silica and metals (i.e. aluminium, zirconium and beryllium) in 21.2% of patients with sarcoidosis and 0% of controls, further supporting the hypothesis that these antigens may be pathogenetic in a significant minority of sarcoidosis patients [56].
IPF and other ILD subtypes
Occupational exposures are risk factors for several fibrotic ILDs, including those considered idiopathic such as IPF and other idiopathic interstitial pneumonias [57]. Indeed, although these entities are by definition of unknown aetiology, established mechanisms of lung injury support the hypothesis that inhaled exposures might contribute to disease pathogenesis and progression. However, while inferring causality in patients who develop an acute disease immediately after a high-intensity exposure is usually straightforward, determining the likelihood that any given exposure is causative is difficult when chronic low-level exposures occur over many months to years or if multiple exposures co-occur, particularly in the absence of a histological confirmation of the diagnosis. In such cases, the best approach remains suspecting the possibility of an exposure-related ILD and taking a thorough exposure history (e.g. exposure to agents known to cause ILD, cluster of disease in co-workers or age younger than expected).
Over the last two decades, several case–control studies have investigated the association between occupational exposures and IPF. In the largest meta-analysis to date, pooled findings from 11 cohort and case–control studies reported the following population attributable fractions: silica 3%, wood dusts 4%, metal dusts or fumes 8% and vapours, gases, dusts and fumes 26%, indicating that a quarter of IPF cases are attributable to occupational exposures [2]. A recent Australian study used a population-based control group to estimate the risk of IPF diagnosis by occupational exposures [58]. Respirable (smaller than 10 μm) dust (OR 1.38) and asbestos (OR 1.57) were independently associated with increased risk of IPF. Similarly, a case–control study in Italy found that people with self-reported history of exposure to metal dust, metal fumes or organic dust were at higher risk of developing IPF, with longer exposure time conferring an increased risk of disease [59]. More recently, Park et al. performed a systematic review and meta-analysis of eight case–control studies including 3502 patients [57]. They confirmed that exposure to metal (OR 1.83) and wood dust (OR 1.62), pesticide (OR 2.07) and history of farming or agriculture work (OR 1.88) increased the risk of IPF.
For ILDs overall, registry-based studies show that inhaled exposures, including occupational, home and hobbies, are highly prevalent across ILD subtypes, including IPF, connective tissue disease-associated ILD (CTD-ILD) and unclassifiable ILD. Data from two distinct cohorts identified potentially relevant exposures, including avocational and para-occupational exposures, in 65% and 62% of all ILD cases, with higher exposure prevalence in men compared to women, although the contribution of inhalation exposure to disease development or progression could not be established conclusively in all cases [60-65].
Silica exposure is an established risk factor for autoimmune conditions including systemic sclerosis [63-65] but little is known about its relationship with other CTD-associated ILD [66]. In a longitudinal familial pulmonary fibrosis cohort, development of interstitial lung abnormalities was independently associated with self-reported occupational or environmental exposures to aluminium smelting, lead, birds and mould [67]. Occupational rock dust in this cohort was further identified as a risk factor for radiologic honeycombing [68].
Diagnostic approach to patients with suspected occupational ILD
Initial assessment
The possibility of an occupational aetiology should always be considered in the initial assessment of patients with ILD as occupational and idiopathic ILDs may be clinically indistinguishable, and ‘occult’ exogenous causes are easily missed unless an extensive occupational/environmental history is taken. Exposure history should include but is not limited to, job role and details of what activities are undertaken, an estimate of relevant exposures including duration and dose (where possible), latency and disease course. Questionnaires are commonly used in health surveillance programmes and may provide useful information about current or previous exposures. Standardized health questionnaires exist to identify respiratory symptoms suggestive of occupational disease [69, 70], but none is specific to ILD.
