Effectiveness of combined microfocused ultrasound with visualization and subdermal calcium hydroxyapatite injections for the management of brachial skin laxity
Abstract
Background
There is no publication to date on the combined use of microfocused ultrasound with visualization (MFU-V) and calcium hydroxylapatite (CaHA) for brachial skin laxity.
Aim
To assess the effectiveness of combining MFU-V with diluted/hyperdiluted CaHA in a single session for treating brachial skin laxity.
Subjects/Methods
Female subjects who had skin laxity in the brachial regions and who desired non-surgical intervention were enrolled into this prospective, single-arm pilot study. MFU-V (Ultherapy®, Merz North America, Inc. Raleigh, N.C.) was applied using the 4.0 MHz-4.5 mm and 7.0 MHz-3.0 mm depth transducers, followed by subdermal injections of diluted (1:1)/hyperdiluted (1:2) CaHA (Radiesse®, Merz North America, Inc). Subjects were followed for six months after treatment. Objective biophysical skin assessments were conducted using a cutometer (Cutometer® Dual 580 MPA; Courage & Khazaka, Cologne, Germany). Subjective assessments included the arm visual analogue scale (VAS), global aesthetic improvement scale (GAIS), and subject global satisfaction scale.
Results
Twelve subjects participated in the study. The mean R0 reading (measure of skin firmness) progressively improved from 0.515 mm at baseline to 0.433 mm at 24 weeks (p < 0.05 for 12 and 24 weeks). The mean R2 reading (measure of skin elasticity) and mean arm VAS improved significantly from baseline at all visits (p < 0.05 for all). The majority of subjects at each visit showed improved arm appearance and were satisfied with their treatment. Both procedures were well-tolerated.
Conclusions
Combined use of MFU-V with diluted/hyperdiluted CaHA demonstrates significant improvements in both objective and subjective measures of brachial skin laxity.
Video Abstract
Effectiveness of combined microfocused ultrasound with visualization and subdermal calcium hydroxyapatite injections for the management of brachial skin laxity
by Ramirez et al.1 INTRODUCTION
There is a paradigm shift for body contouring treatments.1 Individuals are paying more attention to concerns in visible body areas, including the arms, where sagging and laxity in the brachial area may present as “batwing appearance,”2 and along with this is a higher desire for non-invasive procedures.3 Indeed, data from the American Society for Dermatologic Surgery (ASDS) suggest that the number of invasive and non-invasive body contouring procedures performed has increased by fourfold between 2012 and 2018.4 Brachial skin laxity, in particular, can negatively impact an individual's quality of life.5 The condition can become more apparent as collagen and elastin levels decline with aging and weight loss, which cause the skin to lose firmness and elasticity.2, 6 The demand for improvement in brachial skin laxity is evidenced by the more than 50-fold increase in “upper arm lifts” or brachioplasty from 2000 to 2019 as reported in the Plastic Surgery Statistics Report by American Society of Plastic Surgeons (ASPS).3
Although surgical procedures can improve the appearance of the brachial area, they can be associated with significant complications and extensive scarring often requiring revision surgery.7 Indeed, there has been a rise in demand for non-invasive procedures for the improvement of skin laxity.1 Microfocused ultrasound with visualization (MFU-V; Ultherapy®, Merz North America, Inc. Raleigh, N.C.) and calcium hydroxylapatite (CaHA; Radiesse®, Merz North America, Inc) injections are examples of non-invasive procedures that stimulate collagen and elastin production. Independently, these have been shown to promote dermal remodeling, resulting in lifting and tightening of lax skin primarily for concerns on the face and neck, but there are limited data to suggest that these procedures may have potential applications for use in other body sites.8-11 MFU-V generates focused ultrasound waves that are converted to heat, creating discrete thermal coagulation points at precise depths beneath the skin's surface resulting in collagen denaturation and subsequent new collagen production.12, 13 A small number of studies has demonstrated improvement of brachial laxity with MFU-V.8, 9 These are limited by the predominant use of subjective assessments to evaluate clinical improvements without including objective measurements. An alternative option has been the use of CaHA as a biostimulatory agent.14 Treatment with subdermal injections of diluted or hyperdiluted CaHA has been shown to stimulate neocollagenesis, elastogenesis, and neoangiogenesis, with corresponding increase in dermal thickness and elasticity.15 To date, a handful of case studies has reported the use of CaHA injections to improve skin laxity in the brachial region and other areas.10, 11
In an effort to pursue further improvement in skin tightening especially in body sites, recent studies have examined the effectiveness of combining both MFU-V and CaHA treatments for addressing skin laxity in the neck and buttocks.16, 17 Given that each of these modalities has been shown to improve collagen stores, it is plausible that a combination of both procedures could produce synergistic effects.17, 18 There is, however, no publication to date on the combined use of both modalities in a single treatment session in the brachial region. Given the increasing demand for arm skin tightening procedures, particularly with non-invasive treatments, this prospective pilot study aimed to assess the effectiveness of MFU-V combined with subdermal injections of diluted or hyperdiluted CaHA for improving brachial skin laxity.
