Post-fledging survival of altricial birds: ecological determinants and adaptation
Abstract
enFor altricial young, fledging is an abrupt step into an unknown environment. Despite increasing numbers of studies addressing the post-fledging period, our current knowledge of the causes and consequences of post-fledging survival remains fragmentary. Here, we review the literature on post-fledging survival of juvenile altricial birds, addressing the following main questions: Is low post-fledging survival a bottleneck in the altricial reproductive cycle? What is known of proximate and ultimate causal factors such as trophic relations (food and predation), habitat conditions, or abiotic factors acting in the post-fledging period? We analyzed weekly survival estimates from 123 data series based on studies of 65 species, covering weeks 1–13 post-fledging. As a general pattern, survival of fledglings was low during the first week post-fledging (median rate = 0.83), and improved rapidly with time post-fledging (week 4 median rate = 0.96). For ground-nesting species, survival immediately after leaving nests was similar to egg-to-fledging survival. For species breeding above-ground, survival during the first week post-fledging was substantially lower than during both the nestling period and later post-fledging stages. Thus, the early post-fledging period is a bottleneck of markedly elevated mortality for most altricial species. Predation was the main proximate cause of mortality. Various factors such as habitat, annual and seasonal variation in the environment, and the physical condition of fledglings have been found to affect post-fledging survival. Individual survival depended strongly on physical traits such as mass and wing length, which likely influence the ability of fledglings to escape predation. Trophic relationships at various levels are the main ultimate driver of adaptation of traits relevant to survival during the pre- and post-fledging periods. Spatiotemporal dynamics of food resources determine the physical development of juveniles and, in turn, their performance after fledging. However, predators can cause quick and efficient selection for fledgling traits and adult breeding decisions. Parental strategies related to clutch size and timing of breeding, and the age and developmental stage at which young fledge have substantial effects on post-fledging survival. The intensity and duration of post-fledging parental investment also influences fledgling survival. Post-fledging mortality is therefore not a random and inevitable loss. Traits and strategies related to fledging and the post-fledging stage create large fitness differentials and, therefore, are integral, yet poorly understood, parts of the altricial reproductive strategy.
Supervivencia de aves altriciales luego de salir del nido: determinantes ecológicos y adaptación
esPara juveniles altriciales, salir del nido es un paso abrupto hacia un ambiente desconocido. A pesar del incremento en los estudios investigando el periodo posterior a la salida, nuestro conocimiento actual de las causas y consecuencias de la supervivencia luego de salir del nido es fragmentada. Aquí, revisamos la literatura sobre la supervivencia de aves juveniles altriciales posterior a su salida del nido, con el fin de responder las siguientes preguntas principales: es la baja supervivencia luego de salir del nido un cuello de botella en el ciclo reproductivo altricial? Que sabemos sobre los factores causales últimos y proximales, como las relaciones tróficas (comida y depredación), condiciones del hábitat o factores abióticos que influencian el periodo posterior a la salida del nido? Analizamos estimados semanales de supervivencia de 123 series de datos basados en estudios de 65 especies, cubriendo 1–13 semanas posteriores a la salida del nido. Como patrón general, la supervivencia de los volantones fue baja durante la primera semana posterior a la salida (mediana de la tasa = 0.83) y mejoro rápidamente con el tiempo luego de la salida (semana 4 mediana mediana de la tasa = 0.96). Para especies que anidan en el suelo, la supervivencia inmediatamente después de salir del nido fue similar a la supervivencia de huevo a volantón. Para especies que anidan por encima del suelo, supervivencia durante la primera semana posterior a la salida fue substancialmente menor que durante los periodos de anidación y periodos tardíos posteriores a la salida. Consecuentemente, el periodo temprano posterior a la salida del nido es un cuello de botella con una marcada mortalidad elevada para la mayoría de especies altriciales. Depredación fue la principal causa proximal de mortalidad. El hábitat, variación anual y estacional del ambiente y la condición de los volantones han sido reportados como factores que afectan la supervivencia posterior a la salida del nido. La supervivencia individual dependió fuertemente de caracteres físicos como masa, longitud del ala, los cuales probablemente influencian la habilidad de los volantones de escapar a los depredadores. Relaciones tróficas en varios niveles son el factor ultimo de adaptación de caracteres relevantes para la supervivencia durante los periodos previos y posteriores a la salida del nido. La dinámica espacio-temporal de los recursos alimenticios determinan el desarrollo físico de los juveniles y por lo tanto su rendimiento después de salir del nido. Sin embargo, los depredadores pueden causar selección rápida y eficiente para los caracteres de los volantones y de las decisiones de reproducción en los adultos. La intensidad y la duración de la inversión parental posterior a la salida del nido también influencia la supervivencia de los volantones. La mortalidad posterior a la salida del nido es por lo tanto no aleatoria y una perdida inevitable. Los caracteres y las estrategias relacionadas con la salida del nido y la etapa posterior a esta salida, crean diferencias grandes en la aptitud y por lo tanto son una parte integral pero poco comprendida de la estrategia de reproducción altricial.
Many physiological and behavioral adaptations within the gradient from precocial to altricial avian reproduction have been described (Ricklefs 1968, Ricklefs and Bloom 1977). Precocial species produce well-developed hatchlings able to move and forage on their own. Parents provide limited post-hatching care, e.g., guiding young to food resources and protecting them from predators (e.g., Dreitz 2009, Rickenbach et al. 2011). The period of chick growth and coherence of families may nevertheless be remarkably long and the precocial reproductive strategy is generally described as “slow”, with relatively slow chick growth rates and low parental investment per chick. Among altricial species, reproductive investment appears to be more equally balanced between the sexes. Mates can cooperate in provisioning broods and, as a result of intensive parental care, nestlings grow at rapid rates (Starck and Ricklefs 1998). One potential fitness advantage of the “fast” altricial breeding strategy is that more than one brood can be raised per season. A large range of morphological, physiological, and behavioral species-specific traits is related to the altricial reproductive strategy (Dial 2003). In the altricial ontogeny, fledging is a unique step. Across species, the timing of fledging varies with the age and the physical development of fledglings. Within-species variation in these traits is small (Roff et al. 2005, Martin 2015a,b), suggesting that the stage at which juveniles leave nests is fitness-relevant and has evolved in response to specific factors. The duration and extent of parental care after young fledge also varies considerably among species.
In the context of the ecology and evolution of reproductive systems, altricial birds have been studied extensively, but with a greater focus on the pre-fledging period. Three main points have been the focus. First, studies within and across species show that energetic limitations may drive selection on life-history functions ranging from nest characteristics, number of clutches, and clutch size, to the timing of breeding and food provisioning (e.g., Martin 1987, Newton 1998, Charnov 2000, Verhulst and Nilsson 2008). Second, predation on parents and offspring has been found to exert selection pressure on reproductive traits, e.g., growth rates and timing of fledging (Martin 2009). Third, avian evolutionary ecology is adjusted based on habitat and the availability of resources (Verhulst and Tinbergen 1991, Svensson and Nilsson 1995, Naef-Daenzer et al. 2004, Clutton-Brock and Sheldon 2010, Fuller 2012). In this context, the study of the plasticity, heritability, and adaptation of nestling traits in relation to nesting characteristics and habitat conditions has been prominent (Ricklefs 1977, van Noordwijk et al. 1980, 1995, Roff et al. 2005).
