Intralesional injection of platelet-rich plasma versus steroid in the treatment of oral lichen planus
Abstract
Background
Oral licen planus (OLP) is a chronic inflammatory disease and may have immunological background. Both intralesional injection of PRP and steroids succeeded in treating and decreasing recurrence of the disease.
Patients and Methods
Twenty-four participants with clinically diagnosed as OLP were enrolled in this study. We separated the patients in 2 groups, 12 patients in group A were treated by intralesional PRP every two weeks for 2 months or stopped if healing occurred earlier. Group B (12 patients) treated by intralesional Triamcinolone Acetonide (TA) (20 mg) every two weeks for 2 months or may be less if healing occurred earlier. The response of OLP lesions to treatment was evaluated by reduction of lesional areas, REU scores, and NRS scores. The patients with complete response (CR; 80%–100% reduction in the lesion area) were followed for 3 months biweekly.
Results
There was a statistically significant decrease in REU and pain score in both groups after treatment compared to before. There was a statistically increase in frequency of side effects among patients received PRP especially pain compared to those treated by steroid. Also, recurrence of the disease after treatment during follow-up for 3 months was more significant among patients treated by PRP.
Conclusion
Intralesional PRP is a good and safe modality for treatment of OLP and intralesional TA. However, there were some side effects and recurrence of disease after follow-up for three months in patients treated by PRP more than those treated by TA.
1 INTRODUCTION
Oral licen planus (OLP) is a chronic inflammatory disease affecting the oral mucosa. The role of autoimmunity in the pathogenesis of OLP is evident by the presence of autocytotoxic T cells in OLP lesions Roopashree et al.1
Although various therapeutic modalities have been used for management of OLP, none has been conclusive in treating and preventing recurrence Córdova et al.2 Topical, intralesional, and systemic steroids achieve good results but due to their side effects and resistant cases, many other treatments were used as azathioprine, retinoids, cyclosporins, pimecrolimus, and tacrolimus Piñas et al.3 It was found that deficient transforming growth factor-α1 (TGF-α1) may predispose to autoimmune lymphocytic diseases and subsequently administration of TGF-α1 may have a therapeutic effects in those diseases including OLP Sugerman et al.4 This has raised the question of whether the application of biomolecules obtained from the patient's autologous blood and contained such growth factor at OLP lesions could treat the disease Mutafchieva et al.5
Autologous platelet-rich plasma (PRP) is a concentration of human platelet that is threefold to fivefold more higher than normal concentration in the whole blood, this product contains large amounts of growth factors as PDGF, TGF, VEGF, and FGF which may help in treating OLP lesions Emer.6 The aim of our work was to evaluate the efficacy and safety of intralesional steroid and PRP in treatment of OLP.
2 PATIENTS AND METHODS
This study included 36 persons (24 OLP patients and 12 control).The following patients were excluded: those with a history of topical or systemic therapy for OLP in the past 4 weeks prior to the study, with a history of medication causing lichenoid reaction, with a history of immunosuppression, with bleeding tendency and/or on anticoagulant therapy, those with anemia, with oral candidiasis, with active oral herpes simplex or history of recurrent oral herpes simplex, and pregnant or lactating women. The present study was performed with approval from the Institutional Review Board (IRB) vide approval number 4744. A written informed consent form approved by the IRB was obtained from every participant. The study was conducted in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for studies involving humans.
Surface area of erythema and ulceration were measured using a ruler, and were estimated in squared millimeter. The maximum diameter of the lesion and the maximum width of the lesion, perpendicular to it, were measured. The lesion area was obtained by the multiplication of the two values in squared millimeter. The response of OLP lesion to treatment was evaluated on the basis of reduction in its area. The response was classified as complete or almost complete response (CR: 80%–100% reduction), good response (GR: 50%–79% reduction), partial response (PR: 20%–49% reduction), no response (NR: <20% reduction) or worsening.
