EGCG inhibits hypertrophic scar formation in a rabbit ear model
Yajuan Song and Tong Wang contributed equally to this work.
Abstract
Objective
Hypertrophic scarring is a common skin fibro-proliferative disease, but currently there has no satisfactory drugs for anti-scar treatments. Previous study showed that epigallocatechin gallate (EGCG), the main catechin in green tea, improved wound healing and tissue fibrosis in both rats and mice. In the present study, the therapeutic effects of EGCG on hypertrophic scar were analyzed using a rabbit ear hypertrophic scar model.
Materials
A rabbit ear model of hypertrophic scarring was used. DMSO, 0.5 mg EGCG/wound, 1.0 mg EGCG/wound or triamcinolone were injected subcutaneously once a week for 4 weeks. The scar elevation index (SEI) was measured using HE staining images, the collagen fibers were examined by Masson’ trichrome staining images, and the number of capillaries in hypertrophic scar were calculated by CD31 staining images. The mRNA levels in the scar tissues were detected by quantitative real-time polymerase chain reaction (qRT-PCR).
Results
Gross observation and histological evaluation showed the inhibitory effects of EGCG on hypertrophic scar formation at both doses, and decreased scar height and SEI were detected. EGCG also attenuated the mean collagen area fraction and decreased the number of capillaries in scar tissues. qRT-PCR revealed that EGCG significantly inhibited the mRNA expression of TGF-β1, Col I, Col III, α-SMA, and eNOS.
Conclusion
EGCG may serve as a useful candidate therapeutic drug for hypertrophic scar via inhibiting fibrotic gene expression and suppressing angiogenesis.
1 INTRODUCTION
Hypertrophic scar is a pathological result of abnormal cutaneous wound repair after skin dermis injury, which often occurs after burn, trauma, and surgery. Hypertrophic scar not only influences the appearance of patients but also may lead to dysfunction of injured tissues and/or organs, which seriously influences the quality of life of patients. It is reported that 33%–91% of burn patients and 40%–70% of surgical patients may have secondary hypertrophic scar.1, 2 In developed countries, about 100 million people have scar after surgery every year.3 In the United States, about 12 billion dollars are paid for treating skin scars every year.3, 4 Therefore, it is of great significance to explore a suitable drug for the prevention and treatment of hypertrophic scar.
The most typical features of hypertrophic scar tissue are the differentiation of fibroblasts into myofibroblasts. During this process, it will produce a large amount of extracellular matrix (ECM) partially resulting from the upregulation of transforming growth factor β1 (TGF-β1), collagen I (Col I), collagen III (Col III), and α-smooth muscle actin (α-SMA).5, 6 Drugs with the abilities to inhibit the expression of the above genes may assist in improving hypertrophic scarring.
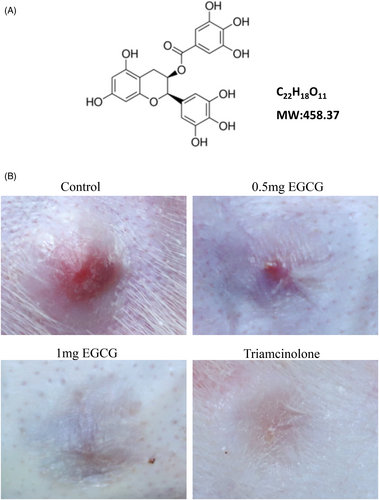
Epigallocatechin gallate (EGCG, Figure 1A), an active compound isolated from green tea,7 exhibits numerous biological properties, including anti-oxidative, anti-inflammatory, and anti-apoptotic.8 Moreover, EGCG has been shown to have the anti-fibrosis effect on myocardial fibrosis,9 lung fibrosis,10 renal fibrosis,11 and skin fibrosis.12 The antifibrotic activities of EGCG on skin were also proved by many researchers. Park et al. found that EGCG suppressed keloids growth and collagen production using a keloid nude mice model13; Syed et al. showed that EGCG inhibited the keloid tissues growth and induced human keloid tissue shrinkage in a human keloid OC model.12 Moreover, both zonal priming and direct EGCG application showed a beneficial role for scar therapy in a double-blind trial.14 These suggest its potential for improving hypertrophic scar and deserve further investigations.