In the stages of the altricial ontogeny (nestling, post-fledging, independent juvenile, and recruit), the moment of fledging represents an abrupt transition and fledglings suddenly face new challenges. Obviously, juveniles enter unknown habitats and must cope with the resources and risks in those habitats. Also, the abiotic environment, such as thermal conditions and exposure to precipitation, may change abruptly. These changes may result in increased mortality during the transition between life-history stages (Low and Pärt 2009). Lack (1948) was among the first to recognize the post-fledging period of altricial birds as a life-history stage with importance for reproductive output and thus highly relevant in population dynamics and life history evolution. Studies by Drent (1984) and Nilsson and Smith (1985) provided empirical evidence confirming the importance of the early post-fledging stage in the survival of young. Although altricial species are known to provide parental care after fledging, little quantitative evidence concerning juvenile survival, parental strategies, and causal mechanisms is available. Most studies have either concentrated on the period from laying to fledging or the entire period from fledging to first breeding. As one implication, the demographic consequences and evolutionary significance of post-fledging survival are unknown for most species. Studies that specifically address the period of post-fledging dependence have been impeded by the difficulty of following juvenile birds and their parents after fledging. Hence, a crucial component of the altricial life history has been insufficiently detailed.
Over the past few decades, new techniques have increasingly provided new opportunities for investigating the fate of young birds after leaving nests, even to track individuals over entire reproductive cycles (Kenward 2001). Tracking large numbers of individuals allows investigators to estimate survival rates accurately and with high temporal resolution (e.g., Cohen and Lindell 2004, Grüebler and Naef-Daenzer 2010a). Accordingly, we now know more about the fate of juvenile altricial birds after fledging, the duration of the post-fledging period (i.e., time between fledging and family break-up; Russell 2000), survival patterns during this period, and the amount of parental care provided to fledglings.
Two recent reviews have addressed selected aspects of juvenile survival during the post-fledging period. These were either concentrated on passerines (Cox et al. 2014) or were based primarily on estimates of first-year survival (Maness and Anderson 2013). These overviews confirmed that post-fledging mortality is high during the first 2 weeks after leaving nests. Accordingly, first-year survival is low compared to that of adults in many species (Maness and Anderson 2013). Thus, mortality during the post-fledging period likely affects key demographic parameters such as population growth rates and age structures.
Here, we review the recent literature reporting post-fledging survival of the young of altricial species of birds to address survival and the causes of mortality after leaving nests. Are the general patterns related to age and the characteristics of taxa? What is known about influential factors such as habitat conditions, trophic relations, or abiotic factors acting in the early post-fledging period? Under the general hypothesis that life-history traits are subject to evolutionary adaptation (Stearns 1992, Roff et al. 2005), we discuss whether juvenile and parental strategies may maximize post-fledging survival as an integral component of reproductive output and thus fitness. We evaluate the importance of the post-fledging phase with respect to the cascade of life-history stages in the ontogeny of young and the reproductive cycle of parents.
Specifically, our objective was to provide a comprehensive synthesis of the importance of the post-fledging period as a life-history stage in the context of the altricial reproductive strategy. We thus focus our review on studies reporting juvenile survival rates with high temporal resolution allowing inference on short-term variation in survival relative to time post-fledging. We characterize patterns and processes that are directly related to the life-history step of fledging. We address the following specific questions: (1) Because fledging is the (irreversible) start of life outside the nest, what determines the timing of this step in relation to the ontogeny of young? (2) What are the general temporal patterns in survival rates in relation to juvenile ontogeny, to season, or to environmental factors? (3) What are the major ecological determinants of survival during the post-fledging period? (4) Which individual qualities affect post-fledging survival? (5) Does post-fledging survival vary in relation to the individual traits of parents and chicks such as the timing of breeding and the physical condition of fledglings? In this context, post-fledging survival is regarded as a potential determinant of parental fitness.
Methods
Fledgling developmental stage
We re-analyzed data in Remeš and Martin (2002). These data include estimates of nest survival and relative development of fledglings of 115 species of songbirds. To determine if the developmental stage of fledglings is related to rates of nest predation and to nesting characteristics, we included nest types in our analysis. Our four categories of nest types were ground nests, open exposed nests (close to ground, e.g., in shrubs), open secure nests (e.g., trees, cliffs, and buildings), and cavities. The respective data were collected from www.allaboutbirds.org (Cornell Lab of Ornithology). Analysis of covariance was used to analyze the relationship between relative fledgling mass (fledging mass/adult mass) and adult mass for species in the four nest-type categories. We restricted our analyses to species with body masses below 100 g and excluded two species with extremes in relative fledging mass (Gray-crowned Rosy-Finch, Leucosticte tephrocotis, and Western Meadowlark, Sturnella neglecta).
Post-fledging survival
We searched the large literature databases (JSTOR, Wiley, Wildlife worldwide, Springer Link, Ornithological Worldwide Literature, Searchable Ornithological Research Archive, and Google Scholar) for papers with the relevant keywords (“survival”/“mortality” combined with “fledgling”, “juvenile”, “chick”, “fledging”, “post-fledging”, “first-year”, “age-dependence”, and “independence”). Except for the focus on altricial species, no restriction to taxa was made, i.e., data from studies of small passerines to large birds of prey were included as long as either at least one estimate of survival for the first 2 weeks post-fledging or a series of repeat estimates at weekly or shorter intervals were reported. Reference lists in publications were also checked for additional resources. As a result, we detected some unpublished theses and several papers that were not captured by the key-word search, but that contained post-fledging survival estimates as supplementary information.
We included all quantitative evidence on post-fledging survival, ranging from Cormack-Jolly-Seber estimates for local survival rates to raw proportions of surviving birds calculated from life tables. Similarly, we included data obtained using several observation techniques such as radio-tracking or visual re-observation of marked individuals. We included estimates given relative to time after fledging, but excluded survival rates estimated for seasonal periods because these cannot be accurately related to age post-fledging. We also ignored information given at an ordinal or nominal scale. To extract numerical values from graphically represented data, we used enlarged photographs of published figures and the software package DigitizeIt (Bormann, Braunschweig, Germany).
Raw data were entered in an Excel database and, if necessary, interpolated from the originally used time intervals to the closest weekly interval (e.g., a survival estimate of 0.87 to day 10 post-fledging was calculated as 0.87(7/10) to day 7). These secondary estimates were used as statistical units and analyzed using general linear models in Statistica Ver.12 (StatSoft Inc., Tulsa, OK).
The primary database included 974 papers matching one or more of the keywords. Of these, we collected 77 references providing point estimates of post-fledging survival for 65 species. In total, we obtained 478 point estimates of weekly survival rates originating from 123 observation series. Depending on species, these estimates covered a period of up to 13 weeks post-fledging (references in Appendix 1).
Statistical analysis
Despite the fair number of observation series from a considerable sample of species, the data set was highly inhomogeneous (descriptive results provided). Therefore, finding an appropriate design for statistical analyses was difficult, and including all potential variables and factors was impossible. We based the analysis on the general hypothesis that the major proximate determinants of post-fledging survival are in the range of individual characteristics and environmental factors. Consequently, the analysis reveals relationships with proximate determinants of post-fledging survival without fully disentangling potential influential factors.
We used General Linear Mixed Models (GLMM) fit by the Laplace approximation to examine factors associated with post-fledging survival. Weekly post-fledging survival estimates were entered as arcsin square root transformed response variables. Time interval, adult body mass, trophic level (predatory or non-predatory), and nest site (ground, open exposed, open secure, or cavity) and the two-way interactions of species characteristics with time interval were included in the model as focus fixed effects. Control variables integrated as fixed effects were the field observation method (visual or telemetry) and the method of analysis (with or without estimation of observation probability). To account for the dependency of survival estimates of different age classes from the same cohort and of different cohorts from the same species, we added the identity of cohort and species as additive random factors. Models were fitted in the program R (R Development Core Team 2013) using the functions glmer from the library lme4 (Bates 2005, 2014). We calculated 95% credible intervals (CrI) for the model parameter estimates as the 2.5% and 97.5% quantiles of 5000 random values sampled from the joint posterior distribution of the model parameters using the function sim() from library arm (Gelman and Hill 2007). Model results are given in back-transformed survival rates.
