Ozonized glycerin (OG)-based cosmetic products lighten age spots on human facial skin
Abstract
Background
Few cosmetic ingredients are shown to be able to safely remove or lighten facial dark spots once they have formed. OG has been reported to possess oxidation power and exhibit various biological activities such as antibacterial, antiviral, and wound healing promotion.
Aims
This study aimed to clarify the effects of OG on human skin, especially on age spots on the face.
Methods
OG formulations (80 and 800 ppm) were mixed with synthetic melanin in vitro for 4 weeks and then assessed for its ability to degrade the melanin. OG also investigated its effect on gene expression of keratinocyte differentiation markers in vitro to explore the cell maturation. In clinical study for the evaluation of effects of OG formulations on age spots on facial skin, 48 women were measured for the melanin content of them by a Mexameter at 4 and 8 weeks after daily twice application of OG formulations. Adverse events were monitored during the study.
Results
Both OG formulations showed direct melanin degradation in a time-dependent manner, with significant effects observed as early as 6 h. OG formulation at 800 ppm showed higher activity than OG formulation at 80 ppm, and the amount of melanin was decreased by about 40% on Day 14 of the mixing reaction. Differentiation marker studies using human keratinocytes showed that the gene expression of involucrin and serine palmitoyltransferase was upregulated by OG, which was almost equivalent concentration to OG formulation 80 ppm, suggesting that OGs can enhance turnover of the skin epidermis. In clinical study, OG formulations 80 and 800 ppm showed larger decreases in melanin contents at 8 weeks compared with those at 4 weeks and their mean values of △melanin index were −16.7 and −15.2, respectively. Statistically significant differences were detected against respective controls. Number of subjects with a decrease in melanin index from baseline to 4 or 8 weeks increased in both OG formulations 80 and 800 ppm, especially prominent at 8 weeks. There were no adverse events related to treatments of OG 80 and 800 ppm during the study.
Conclusion
The result indicated that applications of OG formulations are safe and effective in lightening age spots on the facial skin.
Video Abstract
Ozonized glycerin (OG)-based cosmetic products lighten age spots on human facial skin
by Hanada et al.1 INTRODUCTION
Ozone is known as an excellent disinfectant because its disinfecting action is powerful and instantaneous, it does not cause environmental pollution, it is extremely safe because it becomes oxygen when reduced, and it does not remain in living organisms or the environment. In addition, ozone has been reported to exhibit various biological activities in addition to disinfection.1 However, ozone has a short half-life, about 30 min even in solubilized ozone water, and its lack of sustained action is a major disadvantage.2 To compensate for this disadvantage, Niinomi et al. developed a cream formulation of ozonized glycerin (OG) by solubilizing ozone in glycerin in 2004.3 It has been shown that this formulation maintains a high concentration of ozone and retains its oxidizing power for more than 2 years in refrigerated storage.
Ozonized glycerin has various physiological effects such as anti-inflammatory, cytoprotective, wound healing, antibacterial, antiviral, and hemostatic effects in cell culture systems and various animal models.3-9 For the skin, it has been reported to promote granulation and epithelial formation, synthesis of extracellular matrixes such as collagen fibers and hyaluronic acid, and suppression of inflammation around ulcers in animal models of wound healing.10 Regarding safety for the application of OG to the skin, none of toxicities was found in animal studies so far.11 In the meantime, OG has been developed for cosmetics by taking advantage of its ability to maintain high ozone concentrations for a long period, but the effects of OG on normal skin tissue have not yet been fully clarified.
One of the current focus of attention in cosmetics development is facial age spots caused by UV exposure, other inflammatory stimuli, and aging. Melanin synthesis, secretion, and deposition in epidermal cells are major factors in the formation of dark spots in face. Although many cosmetic components have been found to inhibit melanin synthesis, no cosmetic components are shown to be able to safely decompose or lighten dark spots once they have formed.12 OG can be expected to chemically eliminate aging spots due to its inherent oxidative bleaching properties and also expected to inhibit melanin synthesis and enhance skin metabolism by inducing antioxidant factors and suppressing inflammatory responses. In this study, we clarified the effects of OG on the skin, especially on age spots in the face.