2 SUBJECTS AND METHODS
2.1 Study design
This was a prospective, open-label, non-randomized, single-arm case series of healthy female subjects from two outpatient clinics in Singapore conducted between September 2019 and August 2020. Subjects underwent one treatment session of MFU-V combined with subdermal injections of diluted or hyperdiluted CaHA for improvement of brachial skin laxity and were followed for 24 weeks over three visits to evaluate the effectiveness of the combination treatment. The study was approved by the relevant ethics committee, and all subjects provided written informed consent before the study commenced. The study was carried out in accordance with the Declaration of Helsinki. Written informed consent for publication of the pre- and post-treatment photographs was obtained from the subject whose photographs were included in the manuscript.
2.2 Study population
Female subjects aged 35–65 years of all skin types and ethnic groups who had skin laxity in the brachial regions, desired lifting and tightening of the upper arm skin through non-surgical interventions, were in good health, and could comply with the study requirements were included in the study. Subjects who met any of the following criteria were excluded from participation: (1) used immunosuppressive drugs or had any active systemic or local skin disease that may alter wound healing; (2) used antiplatelet agents or anticoagulants; (3) had significant scarring or keloid scarring in the proposed treatment areas, or a history of keloid formation; (4) had significant open wounds or lesions, or presence of active implants in the proposed treatment areas; (5) performed non-invasive fat reduction procedures; ablative or non-ablative skin procedures, or surgical procedures to the proposed treatment areas within the past six months; and (6) were pregnant or lactating during screening; and (7) had body mass index (BMI) >28 kg/m2.
2.3 Intervention
MFU-V treatment was first administered as described in previously published studies for brachial skin laxity.8, 9 Diluted or hyperdiluted CaHA was then administered according to the recommendations of published consensus guidelines.14, 19
Analgesia was achieved with oral administration of ibuprofen (800 mg) and application of a topical anesthetic (EMLA cream: a eutectic mixture of local anesthetic—lidocaine 2.5% and prilocaine 2.5%, or BLT cream: benzocaine 20%, lidocaine 6%, tetracaine 4%) one hour before treatment. Oral paracetamol (1 g) or tramadol (50 mg) was given to subjects who were allergic to non-steroidal anti-inflammatory drugs (NSAIDs).
The treatment area was marked with the subject standing upright and with the arms extended at 45 degrees from the body using the lower horizontal border of the underarm area and the outer border of the axilla border as reference points. A standardized ruler that matched the width and height of the MFU-V transducer was used to create a grid as shown in Figure 1A. A thin layer of ultrasound gel was then applied to the subject's arms. Each transducer was placed on the skin and was evenly coupled to the skin surface before treatment was administered. The brachial regions were treated in a standardized pattern as previously described8, 9 at two depths using the 4.0 MHz-4.5 mm depth transducer for deeper penetration to the superficial fascial layer in the first pass, followed by the 7.0 MHz-3.0 mm depth transducer for more superficial penetration to the deep dermis in the second pass. Depending on the surface area to be treated, a total of 180–240 treatment lines (20 lines per 2.5 × 2.5 cm square) were administered to each arm using each transducer (Figure 1A), for a total of 360–480 lines per arm with both transducers, with more lines for patients with larger arm volumes. At least half of the height of the surface area of the inner arm was treated. The treatment protocol was not modified by severity of brachial skin laxity.