Supplementary data
To compare post-fledging survival with the survival of juveniles during the nestling stage, we collected information from the Handbook of Central European breeding birds (Glutz von Blotzheim and Bauer 1985, volumes 10-1 to 14-1). We searched the electronic version of the handbook for the terms “Brutdauer” and “Nestlingsdauer” to find information on the duration of the incubation and nestling period and checked the subsequent paragraphs for estimates of total laying-to-fledging survival rates (information about nest survival was not included). From these values, we calculated the average weekly survival rate for the incubation and nestling stages. If available, we also included data provided in the papers on post-fledging survival. In total, 120 estimates of weekly nestling survival for 60 species were collected. Although the species lists for egg-to-fledging survival and for post-fledging survival were not identical, we considered this unproblematic for the inference at the general level given herein.
Results
Developmental stage at fledging
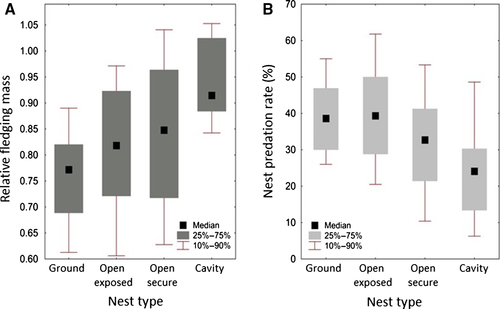
The age and developmental stage at which young birds leave nests are important components of the altricial breeding strategy. For most species of birds, young fledge before they have reached full physical and movement abilities, and are often flightless. Young passerines fledge at ~82% of adult body mass (Remeš and Martin 2002). Young in species under strong predation pressure leave nests at earlier developmental stages and have higher specific growth rates. Our reanalysis of data from Remeš and Martin (2002) revealed additional variation among species with different nest types. For ground-nesting species and species with open exposed nests, slopes were ~0.6 (i.e., nestlings leave nests at 60% of adult mass), whereas the slopes for species that breed in open secure nests and cavities were 0.74 and 0.93, respectively (Table 1, Box 1). These results suggest that the developmental stage at which young birds fledge is adjusted based on environmental factors. Predation is considered the major factor (Box 1). However, questions remain concerning the survival of fledglings to independence. The apparent benefit of leaving nests at an early developmental stage may actually be a disadvantage in terms of survival for the dependent juveniles. Therefore, additional studies are needed to clarify time-survival functions for both the nestling and fledgling life history stages (Roff et al. 2005), and relative to rates of development and allocation of energy to various physical traits.
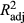 = 0.90, F7,98 = 141.9, P < 0.0001). Dependent variable = fledgling body mass (g), continuous predictor = adult body mass (g), and categorical predictor = nest type (four categories). For ground-nesting species and species with open exposed nests, slopes were ~0.6 (i.e., nestlings leave nests at 60% of adult mass); slopes for species that breed in open secure nests and cavities were 0.74 and 0.93, respectively. Species with an adult body mass below 100 g were included. Data from Remeš and Martin (2002)
= 0.90, F7,98 = 141.9, P < 0.0001). Dependent variable = fledgling body mass (g), continuous predictor = adult body mass (g), and categorical predictor = nest type (four categories). For ground-nesting species and species with open exposed nests, slopes were ~0.6 (i.e., nestlings leave nests at 60% of adult mass); slopes for species that breed in open secure nests and cavities were 0.74 and 0.93, respectively. Species with an adult body mass below 100 g were included. Data from Remeš and Martin (2002)| Parameter | Coefficient | SE | t | P | |
|---|---|---|---|---|---|
| Intercept | 0.15 | 2.035 | 0.1 | 0.94 | |
| Ground | Slope | 0.60 | 0.074 | 8.1 | <0.0001 |
| Open exposed | Slope | 0.59 | 0.029 | 20.3 | <0.0001 |
| Open secure | Slope | 0.74 | 0.042 | 17.6 | <0.0001 |
| Cavity | Slope | 0.93 | 0.079 | 11.7 | <0.0001 |
| Ground | Intercept | 3.21 | 2.761 | 1.2 | 0.25 |
| Open exposed | Intercept | 3.85 | 2.285 | 1.7 | 0.095 |
| Open secure | Intercept | 3.27 | 2.504 | 1.3 | 0.20 |
| Cavity | Zeroed | 0.00 | |||
- Statistically significant coefficients are indicated in bold font.
Weekly survival rates
Data extracted from the literature are not homogeneous in terms of time post-fledging, trophic levels, and nest types. Small species are over-represented, and estimates of survival are concentrated on the first 3 weeks post-fledging (Fig. 1A). For week 4 post-fledging and later, few data are available and primarily from larger species.
Box 1. Controversial Evidence – A Life-History Paradox
Theories of life history evolution predict selection favoring characteristics of fledglings that minimize mortality during the “post-fledging bottleneck.” In reality, however, fledglings appear poorly equipped to face the many challenges of the habitats they enter. Why is this? Remeš and Martin (2002) analyzed 115 species of songbirds and found that “under-developed” fledging is a common pattern. Interestingly, the relationship between predation pressure and fledgling developmental stage is negative. Thus, young of species with high rates of nest predation leave nests at earlier developmental stages and are often flightless when they “fledge,” although their growth rates are faster than those of species with lower rates of nest predation. Remeš and Martin (2002) and Martin (2015a,b) hypothesized an adaptive trade-off between the benefits of extending the nestling period until physical development is completed and the cost of longer exposure of nests to predators. Growth characteristics and relative fledging mass differ among nesting strategies. Species breeding on the ground or in exposed nests fledge at an earlier developmental stage (~60% of adult mass) than species breeding in either secure nests or cavities (74% and 93% of adult mass, respectively). Ground-nesting species have significantly higher rates of nest predation than either open nesters or cavity-nesting species.
In contrast to these between-species analyses, single-species studies show that advanced developmental condition of fledglings is of prime importance for post-fledging survival. Young approaching adult body mass and wing length are better at avoiding predation. These effects are so substantial that an extension of the nestling period by only 1–2 days would result in markedly improved post-fledging survival and reproductive success. Thus, for an individual fledgling, an advanced developmental stage improves its prospects for survival. However, what are the potential costs of extending the nestling period for a few days?
Available evidence, therefore, suggests that predation pressure during the nestling period favors a short nestling stage. However, predation appears to select strongly for high fledgling mass and advanced physical development (see Box 2). Within this trade-off, altricial birds may have evolved their very high nestling growth rates. Additional studies are needed to clarify the details and trade-offs in this adaptive feedback cycle.
A fledgling Song Thrush (Turdus philomelos) has left its nest even though its wings are so incompletely developed that they hardly would help in escaping from a predator. Why do fledglings leave nests at such early developmental stages? (Photo: A. Saunier).
Developmental stage of fledglings relative to nest predation rates. (A) Box plot for median relative fledging mass (fledging mass/adult mass) for four types of nests. (B) Box plot for median rates of nest predation for different types of nests (percent of nests lost before fledging). Ground-nesting species have the highest rates of nest predation and fledge at earlier developmental stages than young of other species (Data from Remeš and Martin 2002).

Similarly, with respect to nest types (Fig. 1B), ground-nesting species are underrepresented compared to species with other nest types, and non-predators are better represented than predatory species (Fig. 1C). As mentioned previously, this inhomogeneity results in restricted potential for statistical analyses. For example, no ground-breeding predators are represented (we considered Burrowing Owls, Athene cunicularia, to be cavity-breeders), and all species with body masses below 100 g were non-predators.