A multidisciplinary approach that includes pulmonologists, radiologists, pathologists, occupational medicine specialists and industrial hygienists, where available, should be pursued. Industrial hygienists and occupational physicians have a key role in preventing occupational ILD (and occupational lung diseases in general). As part of a team, these specialists are trained to recognize, evaluate and control environmental health hazards in the workplace. In this regard, the UK Health and Safety Executive's ‘Dustbuster’ is designed to assess compliance with workplace health regulations and the control measures in place to protect workers’ health, with a focus on preventing occupational lung diseases, especially those caused by construction dust and other hazardous substances (https://www.hse.gov.uk/lung-disease/help.htm). Carlier et al. have recently reported that the integration of a specialized occupational consultation in the multidisciplinary evaluation of patients with ILD resulted in a change in diagnosis in 12.8% (18/141) of cases, with 15.6% of patients (22/1141) being eligible to claim compensation for an occupational disease [71]. A diagnosis of occupational ILD carries legal consequences; indeed, once the occupational origin of the disease has been confirmed, patients can claim compensation. This is the case, for instance, of patients initially diagnosed with IPF who are eventually reclassified as asbestosis following multidisciplinary evaluation.
Tools for detecting occupational exposures
Several tools are available for exposure identification in patients with suspected or confirmed occupational ILD, but there is limited evidence guiding their application in clinical practice. A comprehensive patient history supplemented by validated relevant questionnaires to identify potential exposures is an essential part of the clinical assessment. Clinical improvement following antigen avoidance may inform about the causative exposure in HP. Environmental assessment by occupational specialists and/or industrial hygienists may identify otherwise unrecognized exposures and antigens. Occupational environmental sampling may be informative, but these tools are not standardized. In HP, positive serum-specific immunoglobulin testing indicates exposure but does not prove causality, and negative test results do not exclude specific antigens as potential causes. Specific inhalational challenge and lymphocyte proliferation testing provide information on immunologic sensitization but do not establish causality. Overall, a multidisciplinary approach integrating clinical history, questionnaires, environmental assessment and sampling, and in selected cases, immunologic testing, provides the most comprehensive approach to exposure evaluation in patients with suspected occupational ILD [72, 73].
Clinical features
Occupational ILDs affect workers of both genders and all ethnicities, although they are more common in men due to historical working practices. The latency is variable; for many ILDs such as the pneumoconioses, it can be several years but for HP, CBD and hard metal disease, it can be much shorter. Similarly, acute and accelerated silicosis and ILD secondary to inhalation of fumes and toxic gases may have a short latency. Similar to idiopathic ILD, dyspnoea and cough are the most frequent presenting symptoms but wheezing and chest tightness may also occur. Patients with HP may present with chronological symptoms with onset after job or specific task initiation. Symptoms may progress over time and may demonstrate temporal variation with improvement on weekends, holidays, or when working in different areas. Lung auscultation is often normal and generally nonspecific but may reveal end-inspiratory squeaks in patients with bronchiolitis, inspiratory crackles in advanced stages of fibrosis or wheezing in large airways obstruction. Pulmonary function tests are an essential component in the initial assessment of patients with occupational ILD but are similarly nonspecific, as they may reveal a restrictive, obstructive or mixed ventilatory defect.
Diagnostic features
The diagnosis of occupational ILD is generally made based on a combination of compatible occupational exposure history and chest imaging, and the exclusion of other conditions that may mimic occupational ILD (Fig. 1). Despite its low sensitivity for detecting early disease [74], chest radiograph remains a widely used imaging test for screening of occupational ILDs and for making a diagnosis, particularly in low-income countries. Chest CT has higher sensitivity for early disease and greater accuracy in defining disease pattern than chest radiograph (Table 2) and represents a pivotal tool in the diagnosis of occupational ILD [75].