2 MATERIALS AND METHODS
2.1 In vitrostudy
2.1.1 Test products
Glycerol (concentration equal of more than 99%) was purchased from Sakamoto Yakuhin Kogyo., Ltd. For the preparation of OG samples, glycerin was ozonized using Ozonizer (Mediplus Co.), which produces ozone from medical grade oxygen.6 Glycerol was used as solvent control for OG. High-concentration formulation (water, pentylene glycol (Symrise), and xanthan gum (DSP Gokyo Food & Chemical)) containing 800 ppm ozonized glycerin (OG, 50% of final concentration of glycerin) and its control formulation without OG, and low-concentration formulation (water, pentylene glycol, xanthan gum, and sodium hyaluronate (Spera Nexus)) containing 80 ppm OG (40% of final concentration of glycerin) and its control formulation were used for melanin degradation assay. Assuming that ozone is dissolved in glycerin, the oxidizing power of OG was measured by iodometric titration method, and then, activity/concentration was expressed in ppm. Hydroxide peroxide solution (10 mM, FUJIFILM Wako Chemicals) was used as a positive control in the assay.
For measurements of mRNA expression of skin cells, OG samples, 1% (20), 2% (40) and 4% (80 ppm), were prepared by diluting 2000 ppm of OG with culture medium. Glycerin was also prepared by dilution as a control to match the final concentration of OG.
2.1.2 Measurement of melanin degradation activity
Synthetic melanin (Sigma-Aldrich) was dissolved in dimethyl sulfoxide (FUJIFILM Wako Chemicals) and diluted 2-fold with ultrapure water to make a melanin solution. Then, mix this solution and the test product in equal amounts, and dispense it into 96-well plates at 100 μl/well and incubated at 37°C. After 6 and 24 h, and 2 and 4 weeks, melanin content in each well of the plate was measured at a 490 nm by spectrophotometer (ImmunoMini, NJ-2300, Biotec).
2.1.3 Measurement of mRNA expression of differentiation markers in human normal epidermal keratinocyte (HNEK)
NHEK cells were seeded in 96-well plates (IWAKI) at a density of 2.0 × 104 cells/100 μL/well using HuMedia-KG2 (Kurabo) and incubated with 0, 1% (20), 2% (40) and 4% (80 ppm) of OG for 24 h at 37°C and 5% CO2. After incubation, the cells were washed with PBS(−), total RNA was extracted using the Ambion Cells-to-CT kit (Thermo Fisher Scientific), and cDNA was synthesized by reverse transcription reaction (37°C, 60 min→95°C, 5 min) using the StepOne Real-Time PCR system (Applied Biosystems, Thermo Fisher Scientific). The mRNAs of involucrin and serine palmytoiltransferase, differentiation markers of NHEK, were amplified using the primer (Takara) below, and relative quantification was performed using the ΔΔCt method. The relative expression levels of each gene were normalized to the house keeping gene, GAPDH. The primer sequences are as follows: Homo sapiens involucrin (IVL), mRNA (Gene ID of NCBI database: 3713), forward GCTGGAGCAGCCTGTGTTTG (start 1663) and reverse CTGGACACTGCGGGTGGTTA (start 1821); Homo sapiens serine palmitoyltransferase long chain base subunit 2 (SPTLC2), mRNA (Gene ID: 9517), forward GCCTGTCAGCAGCTCATACCAA (start 1720) and reverse GGCCTGTCCAGTAGA GGTACCAA (start 1840); Homo sapiens glyceraldehyde-3-phosphate dehydrogenase (GAPDH), transcript variant 1, mRNA (Gene ID: 2597), forward GCACCGTCAAGGCT GAGAAC (start 361), and reverse TGGTGAAGACGCCAGTGGA (start 498).
2.2 Clinical study
2.2.1 Test products and application to the face
High-concentration formulation (OG formulation 800 ppm) and its control formulation were applied to half the face of the same subject. It was randomly determined which half of the face they would be applied to. In the same way, low-concentration formulation (OG formulation 80 ppm) and its control formulation were applied to half the face of the same subject. After cleansing face twice a day (morning and night), 2 pumps of each formulation bottle (push type, 30 ml) were dispensed into the palm of hand and applied to the designated half of the face. Since the oxidizing power of OG is thought to be mediated by the action of free radicals, its duration of action was set to be short and in accordance with the existing dosage for general cosmetics (twice a day, morning and evening).