For all species, post-fledging survival was low immediately after leaving nests, then steadily increased (Fig. 2) up to the age of 5 weeks post-fledging. The median survival rate for the first week post-fledging was 0.83. For week four, median weekly survival was above 0.95. Thus, across species, about a quarter of fledglings died within 1 month after fledging. However, the variance in survival rates varied strongly among species and within species among different data series. During the first week post-fledging, variation in observed survival rates was particularly large (range = 0.37–1.0). In the low quartile of the data series, weekly survival rates were between 0.37 and 0.68, indicating that, for some species and under certain circumstances, survival rates can be very low (Fig. 2).

The most important main effect in the GLM model is the strong time trend in survival rates (Table 2, Fig. 3). Rates for the first week post-fledging were significantly lower than for subsequent weeks (main effect; Figs. 3A and B). We found a substantial additional effect of the trophic state of species. Non-predatory species had lower first-week survival than predatory species (Fig. 3A). This interaction effect approached significance (P = 0.07). The temporal development of weekly survival rates also differed among species with different nest types (interaction week*nest site; Fig. 2B). Ground-nesting species and species with open, exposed nests had lower first-week survival than species with open, secure nests and cavity-nesting species. The model results suggest that these differences were not due to the larger average body mass of predatory birds because adult mass had no significant additive effect on survival rates.
| Fixed effects | Coefficient | SE | t | P |
|---|---|---|---|---|
| Intercept | 0.0892 | 0.0017 | 51.6 | |
| Week 1 | Zeroed | <0.001 | ||
| Week 2 | 0.0067 | 0.0014 | 4.7 | |
| Week 3 | 0.0069 | 0.0016 | 4.3 | |
| Week 4 | 0.0021 | 0.0019 | 1.1 | |
| Week 5 and later | 0.0058 | 0.0015 | 3.8 | |
| Observation by telemetry | Zeroed | 0.086 | ||
| Visual observation | 0.0016 | 0.0012 | 1.4 | |
| Observation probability ignored | Zeroed | 0.23 | ||
| Observation probability estimated | −0.0015 | 0.0011 | −1.4 | |
| Adult body mass | 2.990e-06 | 2.597e-06 | 1.2 | 0.67 |
| Trophic state: Non-predatory | Zeroed | 0.28 | ||
| Trophic state: Predatory | 0.0056 | 0.0026 | 2.2 | |
| Nest site: cavity | Zeroed | 0.85 | ||
| Nest site: ground | −0.0038 | 0.0024 | −1.6 | |
| Nest site: Open exposed | −0.0031 | 0.0019 | −1.7 | |
| Nest site: Open secure | −0.0025 | 0.0024 | −1.1 | |
| Week 1 * Adult body mass | Zeroed | 0.74 | ||
| Week 2 * Adult body mass | −8.970e-07 | 2.928e-06 | −0.3 | |
| Week 3 * Adult body mass | −18182e-06 | 3.077e-06 | −0.4 | |
| Week 4 * Adult body mass - | −2.554e-06 | 2.830e-06 | −0.9 | |
| Week 5+ * Adult body mass | −2.805e-06 | 2.617e-06 | −1.1 | |
| Week 1 * Trophic state: Predatory | Zeroed | 0.072 | ||
| Week 2 * Trophic state: Predatory | −0.0067 | 0.0026 | −2.6 | |
| Week 3 * Trophic state: Predatory | −0.0044 | 0.0027 | −1.6 | |
| Week 4 * Trophic state: Predatory | −0.0019 | 0.0026 | −0.7 | |
| Week 5 * Trophic state: Predatory | −0.0040 | 0.0023 | −1.7 | |
| Week 1 * Nest site: Ground | Zeroed | 0.010 | ||
| Week 2 * Nest site: Ground | 0.0021 | 0.0023 | 0.9 | |
| Week 3 * Nest site: Ground | 0.0017 | 0.0025 | 0.7 | |
| Week 4 * Nest site: Ground | 0.0088 | 0.0030 | 3.0 | |
| Week 5 * Nest site: Ground | 0.0054 | 0.0032 | 1.7 | |
| Week 1 * Nest site: Open exposed | Zeroed | |||
| Week 2 * Nest site: Open exposed | 0.0012 | 0.0018 | 0.7 | |
| Week 3 * Nest site: Open exposed | 0.0022 | 0.0020 | 1.1 | |
| Week 4 * Nest site: Open exposed | 0.0071 | 0.0023 | 3.1 | |
| Week 5 * Nest site: Open exposed | 0.0060 | 0.0019 | 3.2 | |
| Week 1 * Nest site: Open secure | ||||
| Week 2 * Nest site: Open secure | 0.0033 | 0.0022 | 1.5 | |
| Week 3 * Nest site: Open secure | 0.0015 | 0.0026 | 0.6 | |
| Week 4 * Nest site: Open secure | 0.0060 | 0.0027 | 2.2 | |
| Week 5 * Nest site: Open secure | 0.0036 | 0.0027 | 1.3 | |
| Random effects | ||||
| CurveID | 7.050e-06 | 0.0027 | ||
| Species | 9.682e-06 | 0.0031 | ||
| Residual | 2090e-05 | 0.0046 | ||
- Dependent variable: ArcSin(Sqrt(weekly survival rate)), N = 478 estimates, 123 data series, 65 species.
- Statistically significant coefficients are indicated in bold font.
Inference from single-species studies
In studies where survival was estimated for shorter re-encounter intervals, several investigators reported >10% daily mortality during the first 2 d after leaving nests (Naef-Daenzer et al. 2001, Low and Pärt 2009, Balogh et al. 2011, Sim et al. 2013). This suggests that a marked decline in survival can occur as a direct consequence of fledging. Various potential covariates that modify the general pattern have been tested, most frequently the effects of age, habitat, and body condition. An overview is provided by Cox et al. (2014). Here, we discuss examples of potential extrinsic and species-specific causes of variation in post-fledging survival.
Comparatively low post-fledging survival rates (weeks 1–2) have, for example, been reported for Florida Scrub-Jays (Aphelocoma coerulsecens, Woolfenden 1978), Whinchats (Saxicola rubetra, Tome and Denac 2012), and Ring Ouzels (Turdus torquata, Sim et al. 2013), and particularly high post-fledging survival has been reported for Burrowing Owls (Davies and Restani 2006), Red-bellied Woodpeckers (Melanerpes carolinus, Cox and Kesler 2012), and Dusky Flycatchers (Empidonax oberholseri, Vormwald et al. 2011). Four species in South Africa had first-week survival rates close to 1.0, including Bar-throated Apalises (Apalis thoracica), Cape Robin-Chats (Cossypha caffra), Cape Penduline Tits (Anthoscopus minutus), and Karoo Prinias (Prinia maculosa) (Lloyd and Martin 2016).
Effects of nest type
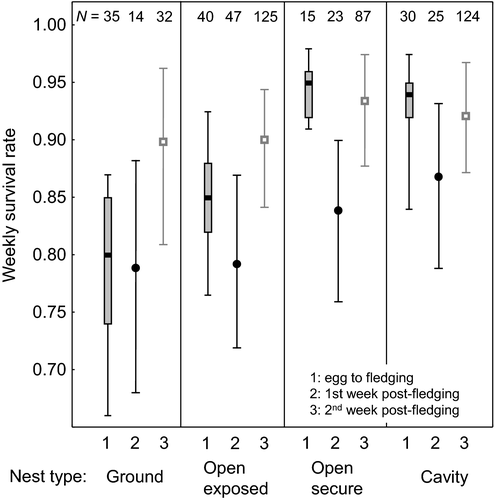
Comparison of post-fledging survival with egg-to-fledging survival rates reveals that survival after leaving nests is similar to that during the nestling period for ground-nesting species. In contrast, survival during the first days after fledging for open-nesting species and cavity-nesting birds is markedly lower than during the nestling period (Fig. 4), but fledgling survival after 2 weeks post-fledging is high. The pattern for species with exposed open nests is intermediate, with slightly higher egg-to-fledging survival than for ground-nesting species.