| Pattern of radiological presentation | Occupational diseases |
|---|---|
| Alveolar pattern |
|
| Interstitial/variable degree of fibrosis |
|
| Nodular pattern |
|
| Airways diseases |
|
| Sarcoid-like reaction |
|
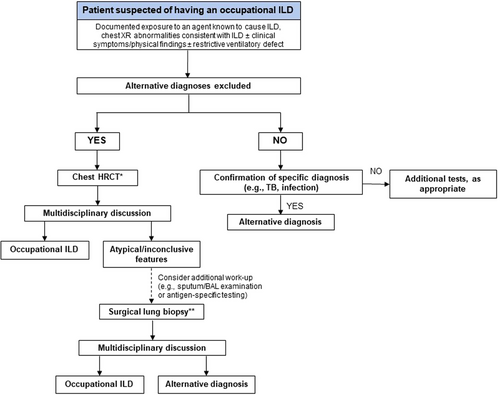
The inhalation of inorganic particles may induce variable degrees of epithelial injury, and fibrogenic and hypersensitivity reactions, leading to pleomorphic and rarely specific CT abnormalities (Table 2). Radiographic features of asbestosis are nonspecific and may be indistinguishable from those of IPF (Fig. 2, Panels A,B); therefore, imaging should always be interpreted according to the exposure history. Asbestosis also shares several histopathological features with IPF [76, 77] (Fig. 2, Panels C,D), with mineralogical studies supporting the concept of asbestosis-IPF misclassification by revealing high fibre burdens in the lung tissue of patients initially diagnosed with ‘IPF’ and revision of the diagnosis to ‘asbestosis’ [78]. The diagnosis of asbestosis requires the presence of interstitial fibrosis coupled with the identification of ≥2 asbestos bodies/cm2 counted on 5-μm thick iron-stained sections [79], which correspond to approximately 2000 asbestos bodies/g of dry lung. However, a confirmatory lung biopsy is rarely needed, and claims are generally made based on compatible clinical-radiological presentation and history of asbestos exposure [80-82]. Notably, marked differences exist in the propensity of different asbestos fibre types to cause asbestosis, with commercial amphibole (amosite and crocidolite) being two or three orders of magnitude more potent than chrysotile. It is estimated that, compared to the levels of exposure required to cause pleural plaques, those required for asbestosis to occur are approximately 2.5-fold higher for chrysotile and 10-fold higher for amosite [79, 83].
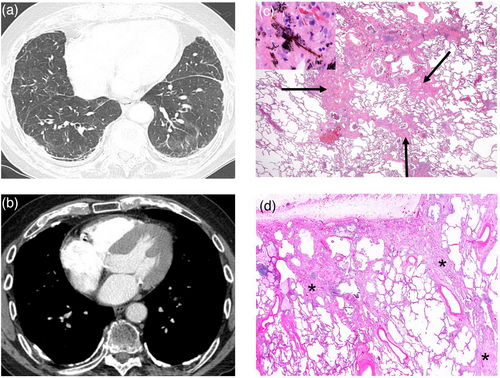
A high level of exposure may cause alveolar proteinosis, which manifests as ‘crazy paving’ and ground glass opacities, as in acute silicosis (Fig. 3, Panels A,B), or Indium-induced ILD [84, 85]. Non-fibrotic HP may also manifest acutely following exposure to antigens to which the individual is sensitized and hyper-responsive [86]. Long-term, cumulative exposure may be associated with ground glass opacities, subpleural lines and traction bronchiectasis, sometimes with coexisting honeycombing, as in asbestosis or in dust-related diffuse fibrosis (Fig. 4, Panels A,B) [87]. Silica- or silicate-induced diseases can manifest with a nodular pattern, ranging from simple silicosis to PMF, depending on the size of nodules (below or above 1 cm, respectively) (Fig. 3, Panels C,D). Inhalation of ketone butter flavouring diacetyl (as in food-flavouring workers, i.e. ‘popcorn worker's lung’) [88] or sulphur mustard (gas) (as in the US military exposed during the Iran–Iraq War) [89] may result in constrictive bronchiolitis, which is characterized pathologically by subepithelial scarring leading to narrowing of the airway, and radiologically (i.e. CT) by mosaic attenuation/air trapping and centrilobular nodules [90]. Typically, constrictive bronchiolitis causes expiratory flow limitation. Similarly, nylon flock workers’ disease is characterized pathologically by lymphocytic bronchiolitis and, on CT, by patchy ground glass opacities, consolidation and diffuse centrilobular micronodules [91, 92].
