Late results of right ventricular outflow tract reconstruction with a bicuspid expanded polytetrafluoroethylene valved conduit
Abstract
Background and Aim to Read
We report the results of a bicuspid expanded polytetrafluoroethylene (ePTFE) valved conduit used for right ventricular outflow tract reconstruction (RVOTR).
Methods
Between November 2005 and February 2009, 12 conduits were used for RVOTR. The mean age and weight of patients were 43.5 ± 46.4 months and 13.4 ± 8.6 kg. The main diagnosis was tetralogy of Fallot with pulmonary atresia in eight patients. The most common conduit size was 18 mm. The mean follow-up was 88.0 ± 35.9 months.
Results
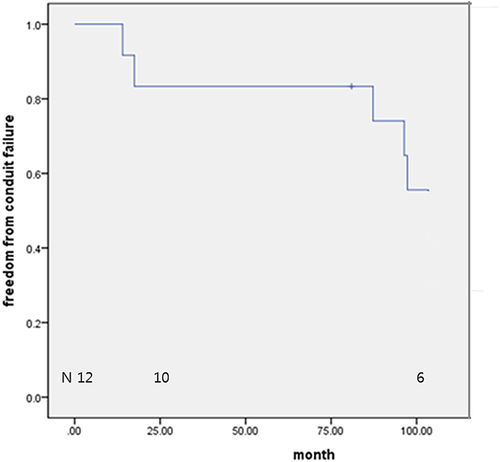
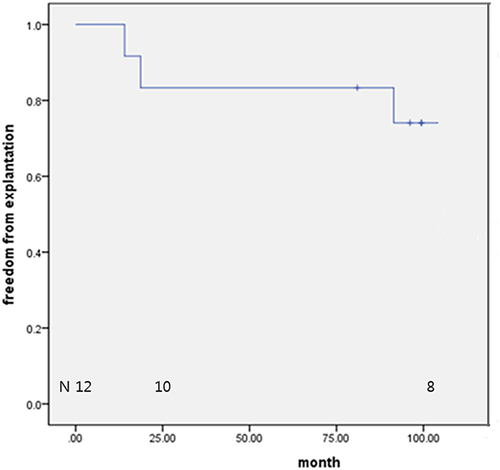
There were no operative and late mortalities. At discharge, the mean peak systolic pressure gradient across the RVOT was 14.1 ± 11.3 mmHg. There was no conduit valve regurgitation in nine patients. At the latest echocardiography (mean follow-up: 84.3 ± 35.5 months), the mean peak systolic pressure gradient across the RVOT was 59.7 ± 20.2 mmHg, and there was no conduit valve regurgitation in six patients. Freedom from conduit malfunction was 100% and 83.3%, at 1 and 8 years, respectively. Two conduits were explanted due to sternal compression and four from conduit malfunction. Freedom from explantation was 83.3% and 74.2% at 2 and 8 years, respectively.
Conclusions
ePTFE bicuspid valved conduit has good late function in terms of valve regurgitation, but the pressure gradient across the conduit increases with time, which is the main cause of conduit failure and explantation.
1 INTRODUCTION
Conduit selection for right ventricular outflow tract (RVOT) reconstruction is a major challenge in the treatment of congenital heart diseases with RVOT obstruction. Xenografts and homografts have been used for long time, but their longevity is limited due to early degeneration or calcification especially in young patients.1-6 Moreover, the availability of these conduits can be limited. Replacement with a bioprosthetic or mechanical valve is sometimes required when the patient reaches late adolescence or early adulthood. However, for neonates there is no ideal conduit. To expand current conduit alternatives, we developed a bicuspid expanded polytetrafluoroethylene (ePTFE) valved conduit that is composed of a standard stretch PTFE graft and bicuspid cusps that are made of a thin (0.1 mm) PTFE membrane (W.L. Gore & Associates, Inc., Flagstaff, AZ). When the ePTFE membrane was used, significant calcification was not demonstrated in monocuspid, bicuspid, and tricuspid valved RVOT reconstruction.7-10
The purpose of this study is to investigate the long-term functional results of this valved conduit.