Methodological aspects
With respect to methods used, our data set was also very diverse. Radio-telemetry was frequently used in studies of larger species, whereas studies of smaller species included survival estimates based on detection of re-observation probability. In the current data set, we found no significant difference between studies using telemetry or visual detection or studies with or without detection of re-observation probability (Table 2).
Causes of post-fledging mortality
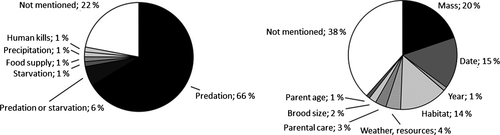
Almost all authors who referred to causes of mortality after fledging note that predation was the major cause, even for “apex-predators” such as Northern Goshawks (Accipiter gentilis, Wiens et al. 2006) and Great Eagle Owls (Bubo lacteus, Rohner 1996). Thus, predation of fledglings appears to be an important determinant, at least as the proximate mechanism (Fig. 5). Relatively few authors discuss potential intermediate causes that influence post-fledging survival rates. Among the factors modulating post-fledging predation rates, fledgling mass (20% of stated effects), fledging date and annual variation (16% combined), and habitat structure (14%) were the most-frequently mentioned factors. Effects of brood size, parent condition, and weather (7%) on post-fledging survival have rarely been addressed. Authors of four papers reported effects of post-fledging parental care on juvenile survival (3%; Styrsky et al. 2005, Grüebler and Naef-Daenzer 2010a,b, Tarwater and Brawn 2010).
Individual characteristics
As summarized above, altricial fledglings are normally not fully developed when they fledge. The analyses of Remeš and Martin (2002) suggest that the age at which young fledge is a specific trait with limited variation compared to, for example, clutch size or the timing of reproduction (see also Woolfenden 1978, Nur 1984, Naef-Daenzer et al. 2001, Deguchi et al. 2002, Dunn et al. 2010). Strikingly, fledglings leave nests at earlier developmental stages when (nest) predation rates are high.
For some species, developmental traits (mass and wing length) were found to be major determinants of post-fledging survival. Naef-Daenzer et al. (2001) showed that fledging mass and seasonal timing of fledging contributed to large variation in early post-fledging survival of Great (Parus major) and Coal (Periparus ater) tits, with no individual in the category of <80% relative fledging mass surviving to independence. For Barn Swallows (Hirundo rustica), wing length was a strong predictor of post-fledging survival (Grüebler and Naef-Daenzer 2010a,b). Several experimental tests have provided evidence that energy flow to the nestlings is a main determinant of fledgling condition and, thus, survival prospects (e.g., Nager 1993, Naef-Daenzer and Keller 1999, Perrig 2015). Therefore, environmental conditions during reproduction and the provisioning performance of parents are crucial predictors of post-fledging survival rates that act via the developmental stage of fledglings.
We found one example for a deviating life history (Tarof et al. 2011). Purple Martins (Progne subis) have an extraordinarily long nestling period of ~4 weeks and, in contrast to most species, fledglings fly instantly over remarkable distances. The authors report a first-week survival rate of 1.0 and a rate of 0.73 to the onset of migration at an age of ~3 weeks, which is markedly higher than reported for other species of swallows (Tarof et al. 2011). For Barn Swallows, by comparison, the rates were 0.81 and 0.40, respectively (Grüebler and Naef-Daenzer 2010a,b).
Parental breeding traits
As introduced in Box 2, parental breeding decisions have been the focus of studies of the evolutionary ecology and reproductive performance of several species (e.g., van Noordwijk et al. 1995, Verboven and Visser 1998, Dawson 2008, Dixon and Haffield 2013). Although investigators have reported differential survival relative to fledgling condition/quality (mainly estimated as fledging mass; e.g., Krementz et al. 1989, Yackel Adams et al. 2001, 2006, Perrig et al. 2014), few have analyzed the potential mechanisms involved (e.g., timing of breeding or post-fledging parental care). Therefore, except for a few species, the impact of variation in parental reproductive traits, their interaction with environmental factors, and the final reproductive output is unclear.
In the first experimental investigation of the effect of parental breeding decisions, the impact of the duration of parental care during the post-fledging period of Barn Swallows was examined. Using a cross-fostering experiment, Grüebler and Naef-Daenzer (2010a) showed that the duration of post-fledging parental care varied markedly among pairs and was the major determinant of post-fledging survival and thus the number of independent offspring produced. Parents caring for their fledglings for 14 d produced 2.5 times more independent offspring than pairs providing 6 d of post-fledging care. With a longer period of parental care, the peak in chick mortality was lower and occurred later. Analyzing timing effects on second broods, Grüebler and Naef-Daenzer (2008, 2010b) found a strong decline in survival of second-brood juveniles with date, particularly during the post-fledging period. Thus, parents have to trade off the benefits of caring longer for first broods against the costs of delaying the start of second broods.
Box 2. Controversial Evidence – The Case of Great Tits
The famous long-term investigations of Great Tits (Parus major) in the Wytham Woods (UK), the Hoghe Veluwe (NL), and on Vlieland (NL) were among the first to reveal evidence concerning the ecological determinants of juvenile survival. Later empirical and experimental studies of the adaptation of clutch size, nestling growth, and timing of breeding have accumulated exemplary insight into the reproductive ecology of the species.
Intriguingly, the early evidence was controversial with respect of the causal mechanisms involved. At the population level, band recoveries suggested that the earliest breeders produced the most recruits (Kluyver 1970, Verboven and Visser 1998, Verhulst and Nilsson 2008). In contrast, studies of individual growth performance showed that it was not the earliest, but those that benefited most from the seasonal development of resources (the “caterpillar peak”) that appeared to have the greatest fitness (Perrins 1991, van Noordwijk et al. 1995, Perrins and McCleery 2001). Studies of the breeding ecology and nestling diet of a major predator, the Eurasian Sparrowhawk (Accipiter nisus), revealed the key role of predation. More than half the diet of nestling Eurasian Sparrowhawks may consist of juvenile birds, creating a marked “tit fledgling peak” in their diet (Geer 1982, Newton and Marquiss 1982, Götmark 2002). However, the interacting effects of food availability and predation remained unclear.
In a pioneering study, Drent (1984) tracked families of Great Tits during the first weeks after fledging by relocating families acoustically and by visual observation of color-banded birds. He found that the first days after leaving nests were a critical bottleneck in the life history of Great Tits. This was later confirmed by radio-tracking fledglings, with particularly high mortality rates during the first 2 days after leaving nests (Naef-Daenzer et al. 2001). Predation is the major determinant of fledgling survival, resulting in immediate selection for high fledging mass and early breeding. Selection differentials for fledging mass are particularly high later in the season, when predation rates are maximal (Naef-Daenzer et al. 2001). The condition of fledglings is also a predictor of their mobility and of the size of family home ranges (Naef-Daenzer and Grüebler 2008). Recent research identified Eurasian Sparrowhawks as the agent of selection for fledging mass (Vedder et al. 2014) and breeding phenology (Götmark 2002).

The timing of fledging of Great Tits in relation to the peak in food availability (“caterpillar peak”) and the later peak in predation pressure. Top panel: Declining food availability and increasing predation pressure create a narrow time window for optimal fledging dates. Bottom panel: The frequency distributions of fledging dates of all fledglings (black bars) and of those surviving to independence (grey bars) indicates that the overwhelming proportion of birds fledging outside the optimal time window were eliminated during the post-fledging dependence period (Data from Naef-Daenzer et al. 2001).