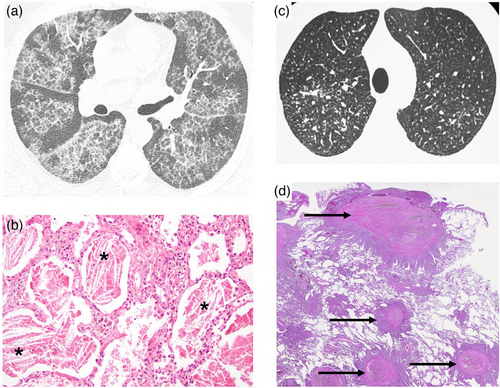
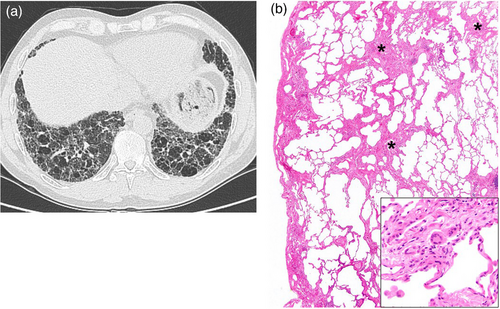
Exposure to beryllium can cause BeS, which is diagnosed by the demonstration of a beryllium-induced immune response via the beryllium lymphocyte proliferation test (BeLPT) [93]. BeS is the immunological precursor to CBD, with a rate of progression from BeS to CBD of 6%–8% per year after initial diagnosis [94]. As the BeLPT does not differentiate between CBD and BeS [95], a definite diagnosis of CBD requires evidence of non-caseating granulomatous inflammation in lung tissue (Fig. 5, Panels A–D).
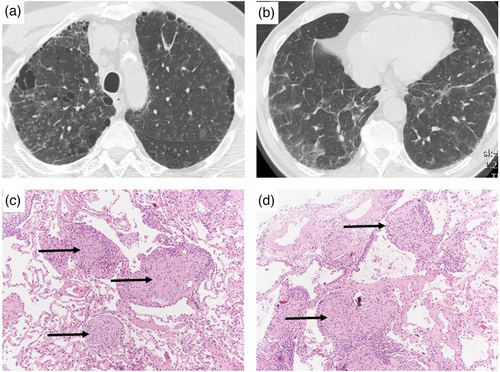
Bronchoalveolar lavage (BAL) plays an important role in the diagnostic workup of patients with ILD, including occupational ILD, particularly when used in conjunction with clinical and CT data, thus reducing the need for more invasive procedures [96]. Electron microscopy (EM) can quantify mineral fibres and particles in BAL (as well as in lung tissue) and is also able to detect detailed elements via X-ray analytical EM [97]. In addition, with regard to the detection of asbestos fibres, EM using BAL has a similar sensitivity as light microscopy using lung tissue [98]. BAL in combination with transbronchial forceps biopsies can also be useful in the evaluation of exposure-related granulomatous lung disease such as CBD, sarcoidosis and non-fibrotic HP. Transbronchial lung cryobiopsy provides pathologists with larger samples of lung tissue compared to conventional forceps biopsies thus serving as an alternative to surgical lung biopsy in certain scenarios [99]. When diagnostic uncertainty persists, surgical lung biopsy may be required to confirm the diagnosis given the potential preventative, therapeutic, prognostic and legal implications of a diagnosis of occupational ILD. In such cases, a thorough review of the lung specimen by an expert pathologist looking for clues to exposures—of which the patient may not be aware, or may have forgotten, given the long latency of some exposure-related disease—by light microscopy, polarized light microscopy or EM, as appropriate, is of outmost importance (Table 3).