In the present study, the role of EGCG in the prevention of hypertrophic scar formation were investigated using a rabbit ear hypertrophic scar model. The results showed that EGCG significantly suppressed hypertrophic scar formation through inhibiting angiogenesis and decreasing the expressions of TGF-β1, Col I, Col III, α-SMA, and eNOS.
2 MATERIALS AND METHODS
2.1 Animals and reagents
Twelve New Zealand white rabbits ranged from 1.5 kg to 2.0 kg were purchased from the animal center of the Fourth Military Medical University, Xian, China. Both male and female rabbits were used for the present study. All experimental animals were caged individually and fed at the temperature of 25 ± 2°C and relative humidity of 55% ± 2%. The experiments and protocols were approved by the animal experiment ethics committee of the Fourth Military Medical University (IACUC-20200609).
EGCG [purify ≥80% (HPLC), catalog number: E4268] was obtained from Sigma-Aldrich. and then dissolved in DMSO (Sigma-Aldrich). Triamcinolone acetonide acetate injection (abbreviation: Triamcinolone, H31021257, 5 ml:50 mg) was purchased from Shanghai General Pharmaceutical Co., Ltd. The mouse monoclonal antibody [JC/70A] to CD31 (ab9498) was bought from Abcam.
2.2 Rabbit ear hypertrophic scarring model establishment
The rabbit ear scarring model was built based on the procedures described in our previous study.15 Briefly, 1.5% (15 g/L) pentobarbital sodium at 2 ml/kg and xoylazine hydrochloride (2%) at 0.5 ml/kg were intravenously injection or intramuscularly injected to anesthetize the New Zealand white rabbits. Then, the rabbit ears were sterilized with iodophor, and four round wounds with a diameter of 1 cm were created on the ventral surface of each rabbit ear with a biopsy punch. Tissues, including epidermis, dermis, and perichondrium, were totally removed. Then, each wound was exposed to air and examined for infections for 3 days. All the wounds showing signs of infection or necrosis were excluded from this study.
2.3 Treatment of hypertrophic scars with EGCG
Twenty three days after operation, all rabbit ear wounds were completely re-epithelialized, then 12 rabbits with 96 wounds were randomly divided into four groups (24 wounds in each group). The wounds were treated intralesionally with different drugs or drug of different doses. Contralateral ears from the same rabbit were used as self-controls: (1) Control group - each wound was treated with 50 μl DMSO. (2) 0.5 mg EGCG group - each wound was treated with 0.5 mg EGCG dissolved in 50 μl DMSO. (3) 1.0 mg EGCG group - each wound was treated with 1.0 mg EGCG in 50 μl DMSO. (4) Triamcinolone group - each wound was injected with 50 μl triamcinolone. Every wound was injected once a week for a total of four times. The dose of EGCG was chosen based on the calculation of dosage previously reported. An equal volume of triamcinolone was used.
Thirty one days after treatment, scar tissues were collected from each group. Half of the specimens were fixed in 4% formaldehyde solution and embedded in paraffin, and half of the specimens were frozen in liquid nitrogen and stored at −80°C.
2.4 Histological quantification of the rabbit ear scars
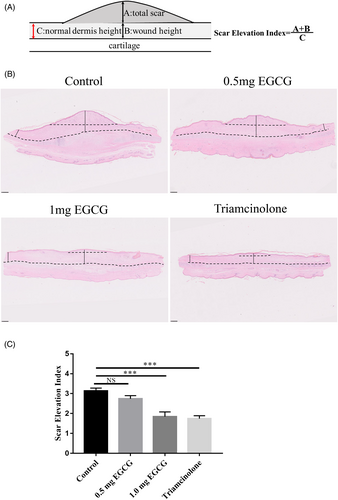
Hematoxylin and Eosin (HE) staining and Masson’ s trichrome staining images of hypertrophic scar tissues were used for scar elevation assessment and collagen fibers analysis, respectively. Scar elevation was obtained by calculating the scar elevation index (SEI) as previously reported.15 The SEI is a ratio of total scar and wound height to the height of nearby normal dermis (Figure 2A). SEI was measured using Image J software 1.52a.