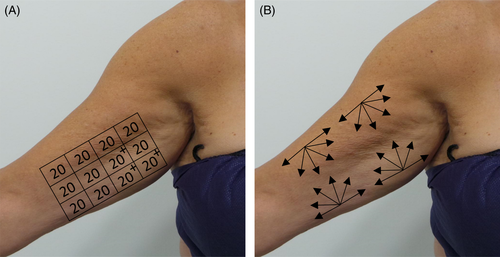
Depending on individual subject's availability, subjects received subdermal injections of diluted or hyperdiluted CaHA immediately or up to one week following MFU-V treatment in the same areas with a 25-gauge cannula using a retrograde fanning technique (Figure 1B) in accordance with the recommendations of published consensus guidelines.14, 19 Two different dilutions were used depending on investigator's assessment of skin thickness. A dilution ratio of 1:2 (1.5 ml of CaHA diluted with 0.3 ml 2% lidocaine and 2.7 ml normal saline, for a total volume of 4.5 ml per arm) was used for subjects with thinner skin. A dilution ratio of 1:1 (1.5 ml of CaHA diluted with 0.3 ml 2% lidocaine and 1.2 ml normal saline, for a total volume of 3 ml per arm) was used for subjects with thicker skin. After injecting CaHA, vigorous massage of the upper arm areas was performed to ensure even distribution of CaHA.
2.4 Study assessments
Subjects were followed for six months after treatment and evaluated at 4, 12, and 24 weeks. The primary outcome evaluated was the change in biophysical skin parameters, specifically skin firmness (R0) and skin elasticity (R2) during the 4, 12, and 24 week follow-up visits as compared with baseline. These parameters were measured using a cutometer (Cutometer® Dual 580 MPA; Courage & Khazaka, Cologne, Germany), which is an established objective assessment of skin firmness and skin elasticity in dermatologic clinical studies.20 The cutometer uses a suction method which creates a negative pressure that draws the skin into a probe and releases it after a defined time. It measures skin firmness (R0: the resistance of the skin to negative pressure, lower R0 value denotes firmer skin) and elasticity (R2: the ability of the skin to return to its original position, higher R2 denotes more elastic skin) using a non-contact optical measuring system.21 Readings were taken at three standardized points on each arm during each visit (Figure 1A), and the average of three measurements was reported for each visit.
Secondary outcomes utilized subjective assessments including the arm visual analogue scale (VAS),10 the global aesthetic improvement scale (GAIS), and subject global satisfaction scale. Standardized photographs of upper arms were taken before treatment and at each visit post-treatment with the subject standing and both arms outstretched at a 45-degree angle from the body using standardized camera angles and room lighting. Two blinded reviewers evaluated photographs of upper arms and provided their assessments using the VAS for upper arms developed by Amselem et al (Type I = no laxity, Type II = mild laxity, Type III = moderate laxity, Type IV = severe laxity, and Type V = very severe laxity).10 The average VAS scores from both reviewers were reported for each visit. In addition, participating investigators compared photographs from each post-treatment visit with baseline and provided their assessments on changes in overall aesthetic appearance after treatment using the GAIS (0 = worsened, 1 = no change, 2 = improved, 3 = much improved, and 4 = very much improved), as previously described.11 Subjects provided their assessments of treatment satisfaction at each post-treatment visit using the subject global satisfaction scale (1 = very dissatisfied to 5 = very satisfied). Adverse events (AEs) were recorded immediately after treatment and at all post-treatment visits.
2.5 Statistical analyses
Descriptive statistics were primarily used to summarize the results. Cutometer values (R0 and R2) and arm VAS scores were summarized using mean and standard deviation. Investigators’ GAIS, subject satisfaction, and AEs were summarized by percentages. The Wilcoxon signed-rank test was used to compare cutometer values and arm VAS scores at each follow-up visit with baseline values. p values < 0.05 were considered statistically significant. R (version 4.0.3, 2020–10–10), and R Studio (version 1.3.1093) were used to perform the analyses.