REU and pain scores were done to all the patients before and after treatment. In REU scoring, the oral cavity was divided into 10 sites: right buccal mucosa, left buccal mucosa, tongue dorsum, tongue ventrum, maxillary gingiva, mandibular gingive, floor of the mouth, hard palatal mucosa, soft palate and tonsil, and labial mucosa (upper and lower together). The severity of the lesion in each site was scored according to the following: (a) Presence of reticular/hyperkeratotic/ white papular (R) lesions as 0 = none and 1 = presence; (b) presence of each erythematous (E) lesions as 0 = none, 1 = lesions smaller than 100 mm2, 2 = lesions from 100 to 300 mm2, and 3 = lesions larger than 300 mm2; (c) presence of each ulcerative (U) lesions as 0 = none, 1 = lesions smaller than 100 mm2, 2 = lesions from 100 to 300 mm2, and 3 = lesions larger than 300 mm2. Each REU score was totaled from all 10 areas, and the total weighted score was a summation of reticulation score, erythematous score (weighted 1.5), and ulcerative score (weighted 2.0): ∑R + ∑ (E × 1.5) + ∑ (U × 2.0) Piboonniyom et al.7
Pain was measured using a numerical rating scale (NRS). Patients were asked to assign a numerical score representing the intensity of their symptoms on a scale from 0 to 10 with 0 being no symptoms and 10 being worst imaginable symptoms Park et al.8
Patients were divided into 2 groups: Group A included 12 patients treated by intralesional PRP, and group B involved 12 patients treated by intralesional triamcinolone acetonide.
Digital photographs before, during, and after treatment of clinical cases were taken using digital camera with resolution 10 Mega Pixels. The camera used in the study is Canon IXUS 9515 digital camera.
In group A, PRP Injection was done at four point of the lesional periphery: superior, inferior, left, and right. Injection was done using 1 ml syringe and 30 guage sterile needles. PRP was injected while still in a liquid state. Pressure with sterile gauze was applied for 3–5 min to avoid bleeding. The injection was performed every 2 weeks for 2 months.
The preparation of autologous PRP was performed with the following method: 3 ml of venous blood was drawn from each patient under sterile conditions and collected into PT tubes (3.2% sodium citrate) and then centrifuged at 222 g for 20 min. The lower red blood cell portion was discarded, and the supernatant with the pellet of platelets collected was approximately 1.5 ml. Three percentage of calcium chloride was added to the PRP in a ratio of 1:10 to induce platelet activation El-Komy et al.9
In group B, intralesional injection of triamcinolone acetonide 40 mg/ml vail mixed with 1 ml of lidocaine 2% with 20 mg/ml of TA final concentration was done. TA was injected using insulin syringe with a 0.5-inch long, 30-gauge needle, as multiple 0.2-ml injections at 1-cm intervals. The injection was administered into the connective tissue below the lesion from adjacent normal mucosa. Pressure with sterile gauze was applied for 3–5 min to avoid bleeding. The injection was performed every 2 weeks for 2 months.
All patients in both groups were followed every 2 weeks for 3 months after the end of the treatment period. Incisional biopsies of the lesions and histopathological examination were performed before and after any of the two therapies.
3 RESULTS
The clinical characteristic of the studied groups regarding age, sex, duration of the disease, and type and site of OLP lesions were illustrated in Table 1.
| Variable | Group A (PRP) (n = 12) | Group B (Steroid) (n = 12) | MW | p | ||
|---|---|---|---|---|---|---|
| Age: (years) | ||||||
| Mean ± SD | 47 ± 13.12 | 52.17 ± 9.93 | 0.63 | 0.54 | ||
| Range | 30–70 | 38–72 | NS | |||
| Variable | n | % | n | % | χ 2 | p |
| Sex | ||||||
| Female | 7 | 58.3 | 7 | 58.3 | 0.23 | 0.89 |
| Male | 5 | 41.7 | 5 | 41.7 | NS | |
| Duration (months) | ||||||
| Mean ± SD | 14.5 ± 12.62 | 8.83 ± 5.52 | 1.54 | |||
| Median | 10 | 8 | 0.12 | |||
| Range | 3–48 | 2–24 | NS | |||
| n | % | n | % | χ 2 | p | |
| Site | ||||||
| Buccal | 7 | 58.3 | 5 | 41.7 | 1.42 | |
| Lower lip | 5 | 41.7 | 6 | 50 | 0.49 | |
| Buccal & soft palate | 0 | 0 | 1 | 8.3 | NS | |
| Type | ||||||
| Erosive | 4 | 6 | 50 | 1.60 | ||
| Reticular | 2 | 3 | 25 | 0.44 | ||
| Mixed | 6 | 3 | 25 | NS | ||
| Other associated lichen lesions | ||||||
| No | 8 | 5 | 41.7 | 3.42 |
0.635 NS |
|
| Yes | 4 | 7 | 58.3 | |||
| Actinic lichen | 1 | 1 | 8.3 | |||
| Cutaneous lichen | 3 | 4 | 33.3 | |||
| Lichen Planus pigmentosus | 0 | 1 | 8.3 | |||
| Genital lichen | 0 | 1 | 8.3 | |||
| Family history | ||||||
| −ve | 12 | 12 | 100 | – | – | |
| HCV antibodies | ||||||
| −ve | 7 | 9 | 75 | 0.75 | 0.39 | |
| +ve | 5 | 3 | 25 | NS | ||
- Abbreviations: MW, Mann–Whitney test; NS, Non-significant (p > 0.05) **: Highly significant (<0.01); SD, Standard deviation; χ2, Chi-square test.