2.2.2 Study design
This was proof-of-concept, double-blind study to evaluate the safety and impact of novel cosmetic products including OG (OG formulations 80 and 800 ppm) on age spots of facial skin. Fifty Japanese participants who met the inclusion criteria were enrolled at research facility (701 Research Inc.), and then, randomly assigned to OG formulation 80 ppm/control and OG formulation 800 ppm/control groups with 1:1 allocation ratio. Subjects were assessed for changes in melanin content of the spot after twice-daily application of OG formulations for 8 weeks. Evaluation visits of subjects were totally 3 on baseline (first visit before treatment), Weeks 4 and 8. The evaluation was based on the fact that turnover of the epidermis of the skin takes approximately 1 month. The study was conducted from mid-April to early June. All subjects provided written informed consent. The study protocol was approved by the Institutional Review Board for Research Clinical Review Center and followed the principles of the Declaration of Helsinki and Ethical Guidelines for Medical and Health Research Involving Human Subjects (Public Notice of the Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labor, and Welfare in Japan).
Women between 40- and 60-year-old, who were concerned about dullness and have age spots of 2 mm to 1 cm in diameter on both the left and right face, and willing to replace their skincare products with the test product, were recruited. The subjects should have no previous experience of severe rashes caused by cosmetics. We excluded subjects who (1) were currently visiting a dermatologist for treatment of skin diseases; (2) were taking medication that could affect this study by judgment of the study physician; (3) have allergic skin diseases such as atopic dermatitis on their face; (4) have skin disease or damage on the skin of the test site; (5) show allergic or idiosyncratic reactions to drugs or cosmetics; (6) may show skin allergy symptoms or those who have skin hypersensitivity; (7) have been hospitalized in the past due to skin problems caused by cosmetics; (8) are likely to be exposed to significant sunlight during the study period; (9) have undergone chemical peeling, laser treatment, phototherapy, and cosmetic surgery on the face within 4 weeks of the start of the study or plan to undergo such treatment during the study period; (10) have used whitening skin care (ascorbic acid or tranexamic acid as the main agent) or whitening supplements within 4 weeks of the start of the study, or whitening supplements within 4 weeks of the start of the study; (11) are pregnant or lactating and those who plan to become pregnant during the study period; (12) are participating in other clinical trials; and (13) are judged unsuitable by the study physician or investigator.
All adverse events were monitored during the study, which were any unfavorable or unintended sign, symptom, or illness that occurs as a result of participation in the study, whether or not causally related to the study product. The study physician determined if the adverse event was associated with the test products or not.
2.2.3 Evaluation
On first visit, skin diseases, scars and damages on the test site were checked. After washing the face with make-up remover (if make-up is worn) and facial cleanser that the subject normally uses, acclimatization was performed in a thermo-hydrostatic chamber (temperature: 21 ± 1°C, humidity: 50 ± 5%) for 20 min, and then, one aging spot site was selected on each facial side. Then, its size was selected should be less than 5 mm. Thereafter, melanin content of the spots was analyzed. Melanin content was measured at aging spot areas on the right and left cheeks of subjects by a Mexameter MX18 (Courage + Khazaka electronic GmbH). Measurements were performed 5 times. Measured value was expressed as melanin index. The maximum and minimum values of 5 data are excluded, and the average value is calculated. The △melanin index was calculated by subtracting the baseline melanin index from those at 4 or 8 weeks. On Weeks 4 and 8, after adverse events on the applied facial sites were assessed and melanin contents measured in the same manner.
2.3 Statistical analysis
Data in figures were expressed as mean ± standard deviation (SE). Statistical significance was evaluated using the paired Student's t-test for comparisons between the data of two time points. Statistical significance was set at p < 0.05, and all analyses were performed using Excel (Microsoft).
3 RESULTS
3.1 In vitrostudies
Ozonized glycerin has an inherent oxidative activity chemically, therefore degradation effect on synthetic melanin was evaluated in simple test system of mixing and reaction at 37°C. Both OG formulations showed direct melanin degradation in a time-dependent manner, with significant effects observed as early as 6 h (Figure 1). OG formulation at 800 ppm showed higher activity than OG formulation at 80 ppm, and the amount of melanin was decreased by about 40% at 2 weeks of the mixing reaction. The degradation activity was almost the same as that of 10 mM hydrogen peroxide. Melanin contents of mixed reaction solutions with OG formulation at 800 ppm or 10 mM hydrogen peroxide reached a plateau after 2 weeks. Probably, this is due to the reduced oxidative capacity of them (oxidative powers were less than 10% from baseline). The reduction of melanin in all controls was less than 10% of those at baseline, except for the OG formulation 80 ppm control after 4 weeks of reaction. These results suggest that OG formulations decompose melanin pigments already formed and deposited in the skin by its oxidative power.