3 RESULTS
3.1 Subject demographics and baseline characteristics
Twelve female subjects participated in and completed the study. Although follow-up data were available for all 12 subjects at 24 weeks, these were available for 11 and 8 subjects at 4 weeks and 12 weeks, respectively, as these visits fell within the period of COVID-19–related movement restrictions in Singapore. Subject demographics and baseline characteristics are summarized in Table 1. The mean age was 51.3 (SD 7.3) years. Three subjects (25%) were Asian and the remaining nine (75%) were Caucasian. The mean BMI was 23.3 (SD 3.1) kg/m2, five subjects (42%) had BMI 20–23 kg/m2, three (25%) had BMI >23 to 25 kg/m2, another three (25%) had BMI >25 kg/m2, and one (8%) had BMI <20 kg/m2. At baseline, the mean arm VAS score (average of both arms) was 2.9 (SD 0.6). All arms (24, 100%) had a VAS score of 2 or more, indicating at least mild brachial laxity (VAS score 2: 3 arms, 13%; VAS score >2–3: 16 arms, 67%; VAS score >3–4: 4 arms, 17%; and VAS score >4: one arm, 4%).
| All subjects (n = 12) | |
|---|---|
| Age (years) | 51.3 (7.3) |
| Ethnicity, n (%) | |
| Asian | 3 (25) |
| Caucasian | 9 (75) |
| Weight (kg) | 62.7 (8.3) |
| Height (m) | 1.6 (0.1) |
| BMI (kg/m2) | 23.3 (3.1) |
| BMI, n (%) | |
| <20 kg/m2 | 1 (8) |
| 20–23 kg/m2 | 5 (42) |
| >23–25 kg/m2 | 3 (25) |
| >25 kg/m2 | 3 (25) |
| aBaseline arm VAS | 2.9 (0.6) |
| bBaseline arm VAS, n (arms) (%) | |
| 1 | 0 (0) |
| 2 | 3 (13) |
| >2–3 | 16 (67) |
| >3–4 | 4 (17) |
| >4 | 1 (4) |
Notes
- Data presented are mean (SD) unless otherwise stated. Arm VAS was reported based on the average score from both reviewers.
- Abbreviations: BMI, Body mass index; SD, Standard deviation; VAS, Visual analogue scale.
- aAverage of both arms
- bPercentage calculated out of a total of 24 arms.
3.2 Impact on objective outcome measures: biophysical skin parameters
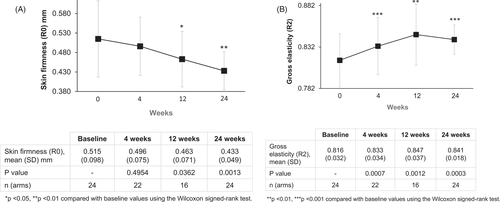
Cutometer readings demonstrated a progressive decrease in the mean R0 reading (the lower the reading, the better the firmness of the skin), throughout the course of the study, suggesting progressive improvement in skin firmness from baseline at all time-points (Figure 2A). This reduced from 0.515 (SD 0.098) mm at baseline to 0.433 (SD 0.049) mm at 24 weeks. Statistically significant improvements were observed from 12 weeks onwards (p < 0.05 for 12 and 24 weeks). In addition, the mean R2 reading (the closer the reading to 1, the better the elasticity of the skin) improved significantly from baseline at all time-points (p < 0.05 for all) (Figure 2B). The mean R2 reading increased from 0.816 (SD 0.032) at baseline to 0.847 (SD 0.037) at 12 weeks, where maximum improvement was observed.
3.3 Impact on subjective outcome measures: aesthetic appearance and subject satisfaction
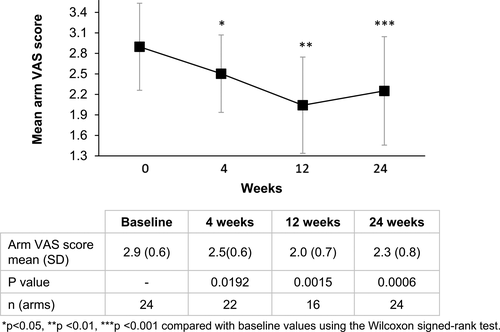
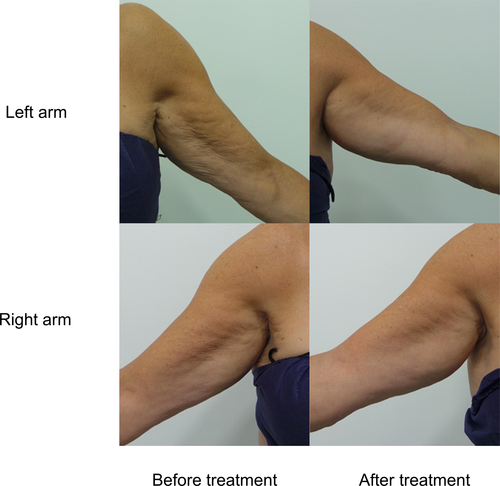
Reviewer-rated arm VAS score improved significantly from baseline at all time-points (p < 0.05 for all) (Figure 3). The mean arm VAS score reduced from 2.9 (SD 0.6) at baseline to 2.1 (SD 0.7) at 12 weeks, where maximum improvement was observed. Figure 4 shows the change in overall skin quality in a subject who had a VAS score of 4 (severe laxity) at baseline. Pre- and post-treatment photographs depict the improvement in overall skin quality and laxity from baseline to 24 weeks after treatment.