Habitat
Several investigators have addressed the question of whether post-fledging survival rates are affected by habitat conditions. For example, Cohen and Lindell (2004) found that survival of young White-throated Robins (Irania gutturalis) was best in forest habitat, moderate in pastures, and poor in coffee plantations. Furthermore, nestlings in pasture nests fledged at an earlier age than those in nests in coffee plantations. Slagsvold et al. (2013) reported altitudinal moves by families of Great Tits, apparently following the phenology of food resources. Rohner (1996) found that survival rates of young Great Horned Owls (Bubo virginianus) varied relative to the abundance of their main prey. Balogh et al. (2011) found a marked correlation between spatial variation in post-fledging survival of Gray Catbirds (Dumetella carolinensis) and predator abundance. Haché et al. (2014) found that survival of young Ovenbirds (Seiurus aurocapilla) depended on spatio-temporal variation in predator abundance. Other investigators have reported no relationship between post-fledging survival and habitat characteristics (Robles et al. 2007), forest management (Moore et al. 2010), or a gradient in urbanization (Ausprey and Rodewald 2011, 2013). However, this may be due to the fact that complex patterns in range use may develop soon after fledging (e.g., Ciudad et al. 2009, Maness and Anderson 2013, Cox et al. 2014).
Evolutionary significance
Several investigators have reported that strong selection for certain reproductive traits may occur (Lindén et al. 1992, Perrins and McCleery 2001, Monrós et al. 2002). van Noordwijk et al. (1995) demonstrated marked selection for timing of breeding and fledging mass. Naef-Daenzer et al. (2001) suggested that this selective pressure likely operates during the early post-fledging period for Great Tits (Box 2). The selection differential for fledging mass in Great Tits was up to 1.5 g (adult body mass = ~18 g) and increased with season, suggesting that being heavier is particularly important later in the breeding season when predation pressure is higher. For Great Tits, predation by Eurasian Sparrowhawks (Accipiter nisus) was identified as the major selecting force (Götmark 2002, Vedder et al. 2014).
Remeš and Martin (2002) made clear that nestling growth rates and, in turn, the developmental stages of fledglings vary broadly among species. They suggested that predation pressure is a major driver of this variation, with higher predation rates favoring faster-growing nestlings and shorter nestling periods. Adaptation within the multiple trade-offs in clutch size, chick growth trajectories, and allocation of energy to various physical traits (e.g., mass vs. wing length) may explain general—even global—patterns in the reproductive strategies of altricial species of birds (Martin 2015b). Because physical traits at fledging have frequently been detected as proximate determinants of juvenile survival, potential causal mechanisms for adaptation may operate during the post-fledging period (Magrath 1991, Wiens et al. 2006, Lloyd et al. 2009, Vitz and Rodewald 2011). Even among cavity-nesting species in primeval habitats, the impact of predation on nest and nestling survival is high (Wesolowski 2002, Wesolowski and Tomialojć 2005), suggesting that the mechanism operates similarly (but with varying intensity) over the entire range of nesting strategies.
As presented in Box 1, Remes and Martin (2002) and Martin (2005a,b) built models for the adaptation of fledging age relative to stage-specific mortality rates based on the hypothesis that nestlings are expected to fledge when time-dependent mortality inside the nest exceeds the rate outside. A critical issue in such models is to know the time-dependent survival functions (Roff et al. 2005). These appear not to be uniformly continuous. In contrast, an abrupt change of survival rates linked with fledging is frequent (saw-tooth function, Low and Pärt 2009, Fig. 5). Actually, broader insight into the form of the survival function from egg to independence is lacking.
Discussion
Three principal conclusions emerge from our review. First, a post-fledging “bottleneck” in survival rates occurs across a broad variety of taxa (Figs. 2, 3, and 4). This period of high mortality may contribute markedly to the relatively low first-year survival of juveniles in many taxa (e.g., Maness and Anderson 2013, Martin 2015b, Newton et al. 2016). Parental traits, post-fledging parental care, and adaptations related to timing of breeding and the age and stage at which chicks fledge likely have substantial effects on the survival of young birds after fledging. Post-fledging mortality is therefore not random and inevitable. The traits and strategies related to fledging and the post-fledging stage create large variation in survival and, therefore, fitness and so are integral parts of the reproductive strategy of altricial species.
Second, trophic relationships, including both food and predation, are major proximate determinants of post-fledging survival and adaptation of particular reproductive strategies. The energy supplied to nestlings predicts post-fledging survival (via nestling growth and fledgling characteristics; Box 1), and this relates mainly to the availability of food and the provisioning performance of parents. However, predation of fledglings is a major proximate cause of mortality. Great Tits (Box 2) illustrate the compound effects of variation in food resources and predation pressure that play a major role in shaping the reproductive traits of altricial species of birds in general and patterns of post-fledging survival in particular. The reproductive cycle of Great Tits is closely linked to both the reproduction and phenology of “food” species and to the life histories of predators whose reproductive cycles are adjusted to coincide with periods of optimal prey availability by similar mechanisms.
Third, comparisons across species indicate that post-fledging mortality is so substantial that it will influence demographic rates such as recruitment and annual survival (van Noordwijk et al. 1995). Post-fledging mortality is likely additive to the mortality during other periods in the first year (e.g., Perrins 1991, Low and Pärt 2009, Perrig 2015).
In a framework of life-history adaptation, the conundrum of apparently counteracting evolutionary pathways persists. Across species, there is a negative relationship between (nest) predation pressure and fledging age and development (e.g., Remeš and Martin 2002, Martin 2015a, Box 1). In other words, with greater nest predation pressure, the ability of fledglings to escape predators declines. In contrast, individual post-fledging survival strongly increases with advanced development (mass and wing length). These apparently counteracting pressures acting on the duration of nestling periods and post-fledging survival require further investigation. Examination of multiple characteristics of fledglings such as relative mass or wing and muscle development would be useful for further analyzing the effects of variation in nestling development on post-fledging behavioral performance and survival.
Open questions remain in at least three areas: (1) Patterns and mechanisms of differential individual post-fledging survival remain poorly explored, and perhaps this review can provide a basis to build testable hypotheses. (2) Individual morphological and behavioral traits that promote post-fledging survival are incompletely quantified. Comparisons across species may provide an approach to explore the importance of particular traits (Penn and Martin 2015). However, our review has revealed that the data available for analyses across species are scattered and inhomogeneous. (3) Hypotheses concerning adaptation of parental reproductive traits via differential reproductive output require additional tests. Few investigators have quantified differential post-fledging survival in relation to variation in parental traits. Experimental approaches for examining these relationships would be highly desirable.
Differential individual post-fledging survival
First, and possibly most importantly, fledglings face poor survival prospects during the first few days after leaving nests. However, the general pattern varies with species characteristics. For predators, the decline in survival rates after fledging is less pronounced than for non-predators (irrespective of adult body mass). Compared to survival during the nestling period, post-fledging survival of ground-nesting species of birds does not appear to change substantially. In contrast, for species that nest above ground or in cavities, survival during the first week post-fledging has been found to be markedly lower than during the nestling period or the later post-fledging period, creating a marked “bottleneck.” However, post-fledging survival is strongly influenced by environmental factors, individual traits, and condition, and by the breeding decisions of parents. Studies of Great Tits (Box 2) have revealed that the reproductive cycles and phenology of food resources as well as of predators can create quick and strong selection pressures (Naef-Daenzer et al. 2001, Götmark 2002). Therefore, parental breeding decisions and individual characteristics result directly in fitness differentials. In combination with the considerable heritability of reproductive traits (Gebhardt-Henrich and van Noordwijk 1991, Freeman-Gallant and Rothstein 1999, MacColl and Hatchwell 2003), this strongly supports a rapid evolutionary feed-back cycle. Data provided in our review and by Maness and Anderson (2013) support the hypothesis that advanced developmental stages of nestlings (mass and wing length) result in higher post-fledging survival. Further analyses, particularly comparisons between and among species with different nesting characteristics, will be needed to reveal the detailed mechanisms.