The arrangement of the collagen fiber was examined by Masson's trichrome staining images. Eight images of dermis were taken at 100 × magnification in each scar. The mean collagen area fraction was measured using Image J software 1.52a.
2.5 Immunohistochemistry
4-μm thick scar tissue sections were used for immunohistochemical staining. After dewaxing, citrate buffer at pH 6.0 was applied for antigen retrieval for 20 min. Then, 5% bovine serum albumin (BSA) in phosphate buffer saline (PBS) was utilized for blockage. 3% hydrogen peroxide was used to quench endogenous peroxidase activity for 15 min. Sections were then incubated overnight at 4°C with anti-CD31 antibody (1:15). After washing with PBS, sections were incubated with an immunoperoxidase conjugate provided by Dako REAL™ EnVision™-HRP Detection Kit (Peroxidase/DAB+, K5007). Images were taken under a Nikon Eclipse E400 photomicroscope at a 100 × magnification. The numbers of capillaries in the scar sections were analyzed using the Image-J software 1.52a.
2.6 qRT-PCR
To determine the mRNA levels in the scar tissues, total RNA was isolated with TRIzol (Invitrogen) according to the manufacturer's instructions and reversely transcribed into cDNA using a HiFiScript cDNA Synthesis Kit (cw2569M, CWBIOTECH). qRT-PCR experiments were conducted with UltraSYBR Mixture (CW0957M, CWBIOTECH). Data were analyzed using the 2−ΔΔCt method. The results from three independent reactions were used to calculate the gene expression of chosen genes, which were normalized to the expression of the GAPDH. The primers were synthesized by Sangon Biotech Co., Ltd., and the sequences are shown in Table 1.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| TGF-β1 | 5′-AGGACGCCAACTTCTGCCT-3′ | 5′-AGGACCTTGCTGTACTGGGTGT-3′ |
| Col I | 5′-TGAGCCAGCAGATTGAGAAC-3′ | 5′-CCAGTGTCCATGTCGCAGA-3′ |
| Col III | 5′-TTCCTTTTGTTCTAATCTTGTCA-3′ | 5′-TAGCACCATTGAGACATTTTGA-3′ |
| α-SMA | 5′-TTCTGCATACGGTCAGCAAT-3′ | 5′-CATCCATGAAACCACCTACA-3′ |
| eNOS | 5′-GTGAGACTTTCTGCGTGGGA-3′ | 5′-CAGACCTGGCAGCAACTGTA-3′ |
| GAPDH | 5′-GAACGGGAAACTCACTGGCAT-3′ | 5′-CCTTCTTGATGTCGTCATACTTAGC-3′ |
2.7 Statistical analysis
Results from each experiment were presented as the mean ± SE of mean (SEM). Differences between groups were determined by analyzing variance (ANOVA) followed by Dunnett's multiple comparisons test using GraphPad Prism 7. p < 0.05 was considered to indicate statistical significance.
3 RESULTS
3.1 Appearance changes of EGCG-treated scar
In the present study, the impacts of EGCG of different doses on rabbit ear hypertrophic scar formation were analyzed. The scar tissues in control group were convex, showing dark-red color and a moderately firm texture (Figure 1B). While the scar tissues in either triamcinolone or 0.5 mg and 1.0 mg EGCG groups were flatter and softer. Scars in triamcinolone group showed almost no bulge compared with the surrounding skin and displayed pale pink color.