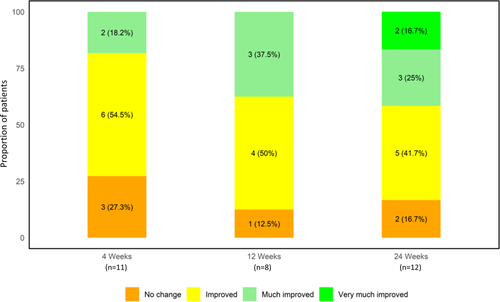
Overall change in arm appearance was assessed using the GAIS (Figure 5). Based on investigator's ratings, over 70% of all subjects showed an improved aesthetic appearance compared with baseline at all time-points throughout the study. At 4 weeks, 73% of subjects (8/11) were assessed to have improvement of brachial laxity. By 12 and 24 weeks, 88% (7/8) and 83% (10/12) of subjects were assessed to have improved, respectively. Furthermore, over 37% of subjects were reported to have “much improved” and “very much improved” brachial laxity by 12 and 24 weeks. There were no reported cases with worsening of brachial laxity.
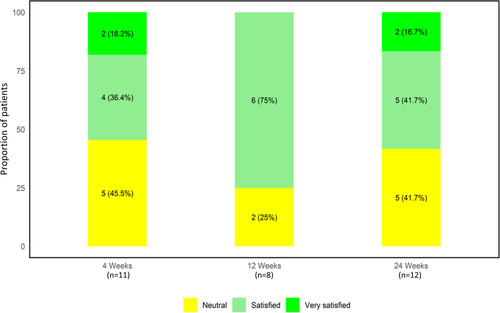
When asked to rate their overall satisfaction with the treatment, 55% of the subjects (6/11) reported they were satisfied at 4 weeks (Figure 6). At the 12 weeks, the highest percentage of satisfied subjects (75%; 6/8) was noted. By 24 weeks, 58% (7/12) were still satisfied with the treatment. No subjects were dissatisfied during the study.
3.4 Adverse events
No serious AEs were noted during the study. All AEs were mild and transient in nature. The most common AEs reported were mild bruising (92%; 11/12) and redness (25%; 3/12), which resolved spontaneously within a week. One subject reported mild paresthesia (electric shock sensations) down the left arm, which also resolved spontaneously over two weeks.
4 DISCUSSION
This 24 weeks pilot study assessed the effectiveness of MFU-V combined with subdermal injections of diluted or hyperdiluted CaHA in healthy Asian and Caucasian female subjects with brachial skin laxity. Our findings demonstrate that a single session of the combined treatment modalities was associated with significant improvement in skin laxity and improved aesthetic appearance of the brachial region. Objective outcome measures using a cutometer demonstrated significantly improved skin firmness and skin elasticity after the combination of treatments. Other subjective outcome measures including arm VAS, GAIS, and subject global satisfaction scale demonstrated improvement in brachial laxity and a high degree of subject satisfaction. These encouraging results are especially relevant given the novel findings of this study and the growing demands for such treatments. This study, to our knowledge, is the first to assess single session combined use of MFU-V and diluted or hyperdiluted CaHA treatment for brachial skin laxity. This is of particular importance given the increased concern over laxity in the brachial region as evidenced by the marked increase in demand for upper arm lifts.3 While this was a pilot study, we chose to recruit a diverse group of subjects as far as possible. The study cohort included subjects with a range of BMI from <20 kg/m2 to >25 kg/m2, varying degrees of laxity, as well as Asians and Caucasians subjects.