Individual traits and behavior
Much evidence for the importance of the development of important physical traits is available in the literature, but few data are available concerning post-fledging behavior and range use. Why, for example, are juvenile birds so noisy? Begging for parental care involves considerable costs in terms of increased risk of predation (Redondo and Castro 1992, Haskell 1994). During the post-fledging period, both the need to beg for parental attention and the probability of attracting predators may be accentuated because young birds are no longer bound to a fixed site.
There are important gaps in our knowledge of the behavior and range use of families of birds during the post-fledging period. In seasonal environments, having young fledge at a relatively early stage may be advantageous because family groups can move to areas with rich resources (e.g., Drent 1984). Similarly, frequent and fast movements may reduce the conspicuousness of young to predators (Naef-Daenzer and Grüebler 2008).
Methodological considerations
Studies of the post-fledging period are still limited by technical and methodological difficulties. Although innovative tracking techniques allow following juvenile birds for increasing periods, other fundamental problems associated with large tracking projects persist.
To estimate survival rates with good precision over short intervals requires large sample sizes. An evident issue is that a sample of birds surviving from one re-encounter interval to the next steadily decreases with the duration of the study. If initial sample sizes are small, the increasing uncertainty of estimates for later stages restrains time-dependent modeling. Therefore, testing mechanistic models that improve survival estimates by including large samples and accounting for re-observation probabilities is important (Naef-Daenzer and Grüebler 2014). However, difficulties at the logistic, personnel, and financial levels rapidly increase with the number of birds to be tracked.
Most studies to date have been correlative, revealing general patterns as a basis to build testable hypotheses about ecological mechanisms and evolutionary consequences. We suggest that studies that include cohorts differing in target variables (e.g., habitat) or that use experimental approaches to manipulate target variables (e.g., age of fledging) would greatly advance our knowledge of the pre- and post-fledging life-history stages. Comparison of post-fledging survival in differing habitats has revealed a strong environmental component (e.g., Cohen and Lindell 2004, Balogh et al. 2011). Experimental approaches such as food supplementation (Thorup et al. 2010, Perrig et al. 2014, Perrig 2015) have tested variation in the energy flow to the brood and its consequences for post-fledging survival. A cross-fostering experiment by Grüebler and Naef-Daenzer (2010a) created variation in the experienced duration of post-fledging parental care and gave insight into the immediate effect of post-fledging parental care.
Conclusions
The apparently contrasting evidence for selection for fledging at earlier developmental stages under high predation pressure versus evidence that the most developed chicks survive best illustrates the fact that neither the proximate mechanisms nor the evolutionary consequences of the survival patterns of altricial young are fully understood. Time-survival functions over the nestling and post-fledging stages appear to vary among nest characteristics and trophic modes. To detail these functions, improved models for the adaptive response to predation pressure and to explain general patterns of breeding by altricial birds are needed (Roff et al. 2005, Martin 2015a,b). Many of the causal mechanisms driving the adaptations may operate during the post-fledging period, and the fate of juveniles from fledging to independence can completely alter reproductive success (Naef-Daenzer et al. 2001, Thompson et al. 2001, Keedwell 2003, Grüebler and Naef-Daenzer 2008, Streby and Andersen 2011). Therefore, inference concerning the evolutionary significance of reproductive traits may be misleading if the number of fledglings is used as a proxy for fitness. Understanding the post-fledging determinants of reproductive performance is also important for inferring key parameters of population dynamics (e.g., Grüebler et al. 2014). Further empirical and experimental studies are required before we will be able to incorporate the post-fledging period into a comprehensive life-history framework covering all stages from egg to recruitment into the breeding population.
Acknowledgments
For advice on the concept and analyses or comments on earlier versions of the manuscript, we thank V. Michel, G. Pasinelli, and M. Schaub. B. Almasi, G. Pasinelli, A. Roulin, and M. Schaub contributed unpublished data on egg-to-fledging survival of various species. We are grateful to V. Remeš and T. E. Martin for granting permission to use the data in Remeš and Martin (2002), and G. Ritchison for carefully copy-editing the manuscript. The authors declare that there are no conflicts of interest.
Appendix 1: Sources of raw data, including major cause of post-fledging mortality and modulating factors
| Reference | Species | Major cause of mortality | Modulating factors of mortality | Interval | Trophic level | Nest type |
|---|---|---|---|---|---|---|
| Ausprey and Rodewald (2011) | Acadian Flycatcher (Empidonax virescens) | Predation | Habitat | Weekly | Non-predatory | Open exposed |
| Middleton and Green (2008) | American Dipper (Cinclus mexicanus) | – | Date | Daily | Non-predatory | Open secure |
| Whittaker and Marzluff (2009) | American Robin (Turdus migratorius) | Predation | Habitat | Weekly | Non-predatory | Open exposed |
| Roulin/Almasi, pers. comm. | Barn Owl (Tyto alba) | – | – | 2-weeks | Predatory | Cavity |
| Grüebler and Naef-Daenzer (2010a,b), own data | Barn Swallow (Hirundo rustica) | Predation | Mass, date | Weekly | Non-predatory | Open secure |
| Magrath (1991) | Blackbird (Turdus merula) | – | Mass | Weekly | Non-predatory | Open exposed |
| Ebenmann and Karlsson (1984) | Blackbird (Turdus merula) | – | – | Weekly | Non-predatory | Open exposed |
| Smith (1967) | Black-capped Chickadee (Poecile atricapillus) | – | – | Weekly | Non-predatory | Cavity |
| Keedwell (2003) | Black-fronted Tern (Chlidonias albostriatus) | Predation | Mass | Weekly | Non-predatory | Ground |
| Weggler (2006) | Black Redstart (Phoenicurus ochrurus) | – | – | 8 days | Non-predatory | Open secure |
| Green and Cockburn (2001) | Brown Thornbill (Acanthiza pusilla) | – | – | 2-weeks | Non-predatory | Open exposed |
| Woodward and Woodward (1979) | Brown-headed Cowbird (Molothrus ater) | Predation | – | Weekly | Non-predatory | Open exposed |
| Davies and Restani (2006) | Burrowing Owl (Athene cunicularia) | Predation, starvation | – | Weekly | Predatory | Cavity |
| Todd et al. (2003) | Burrowing Owl (Athene cunicularia) | Predation | Mass | Weekly | Predatory | Cavity |
| Naef-Daenzer et al. (2001), own data | Coal Tit (Periparus ater) | Predation | Mass, date | Daily | Non-predatory | Cavity |
| Korpimäki and Lagerstrom (1988) | Common Raven (Corvus corax) | – | Date | Weekly | Predatory | Open secure |