3.2 Pathological evaluation
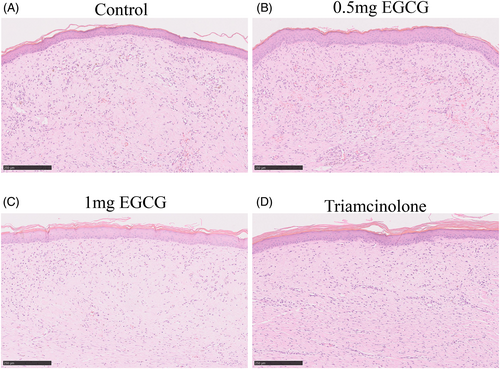
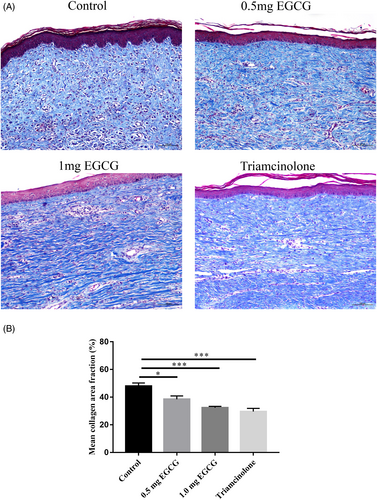
To assess the influence of EGCG of different doses on rabbit ear hypertrophic scar, HE images scanned under NanoZoomer-SQ Digital slide scanner were used. The height of scars was quantified based on the SEI of each scar specimen (Figure 2B). Significant differences were observed between groups (F = 16.25, DF = 31, p < 0.0001). The mean SEI in both 1.0 mg EGCG group and triamcinolone group was significantly lower than that in the control group (1.84 ± 0.24 vs. 3.13 ± 0.15, p < 0.0001; 1.74 ± 0.15 vs. 3.13 ± 0.15, p < 0.0001). No significant difference in SEI was observed between 0.5 mg EGCG group and control group (2.74 ± 0.16 vs. 3.13 ± 0.15, p = 0.25) (Figure 2C). Histologically, the HE images revealed that the control scars were obviously elevated, presenting highly thicker and more tangled collagen fibers in dermis. In contrast, the scar tissues in 0.5 mg, 1.0 mg EGCG groups, and triamcinolone group were thinner and flatter at the scar site (Figure 3, magnification, ×100, scale bar = 250 μm).
The collagen fibers in the control group were denser, thicker, disorganized, and displayed in parallel arrangement, while the cells arranged in a circular or threaded manner. In comparison, collagen fibers in both the 0.5 mg and 1.0 mg EGCG groups and the triamcinolone group were relatively looser, thinner, and more regularly arranged (Figure 4A, magnification, ×100, scale bar = 100 μm). Then, the mean collagen area fraction in all Masson's trichrome staining images was measured by using Image J software 1.52a (Figure 4B). Significant differences were observed between four groups (F = 15.52, DF = 23, p < 0.0001). The results illustrated that the mean collagen area fractions in 0.5 mg EGCG group, 1.0 mg EGCG group and triamcinolone group was significantly lower compared with the control group (38.51 ± 2.41 vs. 47.97 ± 2.24, p = 0.01; 32.37 ± 1.02 vs. 47.97 ± 2.24, p < 0.0001; 29.63 ± 2.28 vs. 47.97 ± 2.24, p < 0.0001).
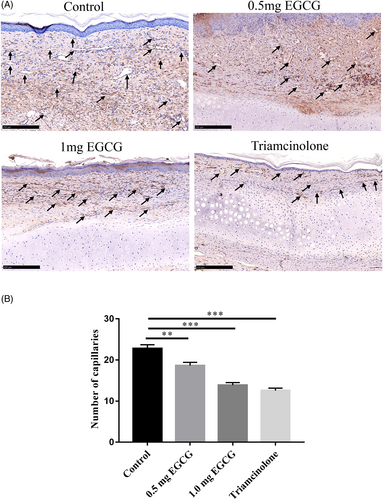
3.3 EGCG reduced the number of capillaries in hypertrophic scars
Images for CD31 staining were applied to study the influence of EGCG on scar angiogenesis (Figure 5A, magnification, × 100, scale bar = 250 μm). Significant differences were observed between the four groups (F = 39.68, DF = 35, p < 0.0001). The capillary numbers in scar tissues of 0.5 mg EGCG, 1.0 mg EGCG group, and triamcinolone group were significantly lower than that in the control group (18.67 ± 0.76 vs. 22.78 ± 0.92, p = 0.0013; 13.80 ± 0.63 vs. 22.78 ± 0.92, p < 0.0001) (12.56 ± 0.60 vs. 22.78 ± 0.92, p < 0.0001) (Figure 5B). This confirmed the inhibitory effects of EGCG on angiogenesis at both 0.5 mg and 1.0 mg in the rabbit hypertrophic scar tissues.