| Silicosis | Chronic beryllium disease | Sarcoidosis | |
|---|---|---|---|
| Occupational exposure | Exposure to silica | Exposure to beryllium metal or metal oxide | Exposure to foreign antigens and inorganic particulates increases sarcoidosis risk |
| Clinical presentation | Dyspnoea, cough, chest pain, fatigue, weight loss | Dyspnoea, cough, fatigue, fever, weight loss | Multiple pulmonary and extrapulmonary manifestations |
| Radiological features | Centrilobular nodules, interlobular septal thickening, large consolidations, ‘eggshell’ calcifications of lymph nodes, architectural distortions, PMF | Upper lobe-predominant micronodules with a perilymphatic distribution, architectural distortion in fibrotic cases | Upper lobe-predominant perilymphatic micronodules bilateral hilar and mediastinal lymphadenopathy, architectural distortion in fibrotic cases |
| Pathological features | Silicotic nodules | Noncaseating granulomas in the lung | Noncaseating granulomas in the lung, lymph nodes and/or other affected organs |
| Management | Primarily avoidance of exposure | Primarily avoidance of exposure | Steroid/immunomodulatory treatment when required |
- Abbreviation: PMF, progressive massive fibrosis.
Management
Management of occupational ILD is focused primarily on prevention, through controlling hazardous exposures in the workplace with adequate ventilation and personal protective equipment. Medical monitoring of exposed workers with effective screening programs is essential. Patients diagnosed with occupational ILD should be removed from further exposure, although whether this leads to any reduction in the rate of progression of the disease is not definitely known. Health-care professionals should have an informed and balanced discussion with the patient about the risks to their health of continuing exposure versus the socio-economic impact of changing their job or ceasing work. All workers should be advised to refrain from cigarette smoking given its potential pathogenic synergy. Additional cornerstones of disease management include treatment of comorbidities, pulmonary rehabilitation, supportive care (e.g. supplemental oxygen) and, in advanced or rapidly progressive cases, lung transplantation [100]. Appropriate advice about compensation schemes and legal aspects, which will vary between countries, should also be pursued. Systemic glucocorticoids are the first-line treatment for sarcoidosis [101] and may be efficacious in HP and CBD but are rarely used for other pneumoconioses. Tumour necrosis factor-α antagonists (i.e. infliximab or adalimumab) may be indicated in sarcoidosis patients with persistently active/progressive disease despite immunosuppressive therapy [102]. Bronchodilators and inhaled glucocorticoids may be used in the presence of coexisting airway disease. The utility of immunosuppressive medications (e.g. mycophenolate mofetil) is unproven, although they may be considered on a case-by-case basis for patients with the suggestion of active inflammation [103]. Whole lung-lavage may be efficacious in acute silicoproteinosis unresponsive to broad-spectrum antibiotics and steroid treatment [104].
Nintedanib is approved for the treatment of non-IPF fibrotic ILDs that progress despite standard management [105] based on data from a randomized placebo-controlled trial in 663 patients with progressive fibrosing ILD, including 39 patients with ‘exposure-related ILDs’ (21 randomized to nintedanib and 18 randomized to placebo) [106]. Subgroup analysis by ILD diagnosis showed that nintedanib reduces significantly the annual rate of decline in forced vital capacity in patients with exposure-related ILDs, with a difference of 252.8 mL/year (95% CI 79.2, 426.5) compared to placebo [107]. Conversely, the role of pirfenidone in the treatment of patients with occupational progressive pneumoconiosis remains to be elucidated [108, 109].
Co-morbidities
The risk for tuberculosis is up to 20-fold higher in silica-exposed individuals compared to those not exposed [110]. This combination (often referred to as silico-tuberculosis) may be refractory to standard therapy, thus requiring specific expertise to guide prolonged multi-drug treatment. Silica exposure might further increase the risk for lung cancer [111] and is associated with CTDs [112]. Asbestos exposure is associated with a high incidence of lung cancer and pleural mesothelioma [113], with mesothelioma also potentially originating from peritoneal serosal membranes [114], as well as colon and laryngeal cancer [114]. Given the risk of ILD exacerbation or progression due to infection, appropriate immunization and aggressive treatment of respiratory infections are recommended [75]. CWP might be associated with an increased occurrence of immune-mediated diseases such as rheumatoid arthritis (Caplan syndrome) [75, 115]. Other general principles of ILD management apply also to patients with occupational ILD (e.g. pulmonary rehabilitation, oxygen and lung transplantation) [116].