| Berkeley et al. (2007) | Dickcissel (Spiza americana) | Predation | Habitat | Weekly | Non-predatory | Open exposed |
| Suedkamp Wells et al. (2007) | Dickcissel (Spiza americana) | Predation | Mass | Weekly | Non-predatory | Open exposed |
| Vormwald et al. (2011) | Dusky Flycatcher (Empidonax oberholseri) | Predation | – | Weekly | Non-predatory | Open exposed |
| Jackson et al. (2011) | Eastern Bluebird (Sialia sialis) | Predation | Mass | Daily | Non-predatory | Cavity |
| Kershner et al. (2004) | Eastern Meadowlark (Sturnella magna) | Predation | – | Weekly | Non-predatory | Ground |
| Suedkamp Wells et al. (2007) | Eastern Meadowlark (Sturnella magna) | Predation | Mass | Weekly | Non-predatory | Ground |
| Eraud et al. (2011) | Eurasian Collared Dove (Streptopelia decaocto) | Predation | – | Weekly | Non-predatory | Open exposed |
| Krementz et al. (1989) | European Starling (Strurnus vulgaris) | – | Mass, date | Weekly | Non-predatory | Cavity |
| Hetmanski (2007) | Feral Pigeon (Columba livia domestica) | Starvation | – | Monthly | Non-predatory | Open secure |
| Zelenak et al. (1997) | Ferruginous Hawk (Buteo regalis) | – | – | Weekly | Predatory | Open secure |
| Woolfenden (1978) | Florida Scrub-Jay (Aphelocoma coerulescens) | – | – | 11d | Non-predatory | Open exposed |
| McIntyre et al. (2006) | Golden Eagle (Aquila chrysaetos) | – | – | 7-weeks | Predatory | Open secure |
| Kenward et al. (1999) | Goshawk (Accipiter gentilis) | Killing | – | 1 mt | Predatory | Open secure |
| Hovick et al. (2011) | Grasshopper Sparrow (Ammodramus savannarum) | Predation | – | Weekly | Non-predatory | Ground |
| Balogh et al. (2011) | Gray Catbird (Dumetella carolinensis) | Predation | – | Daily | Non-predatory | Open exposed |
| Rohner (1996) | Great Horned Owl (Bubo virginianus) | Predation, starvation | – | Weekly | Predatory | Open secure |
| Soler et al. (1994) | Great Spotted Cuckoo (Clamator glandarius) | Predation | – | 5-weeks | Non-predatory | - |
| Naef-Daenzer et al. (2001), own data | Great Tit (Parus major) | Predation | Mass, date | Daily | Non-predatory | Cavity |
| Dhondt (1979) | Great Tit (Parus major) | – | – | Weekly | Non-predatory | Cavity |
| Rush and Stutchbury (2008) | Hooded Warbler (Setophaga citrina) | Predation | – | Daily | Non-predatory | Open exposed |
| Lloyd et al. (2009) | Karoo Scrub-Robin (Cercotrichas coryphaeus) | Predation | Mass | Weekly | Non-predatory | Open exposed |
| Yackel Adams et al. (2006) | Lark Bunting (Calamospiza melanocorys) | Habitat | Date | Weekly | Non-predatory | Ground |
| Salinas-Melgoza and Renton (2007) | Lilac-crowned Parrot (Amazona finschi) | Predation | – | Weekly | Non-predatory | Open exposed |
| Naef-Daenzer et al. (2016), own data | Little Owl (Athene noctua) | Predation | Mass | Bi-weekly | Non-predatory | Cavity |
| Tome and Denac (2012), Tome (2011) | Long-eared Owl (Asio otus) | Predation | – | Weekly | Predatory | Open exposed |
| Nilsson and Smith (1985) | Marsh Tit (Poecile palustris) | – | – | 11-daily | Non-predatory | Cavity |
| Robles et al. (2007) | Middle Spotted Woodpecker (Leiopicus medius) | Predation | Habitat, date | Weekly | Non-predatory | Cavity |
| Low and Pärt (2009) | New Zealand Stitchbird (Notiomystis cincta) | Predation, starvation | Mass | Weekly | Non-predatory | Cavity |
| Ausprey and Rodewald (2011) | Northern Cardinal (Cardinalis cardinalis) | Predation | Habitat | Weekly | Non-predatory | Open exposed |
| Wiens et al. (2006) | Goshawk (Accipiter gentilis) | Food | Mass, year | Weekly | Predatory | Open secure |
| Haché et al. (2014) | Ovenbird (Seiurus aurocapilla) | Predation | – | Weekly | Non-predatory | Open secure |
| King et al. (2006) | Ovenbird (Seiurus aurocapilla) | Predation | Habitat | Weekly | Non-predatory | Open secure |
| Streby and Andersen (2013) | Ovenbird (Seiurus aurocapilla) | Predation | Mass | Weekly | Non-predatory | Open secure |
| Vitz and Rodewald (2011) | Ovenbird (Seiurus aurocapilla) | Predation | Mass | Weekly | Non-predatory | Open secure |
| Powell et al. (2002) | Peregrine Falcon (Falco peregrinus) | – | – | Weekly | Predatory | Open secure |
| McFadzen and Marzluff (1993) | Prairie Falcon (Falco mexicanus) | Predation | – | Weekly | Predatory | Open secure |
| Tarof et al. (2011) | Purple Martin (Progne subis) | (predation) | – | 1 and 3 weeks | Non-predatory | Open secure |
| Cox and Kesler (2012) | Red-bellied Woodpecker (Melanerpes carolinus) | Predation | – | Weekly | Non-predatory | Cavity |
| Sim et al. (2013) | Ring Ouzel (Turdus torquatus) | Predation | Brood size | 4 days | Non-predatory | Open exposed |
| Moore et al. (2010) | Rose-breasted Grosbeak (Pheucticus ludovicianus) | Predation | Habitat | Weekly | Non-predatory | Open exposed |
| Dybala et al. (2013) | Song Sparrow (Melospiza melodia) | Precipitation | Food | Weekly | Non-predatory | Open exposed |
| Johnston (1956) | Song Sparrow (Melospiza melodia) | 2 weeks | Non-predatory | Ground | ||
| Styrsky et al. (2005) | Spotted Antbird (Hylophylax naevioides) | Predation | Clutch size | Weekly | Non-predatory | Open exposed |
| Shipley et al. (2013) | Spotted Towhee (Pipilo maculatus) | Predation | Habitat | Weekly | Non-predatory | Ground |
| Rivers et al. (2012) | Swainson's Thrush (Catharus ustulatus) | Predation | Mass, date | Daily | Non-predatory | Open exposed |
| Overskaug et al. (1999) | Tawny Owl (Strix aluco) | Predation, starvation | – | Weekly | Predatory | Cavity |
| Sunde (2005) | Tawny Owl (Strix aluco) | Predation | Date | Weekly | Predatory | Cavity |
| Coles and Petty (1997) | Tawny Owl (Strix aluco) | Predation | – | Weekly | Predatory | Cavity |
| Wightman (2009) | Western Bluebird (Sialia mexicana) | Predation | – | Weekly | Non-predatory | Cavity |
| Tarwater and Brawn (2010) | Western Slaty-Antshrike (Thamnophilus atrinucha) | Predation | Timing, mass | Weekly | Non-predatory | Open exposed |
| Tome and Denac (2012) | Whinchat (Saxicola rubetra) | Predation | – | Weekly | Non-predatory | Ground |
| Cohen and Lindell (2004) | White-throated Robin (Irania gutturalis) | Predation | Habitat | Weekly | Non-predatory | Open exposed |
| Hylton et al. (2006) | Wood Stork (Mycteria americana) | – | – | Point | Non-predatory | Open secure |
| Anders et al. (1997) | Wood Thrush (Hylocichla mustelina) | Predation | – | Weekly | Non-predatory | Open exposed |
| Powell and Frasch (2000) | Wood Thrush (Hylocichla mustelina) | Predation | – | 3-week | Non-predatory | Open exposed |
| Schmidt et al. (2008) | Wood Thrush (Hylocichla mustelina) | Predation | – | Weekly | Non-predatory | Open exposed |
| Vega Rivera et al. (1998) | Wood Thrush (Hylocichla mustelina) | – | – | 10 d, 21 d | Non-predatory | Open exposed |
| Sullivan (1989) | Yellow-eyed Junco (Junco phaeonotus) | Predation | – | Weekly | Non-predatory | Ground |
| Lloyd and Martin (2016) | Bar-throated Apalis (Apalis thoracica), Cape Robin-chat (Cossypha caffra), Cape Penduline Tit (Anthoscopus minutus), Karoo Prinia (Prinia maculosa), Grey-backed Cisticola (Cisticola subruficapilla), Karoo Scrub Robin (Cercotrichas coryphaeus), Cape Grass Warbler (Sphenoaeacus afer), Chestnut-vented Warbler (Sylvia subcaerulea) | – | – | Weekly | Non-predatory | Open exposed |