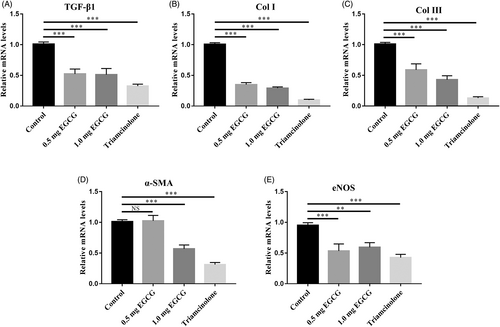
3.4 EGCG decreased the mRNA expression of TGF-β1, Col I, Col III,α-SMA, and eNOS
TGF-β1, Col I, Col III, α-SMA, and eNOS are important for hypertrophic scar formation. TGF-β1 mainly functions in excessive accumulation of collagens and ECM during hypertrophic scar formation. As shown in Figure 6, EGCG reduced TGF-β1 gene mRNA expression in 0.5 mg EGCG group, 1.0 mg EGCG group, and triamcinolone group in contrast to the control group respectively (0.52 ± 0.08 folds, 0.51 ± 0.10 folds, 0.33 ± 0.03 folds; Figure 6A, p < 0.0001). And the expression levels of Col I and Col III were significantly lower in the 0.5 mg EGCG group (0.35 ± 0.03 folds, 0.59 ± 0.10 folds), 1.0 mg EGCG group (0.29 ± 0.02 folds, 0.43 ± 0.06 folds), and triamcinolone group (0.10 ± 0.01 folds, 0.13 ± 0.02 folds) relative to those in control group (Figure 6B,C, p < 0.0001). α-SMA, a maker of myofibroblasts, displayed decreased gene expression levels in 0.5 mg EGCG group, 1.0 mg EGCG group and triamcinolone group relative to the control group (1.03 ± 0.09 folds, 0.57 ± 0.06 folds, and 0.31 ± 0.03 folds) (Figure 6D, p < 0.0001). eNOS is an extremely important regulator of vascular dynamic balance and illustrated a reduced expression in 0.5 mg EGCG group, 1.0 mg EGCG group and triamcinolone group (0.53 ± 0.11 folds, 0.59 ± 0.08 folds, and 0.43 ± 0.05 folds) (Figure 6E, p < 0.0001).
4 DISCUSSION
Hypertrophic scar is a common skin fibrotic disease secondary to skin injury, which often causes adverse effects to patients. Due to the unclear pathogenesis of hypertrophic scar, the effects of currently used medications on hypertrophic scar is not ideal. Therefore, there is an urgent need to explore safe, feasible, and effective drugs to prevent and treat hypertrophic scar. In this study, we revealed the inhibitory effects of EGCG on hypertrophic scar formation using a rabbit ear hypertrophic scarring model.
Using this rabbit ear scarring model, we found that EGCG inhibited fibroblast activation and lowered collagen density through decreasing TGF-β1, Col I, Col III, and α-SMA gene expression. Previous studies confirmed that EGCG significantly inhibited Col I expression in mast cell-stimulated keloid fibroblasts.16 Besides, EGCG also showed strong anti-fibrotic functions by suppressing ECM accumulation and keloids growth in a keloid nude mice model and a human keloid OC model.12, 13 These findings were consistent with our study. Moreover, EGCG-containing wound dressings appeared to benefit wound healing in both animal model and human skin.14, 17-23 This suggests that EGCG treatment may prevent scar formation after wound healing.
In the present study, the anti-scar effects of EGCG were confirmed using a rabbit ear scarring model, which is not reported previously. Among the existed scar research models, the rabbit ear hypertrophic scarring model reproduces many characteristics of human hypertrophic scar and is widely used for exploring anti-scar drugs. It was first built by Morris in 1997.24 Then, this model was modified by numerous researchers.15, 25 The scars in rabbit ear showed skin bulge and thickening, local congestion, and swelling. A large number of proliferative fibroblasts and inflammatory cells are also present in the scar tissues. More importantly, obvious collagen deposition, disordered arrangement of collagen fibers, and the histomorphology can also be found in the scars. These are similar to the pathological changes of human hypertrophic scars. Therefore, this rabbit ear model become one of the most widely used models in the field of hypertrophic scar study, especially in drug therapy, such as flavonoid monomer,26-28 quinone monomer,29 terpenoids,30-34 and other drugs.35-37 These findings in the rabbit ear scar model effectively suggest the potential inhibiting effects of EGCG on human hypertrophic scar.