- Bold values are p values of statistical significance.
There were no statistically significant differences between the studied groups in REU and pain score (NRS) before or after treatment. Regarding difference between before and after in each group, there was a statistically significant decrease in REU and pain score in both groups after treatment compared to before (Table 2).
| Variable | Group A (PRP) (n = 12) | Group B (Steroid) (n = 12) | MW | p |
|---|---|---|---|---|
| REU before | ||||
| Mean ± SD | 3.96 ± 2.24 | 3.38 ± 2.05 | 0.90 | |
| Median | 3.5 | 3 | 0.37 | |
| Range | 1–8.5 | 1–9 | NS | |
| REU after | ||||
| Mean ± SD | 2.38 ± 2.20 | 1.58 ± 1.95 | 1.26 | |
| Median | 1.75 | 1.5 | 0.21 | |
| Range | 0–8.5 | 0–5 | NS | |
| p# | 0.03* | 0.01* | ||
| NRS for pain (score 0–10) before | ||||
| Mean ± SD | 5.83 ± 2.41 | 6.25 ± 2.38 | 0.47 | |
| Median | 5 | 5.5 | 0.64 | |
| Range | 2–10 | 2–10 | NS | |
| NRS for pain (score 0–10) after | ||||
| Mean ± SD | 3 ± 2.66 | 2.58 ± 3.45 | 1.12 | |
| Median | 2 | 1.5 | 0.26 | |
| Range | 0–10 | 0–10 | NS | |
| p# | 0.007** | 0.005** | ||
- Abbreviations: MW, Mann–Whitney test; NRS, numerical rating scale scoring for pain (range from 0 to 10); NS, Non-significant (p > 0.05) * Significant (p < 0.05) ** Highly significant; #, Paired Wilcoxon test (p < 0.01); REU, R reticular/hyperkeratotic, E erythema/erosion, U ulcerative; SD, Standard deviation; χ2, Chi-square test.
- Bold values are p values of statistical significance.
Response to treatment was measured according to the reduction in the lesional area No < 20%, Partial (20%–49%), Good (50%–79%), and Complete (80%–100%; Figures 1 and 2). There were no statistically significant differences between the studied groups in therapeutic response (Table 3).
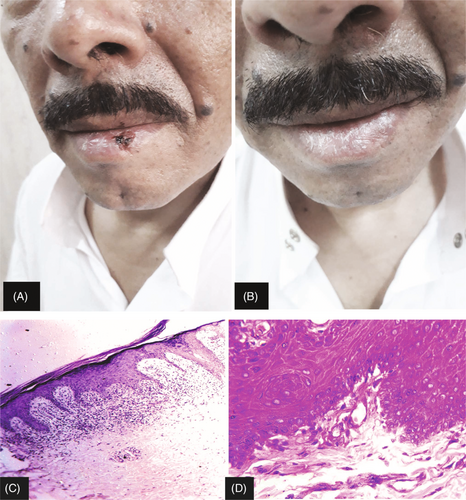
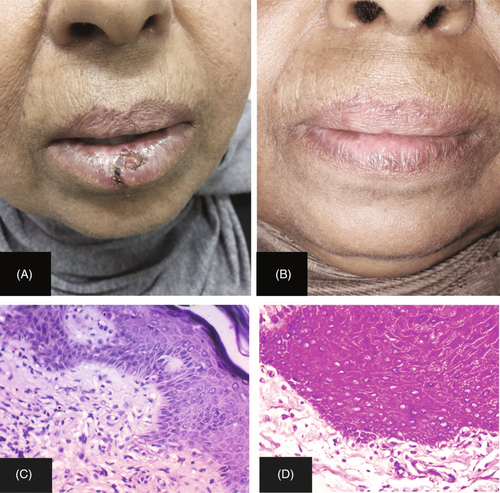
| Variable | Group A (PRP) (n = 12) | Group B (Steroid) (n = 12) | χ 2 | p | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Response: (According to reduction in the lesional area) | ||||||
| No < 20% | 4 | 33.3 | 4 | 33.3 | ||
| Partial (20%–49%) | 2 | 16.7 | 0 | 0 | 0.36 | |
| Good (50%–79%) | 3 | 25 | 2 | 16.7 | 3.2 | NS |
| Complete (80%–100%) | 3 | 25 | 6 | 50 | ||
| Side effects | ||||||
| No | 4 | 33.3 | 9 | 75 | ||
| Yes | 8 | 66.7 | 3 | 25 | ||
| Pain | 6 | 50 | 0 | 0 | 4.20 | 0.04* |
| Infection | 2 | 16.7 | 2 | 16.7 | ||
| Bleeding | 0 | 0 | 1 | 8.3 | ||
- Abbreviations: χ2, Chi-square test; NS, Non-significant (p > 0.05).