2 PATIENTS AND METHODS
We retrospectively reviewed data of patients who underwent RVOT reconstruction using an ePTFE bicuspid valved conduit between November 2005 and February 2009 at Pusan National University Hospital and Pusan National University Children Hospital. The patients’ clinical records were reviewed for preoperative, intraoperative, post-operative, and follow-up data. The study was approved by the local institutional review board.
2.1 Patient profile
Twelve bicuspid ePTFE valved conduits were implanted in 12 patients with a mean age of 43.5 ± 46.4 months (range: 0.2-163.5 months, median: 25.3 months) and mean body weight of 13.4 ± 8.6 kg (range: 3.0-35.0 kg, median: 11.4 kg). The diagnoses were tetralogy of Fallot (TOF) with pulmonary atresia in eight patients, TOF with absent pulmonary valve syndrome in one, transposition of great arteries in one, and right ventricular outflow conduit stenosis in two. The conduit sizes varied between 12 and 20 mm in diameter, based on the patient's body size: 12 mm in one patient, 14 mm in one, 16 mm in two, 18 mm in six, and 20 mm in two.
2.2 Surgical technique
We used two techniques to make the bicuspid valved conduit, as shown in Figures 1 and 2. In one technique, which was used for the initial two patients, two curvilineal incisions were made, and appropriately designed valved cusps (with an ePTFE membrane that was 0.1-mm thick) were introduced into the lumen of the conduit through separate incisions. The cusps were attached to the incisions by closing the incision together with the cusps (Figure 1). In the other technique, which was applied to the remaining 10 patients, we divided the conduit into two parts: the valve and the conduit. We made two longitudinal incisions of appropriate lengths at the bilateral sides of the valve portion. The appropriately designed two layers of the ePTFE membrane that had commissures were put into the conduit through the incisions, and the two incisions were closed together with the ePTFE membranes to attach the valve cusp to the conduit. Then this valve portion was connected to the beveled end of the conduit portion by suturing the cusp membrane together, after the two layers of the membrane were opened. The bicuspid valved conduit was created by connecting the valve and conduit portion of the conduit (Figure 2).
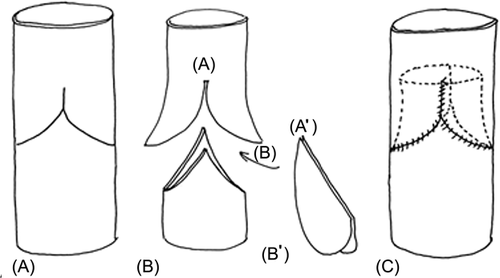
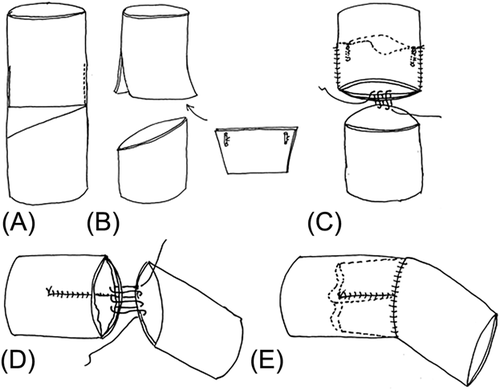
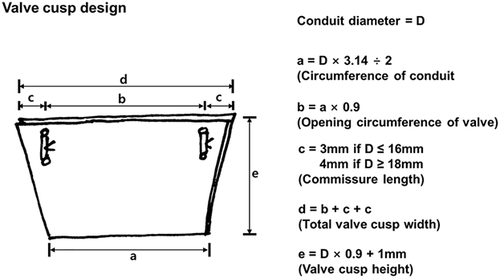
The conduit was prepared under aseptic conditions in an operating room, and was used after ethylene oxide gas sterilization was performed. The smallest conduit that could be manufactured using this technique was 12 mm in diameter. Figures 1 and 2 show how the valved conduit was made and Figure 3 shows how the cups were designed.