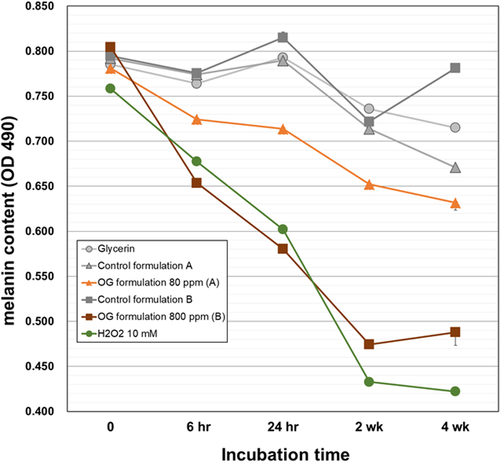
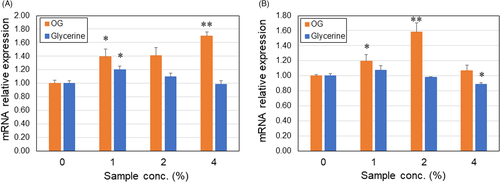
One of the most influential factors on the deposition of age spots is skin cell turnover. Therefore, we investigated effects of OG on mRNA expression levels of differentiation markers of keratinocytes, involucrin (INV), and serine palmitoyltransferase (SPT). The OG significantly increased mRNA expression of both genes in concentration-dependent manner as compared with glycerin control (Figure 2). There was no difference in SPT level between keratinocytes treated with 4% OG and control. The data suggest that OG (~80 ppm) promotes maturation of human keratinocytes and this probably contributes to enhance skin cell turnover.
3.2 Clinical study
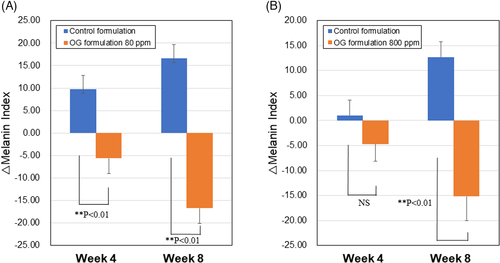
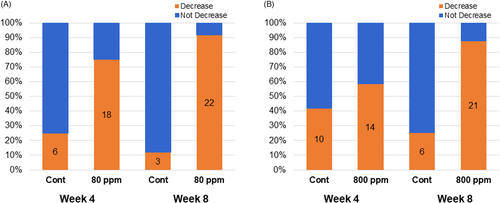
Based on the melanin degradation activity in vitro, we next investigated effects of OG formulations on age spots on faces of healthy women. Totally, 48 subjects were completed with study (each 24 subjects/group). OG concentrations with low activity (80 ppm) and high activity (800 ppm) were selected based on the results of melanin degradation tests. They were measured for melanin content of age spots by a Mexameter at 4 and 8 weeks after daily twice applications of OG formulations. At 8 weeks, OG formulations 80 and 800 ppm showed larger decreases in melanin contents compared with those at 4 weeks and their mean values of △melanin index were −16.7 and −15.2, respectively. Statistically significant differences were detected against respective controls (A and B in Figure 3). At 4 weeks, only OG formulation 80 ppm showed a significant decrease in melanin content (A in Figure 3). Between OG formulations 80 and 800 ppm, there is no significant difference in Δmelanin index either at 4 and 8 weeks. On the contrary, the △melanin index of controls elevated from 4 to 8 weeks, probably due to increase in UV radiation level during study period from spring to early summer in Japan. Number of subjects with a decrease in melanin index from baseline to 4 or 8 weeks increased in both OG formulations 80 and 800 ppm, especially prominent at 8 weeks (A and B in Figure 4). There were no adverse events related to treatments of OG 80 and 800 ppm during the study. The result indicated that applications of OG formulations are safe and effective in lightening age spots on the facial skin.