Usually, microvessels contribute to hypertrophic scar formation after wound healing. Zheng et al. found that during the hypertrophic process of scarring, the number of microvessels increased significantly, while during the scar regression process, most of the blood vessels were partially or completely occluded.38 Targeting blood vessels has been clarified to inhibit scar formation in several investigations. Shen et al. showed the potential role of anti-VEGF antibodies in scar management through inhibiting scar angiogenesis.39 Ren et al. found that endostatin suppressed hypertrophic scar formation in a rabbit-ear scarring model through reducing scar vascularization and angiogenesis.40 Previously, we also found the anti-angiogenic effect of UA (usnic acid) using the rabbit ear scarring model.41 Huang et al. revealed that gambogenic acid (GNA) hindered HS formation by inhibiting angiogenesis, inflammation, and growth factor expression.42 In a double-blind, randomized trial, the authors showed that topical application of EGCG exhibited a beneficial role for scar therapy via reducing VEGFA and CD31 expression.14 In this study, we found that EGCG dramatically reduced the number of capillaries in the scar tissues, which is conducive to inhibiting scar formation.
TGF-β1, Col I, Col III, α-SMA, and eNOS exert critical functions during hypertrophic scar formation. Previous study demonstrated the anti-scar effect of EGCG through lowering the mRNA and protein expression of Col I and Col III after a 4 week treatment using EGCG in a human keloid organ culture model.12 In this study, qRT-PCR results confirmed that the expressions of these genes were also inhibited in the EGCG groups.
In this study, we selected triamcinolone, which is a commonly used clinical therapeutic drug for hypertrophic scar, as a positive control to judge the anti-scar effect of EGCG and provide an experimental foundation for clinical application prospects. Interestingly, the results of this study demonstrated that both 0.5 mg and 1.0 mg EGCG could inhibit the formation of rabbit ear hypertrophic scar. Compared with scars in triamcinolone group, scars in 1.0 mg EGCG group showed similar inhibitory effect. In both triamcinolone and 1.0 mg EGCG group, the scars were soft and smooth, with almost no bulge compared with the surrounding skin. However, the scars in 1.0 mg EGCG group were not as smooth and oily as those in the triamcinolone group. The mean SEI, mean collagen area fractions, and capillary numbers in both 1.0 mg EGCG group and triamcinolone group were significantly lower than that in the control group, while there was no significant difference between these two groups. Significant differences were found in the mRNA expression of Col I, Col III and α-SMA between1.0 mg EGCG group and triamcinolone group, while no significant difference was found in the mRNA expression of TGF-β1 and eNOS between these two groups. These suggest that EGCG and triamcinolone may have similar anti-inflammatory and anti-angiogenic functions. Although 1.0 mg EGCG group showed similar scar inhibition effect to triamcinolone in the rabbit ear hypertrophic scar model, further clinical studies are still needed to verify the effects of EGCG on hypertrophic scar formation.
In summary, our results reveal the inhibitory effects of EGCG on the rabbit ear hypertrophic scarring, this may largely attribute to its anti-angiogenic and anti-fibrotic effects. Therefore, this study presents supportive evidences that EGCG can significantly inhibit hypertrophic scar formation.
AUTHOR CONTRIBUTIONS
Yajuan Song and Tong Wang performed the research, acquired and analyzed data and wrote the manuscript. Liu Yang, Junzheng Wu, Lin Chen, Xiao Fan and Zhe Zhang conducted animal experiments, analyzed data and edited the manuscript. Qing Yang contributed essential reagents. Zhou Yu and Baoqiang Song designed the research study, provided samples and reagents, wrote and edited the manuscript.
FUNDING INFORMATION
This work was supported by the Foundation of National Natural Science Foundation of China (81571906 and 82072182) and Shaanxi Provincial Science Foundation (2020SF-179).
CONFLICT OF INTEREST
No potential conflict of interest was reported by the authors.
ETHICAL APPROVAL
Authors declare human ethics approval was not needed for this study.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.