2.3 Postoperative management
All patients received heparin immediately after the cessation of postoperative bleeding. Anticoagulation with coumadin was then started after oral intake resumed and continued for 3 months. Aspirin was administered indefinitely thereafter.
2.4 Follow-up
Follow-up data were obtained through the patients’ medical records. The function of the valved conduit was evaluated using two-dimensionial echocardiography data that were described in the medical records, and was evaluated for conduit thrombosis, conduit pressure gradient, and degree of pulmonary valve regurgitation. There was 100% patient follow-up.
The mean follow-up duration was 88.0 ± 35.9 months (range: 14.0-130.0 months, median 100.4 months). We defined valved conduit “failure” when the valved conduit gradient was higher than 50 mmHg and when there was greater than moderate valve regurgitation.
2.5 Statistical analysis
Data analysis was performed with SPSS 19.0 software (IBM Corp., Armonk, NY). Continuous data are presented as means with standard deviation and range. The Kaplan-Meier method was used to estimate freedom from conduit malfunction and freedom from conduit explantation for any reason.
3 RESULTS
There were no operative deaths. There were no conduit-related complications or reoperations during the hospitalization period, and late death did not occur. There were no late complications, including thrombotic occlusion, thromboembolism, and endocarditis. At the time of hospital discharge, the mean peak systolic pressure gradient across right ventricular outflow tract was 14.1 ± 11.3 mmHg (range: 0-34.4 mmHg). Conduit valve regurgitation at discharge was none in nine patients, mild in two, and mild-to-moderate in one. Echocardiographic follow-up data were available in all patients; the mean peak systolic pressure gradient across the right ventricular outflow tract after a mean follow-up period of 84.3 ± 35.5 months (range: 12.5-130.0 months) was 59.7 ± 20.2 mmHg (range: 21-82 mmHg). Conduit valve regurgitation at latest follow-up was none in six patients (50%), mild in three, and mild-to-moderate in three.
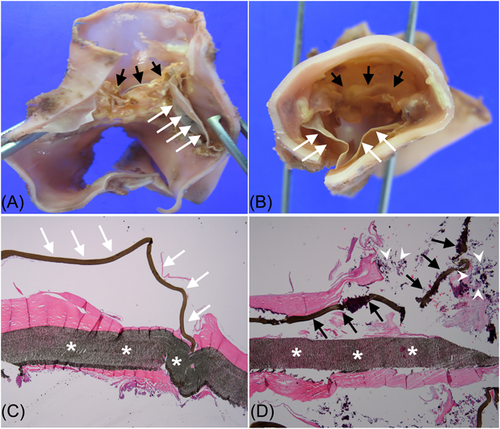
Valved conduit malfunction was noted in nine patients (75.0%) at the latest follow-up. All valve malfunctions were due to an increased pressure gradient, not to conduit valve regurgitation. High pressure gradient in two patients was caused by conduit compression by the sternum. The freedom from valve conduit malfunction rates were 100%, 83.3%, and 74.1% at 1, 2, and 8 years, respectively (Figure 4). Six patients (50%) underwent conduit explantation during the follow-up period. The causes of conduit explantation were conduit stenosis in all patients, among whom two had conduit compression by the sternum. The freedom from valved conduit explantation rates were 83.3% and 74.1% at 2 and 8 years, respectively (Figure 5). A larger size tricuspid ePTFE valved conduit was used for replacement in four patients, and an artificial valve with a hood was implanted on the previous valved conduit in the remaining two patients. Some thickening around the commissure and hinge portion of the cusps were observed on macroscopical examination of the explanted bicuspid valved conduit, even though the free surface and margin of the cusp remained thin. In histopathological examination, the mobile leaflet appeared to be normal; however, the immobile leaflet was covered with calcium and fibrotic tissue (Figure 6).
4 DISCUSSION
ePTFE membranes have excellent biocompatibility and compact microporous structures that impede cellular infiltration.9 These characteristics make the membrane resistant to calcification and provide longer mobility of the valve cusps. However, it is not easy to make valve cusps using an ePTFE membrane because of its lack of distensibility, which is one of the reasons that ePTFE membranes are not frequently used as valve cusps. Furthermore, reports about the long-term results of the monocusp are very limited. In 2011, Yoshida et al8 reported the early results of the bicuspid ePTFE valved conduit. Their early results were excellent, but their follow-up duration was only 6.2 ± 3.9 months.