4 DISCUSSION
Age spots (senile lentigo) are formed by prolonged exposure to ultraviolet light, which increases melanin synthesis in melanocytes and causes excessive accumulation in the epidermis. In addition, aging slows down the metabolic rate of the epidermis, which is thought to cause the long-term accumulation of dark pigmented age spots. Currently, most of whitening cosmetics are designed to inhibit excessive melanin synthesis by suppressing the synthesis or action of tyrosinase enzymes in melanocytes, or to inhibit the proliferation of melanocytes themselves.12 Therefore, these cosmetics may be useful in preventing the formation of new spots prophylactically, but are not effective against them once formed. In this study, it was demonstrated for the first time that OG formulations can directly break down melanin in vitro and lighten age spot that have already formed on facial skin in vivo. Melanin degradation by the bleaching action of OG was reported to be due to the complex process that reversible oxidation of the hydroquinone moieties of melanin followed by irreversible reactions of the monomers that lead to degradation of the melanin polymer.13 In this clinical study, melanin content of age spots decreased with duration of use of both OG formulations and a clear reduction observed at 8 weeks. By using OG formulations for even longer than 8 weeks, the amount of melanin in age spot may be further reduced. Another long-term clinical study is currently being planned.
Differentiation marker studies using human keratinocytes showed that gene expression of INV and SPT was upregulated by OG, which was almost equivalent concentration to OG formulation 80 ppm, suggesting that OGs can enhance the maturation of the skin epidermis. This may contribute to the turnover of skin epidermis that accelerate the fade-out of age spots by OG formulations. We plan to analyze the cornified layer cell area by skin tape stripping method to clarify effect of OG on the turnover speed in future clinical setting.
Some may argue that oxidizing cosmetic products are toxic to the skin. In this study, however, none of adverse effects was found even after daily application of 800 ppm OG formulation to the face for 2 months. In addition, we reported that higher dose of 1000 ppm OG did not show any toxic effects on the skin in animal studies.11 Furthermore, no side effects have been reported in the our company (Mediplus Pharma, Inc.) cosmetic products for atopic dermatitis and sensitive skin formulated with OG that have already been on the market since 2004 (accumulated sales of 1 million units).
Despite the fact that 800 ppm of OG formulation was more active than 80 ppm in melanin degradation in vitro, their effects were similar in human studies. Melanin degradation test in vitro used unpolymerized synthetic melanin, which may have different properties from polymerized melanin accumulated and deposited in the spot area. Therefore, the difference in in vitro degradation activity between 80 and 800 ppm of the OG formulations may not be reflected accurately in clinical. This may be because the permeability of OG into the stratum corneum reaches a plateau at low-concentration of 80 ppm. Absorption distribution study of OG in skin epidermis will clarify this issue in the future. The other important possibility is that OG can induce other effective actions on the skin besides direct melanin degradation. Recently, it has been found that OG has antioxidant and anti-inflammatory effects on the epidermis through activation of the nuclear factor erythroid 2-related factor 2 (Nrf2) transcription factor.14 These actions could be contribute to inhibition of melanin synthesis, cyto-protection of epidermal cells, and improvement of delayed or disordered skin turnover.15-17 It is most likely that these actions work together to lighten the age spots in vivo. It should be necessary to examine the whitening effect of OG on skin areas other than spots in the future study.
Recently, various physicochemical analyses revealed that OG contains a small amount of a novel trioxepane derivative with endoperoxide scaffold, which shows oxidative activity as much as OG. Although we previously assumed that ozone dissolved in glycerin exerted the oxidizing power, it was strongly suggested that the main body of the oxidizing power was this new compound generated as a result of the reaction between glycerin and ozone.14 The trioxepane derivative produces reactive oxygen species (ROS) such as OG and causes oxidative stress on cell and body. Moderate oxidative stress induces the activation of Nrf2 transcription factor, resulted in induction of expression of the antioxidant factors such as heme oxygenase-1 (HO-1) and suppression of inflammatory actions as described before.18 In fact, OG less than 100 ppm, even at concentration as low as 20 ppm, induced antioxidant factors including HO-1, NAD(P)H quinone oxidoreductase 1 (NQO-1), and glutathione (GSH) in epidermal cells and suppress production of cytokines induced by UV-ray.14 Thus, mild oxidative stimulation by OG could elicit beneficial biological effects on the normal skin.
CONFLICT OF INTEREST
None to declare.
AUTHOR CONTRIBUTIONS
All authors' have read and approved the final manuscript. K.H., D.O., R.O., S.K., R.T., and G.S. performed the research. D.O. performed the melanin degradation study. R.O., S.K., and R.T. designed the clinical study. G.S. reviewed the final manuscript. K.H. performed keratinocyte differentiation marker study, analyzed the data, and wrote the paper.
ETHICAL APPROVAL
The clinical study reviewed approval by the local ethical committee (Clinical Research Review Center, Tokyo, Japan, Approval No. CrrC21-010). Informed consent was obtained.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