Given that MFU-V and CaHA stimulate neocollagenesis via different mechanisms, there is considerable rationale for combining both modalities in the same treatment area for enhanced neocollagenesis. MFU-V generates focused ultrasound waves, which cause thermal coagulation at precise depths beneath the targeted skin areas, resulting in collagen denaturation and subsequent collagen remodeling.12, 13 Injecting small amounts of CaHA may then precipitate further collagen building at specific points of injection. The CaHA particles form a matrix, which activates fibroblasts to produce more collagen and elastin.15 Superficial injections of diluted or hyperdiluted CaHA result in skin regeneration, without causing volumization of the brachial tissues. CaHA dilution is titrated according to skin thickness.14, 19 Indeed, limited studies have demonstrated increased neocollagenesis with combined use of MFU-V and CaHA, and these have been for the treatment of other body areas, such as the neck, décolletage, and buttocks.16-18 In the case study by Casabona et al, histological examination of skin samples taken from the inner thighs revealed thicker and denser collagen fibers at 24 weeks when treatments were combined compared with those treated with a single procedure.18 In addition, the skin samples showed no significant differences in immunological patterns between those treated with MFU-V combined with CaHA and those treated with CaHA alone, demonstrating the safety profile of the combined treatment.18 In a subsequent study, significant improvements in skin laxity and cellulite severity in the buttocks and upper thighs were observed 90 days following treatment with MFU-V combined with diluted CaHA.17 Similarly, the combined use of MFU-V and diluted CaHA has been shown to improve skin laxity and lines on the neck and the décolletage.16 The combined approach was well-tolerated and was associated with high subject satisfaction.16, 17
Consistent with these previous studies, our study showed clinically and statistically significant improvement in skin laxity in the brachial area with the combined treatment. In our study, combined use of MFU-V and subdermal injections of diluted or hyperdiluted CaHA was associated with improvements in brachial skin laxity as assessed by both objective and subjective outcome measures. Cutometer assessment of skin firmness (R0) and elasticity (R2), which has been used extensively in the dermatologic literature to measure changes in skin quality due to various factors, including aging,20 demonstrated significant improvements in objective biophysical parameters of the brachial skin. In addition, both reviewer-rated arm VAS and investigator-rated GAIS revealed improvement in arm VAS score with the majority of subjects assessed to have improved arm appearance after treatment.
The cutometer measure for skin firmness (R0 reading) progressively improved throughout the course of the study, with statistically significant improvement from baseline observed from 12 weeks onwards. Notably, it was also at the same time-point of 12 weeks that the greatest improvements from baseline in mean R2 reading (measure of skin elasticity) and mean arm VAS score were observed. The mean arm VAS score improved by almost one grade from a mean score of 2.9 (close to a rating of moderate laxity) to 2.1 (close to mild laxity) after only one treatment session. In addition, the highest percentage of subjects with improved arm appearance was noted at 12 weeks, as assessed by GAIS. These observations coincide with the timeframe required for neocollagenesis as observed in earlier studies, where improvements in neck, décolletage, and cellulite appearance were observed 12 weeks after combined treatment with MFU-V and diluted CaHA.16, 17 Histological examination of skin samples taken from the inner thighs showed more type I and type III collagen in samples treated with MFU-V combined with 1:1 dilution of CaHA compared with untreated samples.17 It should be noted that the improvement in mean arm VAS score from baseline was lower at 24 weeks compared with that at 12 weeks, and the percentage of subjects with improved aesthetic appearance at 24 weeks was lower than that at 12 weeks. The observed trend could possibly be due to the inherent limitations of the subjective outcome measures. Also, photographic assessment of arm appearance can be challenging as it is difficult to capture changes in skin quality on photographs. In light of these drawbacks, objective outcome measures (eg, cutometer assessments to detect biophysical changes in skin quality) become all the more crucial for measuring changes that may not be detected or assessed accurately by more subjective and less sensitive measures.
The use of patient-reported outcome measures, such as the subject global satisfaction scale, allows aesthetic physicians to capture valuable data to understand subjects’ perception of improvement. In this study, the majority of subjects at each time-point reported that they were satisfied with the treatment, and no subjects were dissatisfied. Consistent with the results for R2 value, arm VAS score, and investigator-rated improvement in arm appearance, the highest percentage of satisfied subjects was observed 12 weeks after treatment. A lower percentage of satisfied subjects were noted at 24 weeks than at 12 weeks. It is plausible that subjects developed perception drift22 during the study. In such cases, as commonly perceived flaws become increasingly addressed, subjects become more fixated on previously ignored flaws and start to judge their perceived improvement against a new baseline, making them prone to underestimate the magnitude of improvement. In addition, the follow-up visits of this study fell within the period of COVID-19–related movement restrictions in Singapore, where the public were asked not to leave their homes except for essential purposes. As such, it is possible that some fluctuations in weight may have occurred during this period, subsequently affecting subjects’ satisfaction. Furthermore, subjects were likely to have been distracted and concerned by other issues related to COVID-19 at this time, potentially influencing their mindset regarding the procedure.