Economic burden of occupational ILDs
The economic burden of occupational ILDs in terms of disability, workers’ compensation, health expenditures and lost revenue is difficult to estimate, and very few studies have addressed the problem. This is particularly true for occupational ILDs caused by novel/non-conventional exposures. Moreover, physicians unfamiliar with occupational lung disease may fail to elicit a thorough exposure history, thus missing valuable data. Accordingly, the data that do exist (e.g. disability statistics, death certificates and hospitalization admission diagnoses) may not be accurate or complete [117], and the true burden of exposure-related lung disease is likely to be significantly underestimated. In 2004, in the United States only, estimates of cost for occupational diseases, including respiratory illnesses, exceed $26 billion annually, but the true economic burden is likely to be much higher [118].
Disease prevention
The greatest risk factor for occupational ILD is repeated respiratory exposure to a hazardous agent, indicating the importance and potential benefit of preventative strategies. Disease prevention requires a comprehensive strategy, including environmental monitoring of workplace hazards (often undertaken by occupational or industrial hygienists), health surveillance, education of employers and workforce, implementation of behavioural changes and workplace practices, research and regulation. Most high- and medium-income countries have legal frameworks and agencies that control occupational health and safety, setting standards for workplace/occupational exposure limits. These limits define the level below which no major health risks are expected to occur.
The hierarchy of controls is a widely used system to facilitate primary prevention of disease by eliminating or minimizing exposure to hazards in the workplace [119]. The most effective strategy is complete removal of the hazard. Where this is not possible or reasonably achievable, a stepwise approach is used to reduce exposures by substituting less hazardous materials, using engineering controls (e.g. local exhaust ventilation) and adopting different practices. Use of appropriate respiratory protection, while often the easiest (and least expensive) option, is generally ineffective and should be used only as a last resort. Where there is a residual risk of disease, workers should be enrolled in a respiratory health surveillance program. This is a form of secondary prevention, which aims to detect disease early, frequently prior to onset of symptoms, to allow an implementation of interventions and prevent further disease progression. Such programs involve periodic assessment of the workforce using a variety of tools depending on the industry and relevant exposures. Most commonly, spirometry, generally in conjunction with a questionnaire about respiratory symptoms, is used. This is of most value when spirometric indices are plotted longitudinally; specialist software is available to aid an identification of accelerated decline [120]. In surveillance of silica- and coal-exposed workers, chest X-rays (in conjunction with spirometry, questionnaires and—in some countries—clinical examination) are used. Although high-resolution CT is more sensitive for detection of disease, this must be balanced by the cost, radiation exposure and high detection of incidental findings compared with chest X-rays. In the United States, medical surveillance for beryllium-exposed workers also includes immunological testing for sensitization using BeLPT. Finally, the risk is greater for those working in countries or industries where health and safety are poorly regulated and for temporary workers who may not have the same access to surveillance programs.
Perspectives and closing remarks
Millions of workers are exposed to substances known to cause occupational ILD worldwide, particularly in low–middle-income countries and low SDI regions wherein occupational safety standards may be lacking or suboptimal. In addition, the number of reported associations between novel exposures and ILD continues to grow due to the introduction of new materials and changes in manufacturing practices (Table 4). Data on disease incidence and prevalence remains scarce, whether this is due to under-recognition of occupational causes, under-reporting or both, highlighting the need for more national ILD registries to be collecting country-specific data on the ILD subtypes encountered and more specific training for health-care professionals about the importance of occupational exposures. Ideally, epidemiological studies reporting disease incidence or prevalence by number of workers exposed to a certain agent would allow comparisons between regions, countries, offending agent and type of exposure. A standard nomenclature is also encouraged within these registries with different occupational diseases and their country-specific frequency clearly listed. The emergence of novel potential occupational causes of ILD in recent years emphasizes the need for continuing vigilance.