- The asterisk represents the degree of statistical significance.
4 DISCUSSION
In the present study, there was a statistically significant decrease in REU and pain score (NRS) in both groups after treatment compared to before, but regarding the differences between both groups there were no statistically significant differences between the studied groups in REU and pain score (NRS) before or after treatment.
Platelet-rich plasma is a recent method used to help management of OLP. The present study was done on 12 patients having OLP (4 erosive, 2 reticular, 6 mixed types) used intralesional PRP injection every two weeks for five sessions, and clinical evaluation of the patients was done by REU score and NRS score for pain. Response depended on the percent of reduction in the lesional area (CR: 80%–100% reduction), (GR: 50%–79% reduction), (PR: 20%–49% reduction), and (NR: <20% reduction) or worsening.
The results of the present study showed that 3 patients (25%) showed complete response (two of them showed erosive OLP and one mixed erosive and reticular OLP) to treatment by intralesional PRP and complete disappearance of pain. Platelet-rich plasma gave 66.6% complete response among patients who had erosive OLP.
Regarding side effects of treatment, there was a statistically increase in frequency of side effects among patients received PRP especially pain compared to those treated by steroid. Regarding recurrence of the disease after treatment during follow-up for 3 months, there was a statistically significance increase in frequency of recurrence among patients treated by PRP, all treated patients showed recurrence of OLP after 6 weeks.
On the contrary, in study done by Piñas et al.,3 on four patients had erosive OLP. Platelet-rich in growth factors (PRGF) was infiltrated once weekly, and clinical evaluation of the patients was done by VAS score for pain and reduction in the lesional area. Piñas et al.3 resulted that 2 patients (50%) had complete healing of the lesion and reduction in the lesional area by 80%–90% of the lesion size after single infiltration of PRGF and 2 patients needed another infiltration to achieve complete healing. In all cases, no side effects were noted. No recurrence was noted through the follow-up period of six months following the last infiltration of PRGF.
In another study of Piñas et al.,10 done on 10 patients having erosive form of OLP, treatment protocol was the same as done Piñas et al.3 Significant pain reduction and complete healing of the lesions defined a successful outcome. The result of this study showed that 8 (80%) patients had complete remission of the disease after single infiltration of PRGF, only 2(20%) patients need second infiltration to be symptoms free, and no side effects were recorded during follow-up.
Better results in Piñas et al.3, 10 studies may be due to that all patients who had erosive OLP may gave better results with PRP than other OLP types, also frequent injection of PRP every week may provide sufficient growth factors required for healing of erosive OLP, usage of commercial kits that may be better than the conventional method that was used in our study for preparing PRP.
But if comments done on results of PRP in the treatment of only erosive type OLP, the present study had better results than Pinas et al., 2017 had, this means that PRP is a good therapeutic tool in treatment of erosive OLP.
Another case report study done by Shaik et al.11 The study involved one patient had erosive lichen planus and received intralesional PRP injection once per week for four weeks. The result showed a significant reduction in patient symptoms and clinical presentation of the lesion after one week and complete regression of the lesion after 4th week and complete reduction in the VAS score; no recurrence was observed in follow-up for six months.
Shaik et al.11 results showed better results than the present study may be due frequent injection every 1 week, different sample size of the study.
As regarding to group (B) in our study which include 12 patients of OLP (6 erosive, 3 reticular, 3 mixed type), patients treated by intralesional TA injection (20 mg/ml) every two weeks until healing occurred maximum 2 months, clinical evaluation of the patients done by REU score and NRS score for pain, response depended on percent of reduction in the lesional area (CR: 80%–100% reduction), (GR: 50%–79% reduction), (PR: 20–49 reduction), and (NR: <20% reduction) or worsening.