In this study, combined use of MFU-V and diluted CaHA in a single treatment session was well-tolerated. AEs reported in this study were limited to mild bruising, redness, and paresthesia, and were transient in nature. The safety profile of combined treatment is consistent with that observed in studies which had used MFU-V and diluted CaHA individually in the brachial area or other body areas.8-11
A limitation of this study was the small sample size and the relatively short follow-up period. Both MFU-V and CaHA have individually demonstrated a duration of effect of at least one year.23 Future work should consider studying subjects for a longer period of time to examine whether further improvement can be observed, as well as to assess the durability of the esthetic effects of the combined treatment. We acknowledged that diluted CaHA injection did not immediately follow MFU-V treatment in some patients because they had time constraints. Nonetheless, the investigators ensured that diluted CaHA was injected within one week of MFU-V treatment. This is not expected to have a significant impact on the treatment results as the process of neocollagenesis is known to occur over several months16, 17 and a 1 week interval is unlikely to make an appreciable difference. Next, there is lack of a validated clinical grading scale to assess the severity of laxity in the upper arm area. While the arm VAS developed by Amselem et al10 was published in the literature, it has not been properly validated. In addition, racial/ethnic differences in response to these treatments need to be evaluated. There are recognized differences in skin quality characteristics for Asians vs Caucasians associated with aging,24 and it is possible that certain non-invasive treatments may be more effective for particular racial groups and Fitzpatrick skin types. Arguably, non-invasive procedures, such as MFU-V in combination with CaHA may be more appropriate for individuals with higher Fitzpatrick score given the higher risk of hypertrophic scarring and keloid formation in this population.25 Finally, baseline severity of brachial laxity and patient's BMI may have a potential impact on the response to this treatment combination. However, we did not perform stratified analyses by clinical and demographic characteristics such as severity of laxity and BMI due to sample size constraints. Future studies involving a larger patient population should be considered to assess whether these baseline characteristics have any influence on treatment outcomes and identify individuals most suited for these non-invasive treatments. Another limitation of the study is the use of a standardized treatment protocol at two pre-determined depths of 4.5 mm and 3.0 mm. Further improvement of outcomes may have been achieved with customization of the treatment depths. The real-time ultrasound visualization capability of MFU-V can be used to determine the precise depths of target tissue layers and guide subsequent selection of transducers according to individual's needs. Nevertheless, the results of this study are positive and encourage further investigation. Future work can look into treating the posterior aspect of the upper arms for circumferential tightening and lifting and reduced adverse events.
5 CONCLUSION
This study demonstrates novel findings that combination treatment with MFU-V followed by injection of diluted or hyperdiluted CaHA is effective for the management of brachial skin laxity as demonstrated by clinically and statistically significant improvements in both objective and subjective outcome measures.
ACKNOWLEDGMENTS
Medical writing and editorial support were provided by Tech Observer Asia Pacific Pte Ltd and were funded by Merz Aesthetics.
CONFLICTS OF INTEREST
This study was supported by a research grant from Merz Aesthetics. Sylvia Ramirez and Ivan Puah report no additional conflicts of interest in this work.
AUTHORS CONTRIBUTIONS
Both authors contributed to study conception and design, as well as acquisition and interpretation of data, with the first author contributing to a greater extent in both aspects. They were involved in revising the manuscript for important intellectual content, with the first author contributing to a greater extent, and both have given final approval of the version to be published.
ETHICAL APPROVAL
The study was approved by the Parkway Pantai institutional ethics committee (approval reference: PIEC/2019/024), and all subjects provided written informed consent before the study commenced. The study was carried out in accordance with the Declaration of Helsinki. Written informed consent for publication of the pre- and post-treatment photographs was obtained from the subject whose photographs were included in the manuscript.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