| Exposure | Interstitial lung disease |
|---|---|
| Denim sandblasting | Silicosis |
| Inhaled nanoparticles (polyacrylate) | Interstitial pneumonia |
| Organic chemicals | |
| Isocyanates | Hypersensitivity pneumonitis |
| Polyhexamethylene guanidine phosphate (PHMG) and oligo (2-(2-ethoxy) ethoxyethyl guanidinium chloride (PGH) compounds | Interstitial pneumonia; pulmonary fibrosis |
| 5-chloro-2-methyl-4-isothiazolin-3-one (CMIT) and 2 methyl-4-isothiazolone-3-one (MIT) | Interstitial pneumonia |
| Cross-linked acrylic acid-based polymer (L-PAA) | Interstitial pneumonia; pulmonary fibrosis |
| Military deployment (desert dust particulate matter, burn pit emission products, vehicular diesel exhaust, jet fuel exhaust, oil well fires, debris from detonation, industrial fires, exposures to chemicals, fumes, gases and dusts) | Bronchiolitis obliterans; acute eosinophilic pneumonia; granulomatous pneumonitis; rapidly progressive pulmonary fibrosis |
| Flavouring materials (dyacetyl (2,3-butanedione) and acetylpropionyl (2,3-pentanedione)) | Bronchiolitis obliterans |
| Nylon flock | Nonspecific interstitial pneumonia/fibrosis with bronchiolocentric lymphoid nodules and lymphocytic bronchiolitis; diffuse alveolar damage; organizing pneumonia |
| Indium tin oxide | Interstitial pneumonia, pulmonary alveolar proteinosis |
| Man-made disasters (World Trade Center-related lung disease) | Sarcoidosis; interstitial pneumonia; pulmonary fibrosis |
| Welder's lung (welding fumes from electrode, filler wire and fluxes; base metal, shielding gases; paint or surface coating; metal oxides) | Respiratory bronchiolitis (with risk of progression to desquamative interstitial pneumonia with ongoing exposure) |
Author contributions
Conception of the article; literature search and writing of the manuscript: Paolo Spagnolo, Christopher J. Ryerson, Kerri A. Johannson, Vincent Cottin. Literature search and writing of the manuscript: Sabina Guler, Johanna Feary, Andrew P. Fontenot, Zarir Udwadia, Wim A. Wuyts. Provision of the histological images and writing of the manuscript: Andrew Churg. Provision of the radiological images and writing of the manuscript: Sara Piciucchi. Writing of the manuscript: Tamera J. Corte. All authors approved the final version.
Conflict of interest statement
PS reports grants, personal fees and non-financial support from PPM Services and Boehringer Ingelheim; grants from Hoffmann-La Roche; personal fees from Chiesi, Novartis, Galapagos, Lupin, Pieris, CSL Behring, AstraZeneca, Glycocore Pharma and Menarini outside the submitted work; wife employee of Astra Zeneca. CJR reports grants and personal fees from Boehringer Ingelheim and Hoffmann-La Roche; personal fees from Astra Zeneca, Veracyte, Ensho, Pliant Therapeutics and Cipla outside the submitted work.
SG reports personal fees from Boehringer Ingelheim and Hoffmann-La Roche outside the submitted work. AC reports personal fees from various US law firms in asbestos litigation outside the submitted work. TC reports grants and personal fees from Boehringer Ingelheim and Roche; grants from Biogen and Galapagos; personal fees from BMS, DevPro and Vicore outside the submitted work. WAW reports grants and personal fees from Boehringer Ingelheim; grants from Galapagos and Roche outside the submitted work.
KAJ reports grants and personal fees from Three Lakes Foundation; grants from Chest Foundation, University of Calgary CSM and University Hospital Foundation; personal fees from Boehringer Ingelheim, Hoffmann-La Roche, Pliant Therapeutics and Thryon outside the submitted work. VC reports grants, personal fees and non-financial support from Boehringer Ingelheim; personal fees from Astra Zeneca, Celgene/BMS, CSL Behring, Ferrer/United Therapeutics, Fibrogen, Galapagos, Galecto, GSK, Pliant, Pure Tech, RedX, Roche, Sanofi and Shionogi, outside the submitted work.
The other authors declare no conflicts of interest.