The results showed that 6 patients (50%) had complete response (4 of them had erosive OLP and 2 had mixed erosive and reticular). Intralesional injection of TA gave 66.6% complete response among patients who had only erosive OLP.
No recurrence of the lesion has occurred among patients showed CR after 3 months. Side effects occurred in 25% of cases in the form of infection and bleeding.
Xia et al.12 had a study involved 45 patients with clinical and histologically confirmed ulcerative OLP on bilateral buccal mucosa, one side was used for treatment and the other side for control, single TA injection (of concentration 40 mg/ml) resulted in rapid decrease in VAS score, ulcerative areas and REU scores. Complete response was reported in 84.4% of patients. No side effects were recorded in this study.
Although in the present study, TA (20 mg/ml) injection was given until patients showed complete response maximum 5 sessions. Xia et al.12 study encourages higher concentration of TA (40 mg/ml) with fewer number of sessions; this may explain that severe inflammatory process occurred in OLP need high doses of intralesional corticosteroids. Absence of side effects in Xia et al.12 study may be explained by the higher therapeutic antiinflammatory dose with fewer sessions number as shot therapy reliefing the severe inflammation in OLP lesions.
On addition Lee et al.13 study done on 40 patients compared the efficacy and safety of intralesional TA (20 mg/ml) with TA 0.4% topical solution for the management of OLP. Twenty patients used topical therapy three times a day for six weeks, while the other 20 patients were administered intralesional injection once a week for the first four weeks, and one additional injection after two weeks (five injections in total), patient evaluation was assessed using VAS score for pain and special objective scoring system for OLP by Escudier et al.14
Results showed significant improvement in the previous scores; follow-up was done for one year and showed relapse in 40% in patients. Regarding side effects, only single patients (5%) showed cushingoid features.
Lee et al.13 results were similar to our study, this explained by usage of the same concentration of TA (20 mg/ml) but injection interval was shorter every week, also recurrence was higher than our study that may explained by long period of follow-up (1 year) and this is explained by the chronic nature of the disease with periods of resolution and others with relapse. The difference in the percentage of side effects may be explained by different side effects in both studies, our study resulted that 25% of patients showed infection and bleeding at site of injection but Lee et al., 2013 resulted that 5% of patients showed cushingoid features.
In another study done by Xiong et al.,15 a total of 56 OLP patients were randomly assigned to receive either intralesional injection of 0.5 ml BCG-PSN every other day (31 of 56) or 10 mg TA, a positive-controlled group, (25 of 56) every week for 2 weeks. Patient's evaluation was done by VAS score for pain and lesional area measurement.
The result of Xiong et al.15 study showed that 22 patients (88%) showed complete response and complete recovery of erosive lesions after 10 mg TA injection and complete absence of pain, recurrence was found in 45.5% of patients during 3 months follow-up and side effects occurred in 8% of patients in the form of tingling or burning sensation at the injection site.
The better results of Xiong et al.15 may be due short interval between sessions although low concentration of TA (10 mg/ml); also, Xiang study yielded in high recurrence rate over the result of our study that may be give the upper hand for usage of TA injection in higher dose to ensure long period without recurrence.
The above studies cleared that of usage of higher concentration of intralesional TA every week in OLP showed better results and was effective in reduction of the lesional area and diminution of clinical scoring (REU) and pain sensation because of the anti-inflammatory and immunosuppressive effect of corticosteroid and its regulation on local T-cell-mediated immunologic disorders to a balanced state.
In the present study, regarding comparison of side effects to treatment with PRP and TA, there was a statistically increase in frequency of side effects among patients received PRP especially pain compared to those treated by steroid; this may be due to the dilution of TA 40 mg with lidocaine as local anesthetic. Also, there was statistically significance increase in recurrence rate among patients treated by PRP compared to TA; this may be explained by consumption of growth factors at site of lesion after short period or to the immunosuppressive action of corticosteroids may last for along time.
4.1 Limitations
The small sample size in this study is a major limitation.
CONFLICT OF INTEREST
The authors report no conflict of interest.
ETHICAL APPROVAL
A written informed consent form approved by the institutional review board was obtained from every participant. The present study was performed at Zagazig University hospitals with approval from the Institutional Review Board (IRB) vide approval number ZU-IRB#4641 on May 6, 2018. The study was conducted in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for studies involving humans.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