Miyazaki et al9 produced an excellent ePTFE membrane tricuspid valved conduit with bulging sinuses, but we believe that this method is technically demanding and less reproducible. In particular, the quality of the valves depend on the skill level of the surgeon. Valve cusps that are made using the over-and-over suture technique could also have wrinkles at the cusps, which can produce furrows. These small furrows produce a potential place for collecting fibrin or cells that could hinder cusp motion.
We have simplified the technique of making an ePTFE valved conduit to make it more durable and reproducible. We avoided the over-and-over suturing technique, which can be technically demanding and makes wrinkles on the cusp. Instead, we attached the valve cusps to the conduit with the “incision and suture” technique, in which two incisions were made in the conduit and the incisions were closed together with the ePTFE membrane. Furthermore, the valved conduit that was created by our second technique was designed to have some angulation to fit the natural angulation between the right ventricle and main pulmonary artery.
In Miyazaki et al's11 report of the tricuspid ePTFE valved conduit with a bulging sinus, the rate of freedom from reoperation at 10 years was 95.4%; however, the mean follow-up duration was only 3.6 years. Shinkawa et al12 showed that the 5-year freedom from conduit reoperation rate was 92.7% when using a tricuspid valved conduit, and the rate of freedom from severe conduit insufficiency or a gradient greater than a 50 mmHg was 74.8%. In our series, the two patients who required conduit reoperation within 2 years had conduit compression by the sternum. This is probably related to the failure of the conduit to increase in size as the patient grows. Patient growth, valve cusp design, and decreased valve cusp mobility could contribute to the increase of the pressure gradient across the valved conduit. Increased pressure gradient across the valved conduit caused by patient growth is a natural phenomenon, which is unavoidable for all kinds of contemporary valved conduits. When considering the valve cusp design, our bicuspid valved conduit has taller (longer) valve cusps and a narrower opening area than the tricuspid design. The bicuspid valve is thought to produce more resistance than tricuspid valves. We have changed the valve design from bicuspid to tricuspid since 2009. Some pseudointimal thickening around the commissure and hinge portion of the cusps were observed in macroscopical examination of the explanted bicuspid valved conduit, even though the free surface and margin of the cusp remained thin. It is our opinion that becoming less mobile in one leaflet of bicuspid leaflets could not be avoidable in bicuspid design. And we could observe more pseudointimal thickening and calcification on the immobile leaflet. The mobile leaflet could be kept relatively free of pseudointimal thickening and calcification for a long time. This is another important point that the valve design should be tricuspid in which all three leaflets can be more evenly mobile. We think that this pseudointimal thickening, even if it is mild, could contribute to reduced mobility of the valve cusps and an increase of the pressure gradient over time. Our bicuspid valved conduit was more resistant to the development of late conduit valve regurgitation than other types of conduits. In a study by Yoshida et al,8 three of 18 patients with a bicuspid valved conduit already had more than mild conduit regurgitation at the time of hospital discharge. However, their long-term results have not been reported so far. The tricuspid valved conduit reported by Shinkawa et al12 had moderate or severe conduit valve regurgitation in 20% of their patients. Quintessenza et al13 showed good midterm results in 126 patients. They made the valve of ePTFE bicuspid valve and sutured into RVOT without conduit. Only six patients (4.8%) required replacement because of immobile and calcified leaflets even though the technique for making bileaflet cusps are quite different from ours. Their surgical technique may be a good option when the patients needed valve implantation without conduit.
In conclusion, a bicuspid ePTFE valved conduit can be used in young patients with reasonable durability. However, pressure gradients across the conduit valve tends to increase over time, which limits the durability in these patients. Percutaneous options using the Melody and modified stented bovine bioprostheses may have a role to play in dealing with these stenotic conduit valves.14, 15
CONFLICT OF INTEREST
The authors acknowledge no conflict of interest in the submission.