Different, but the same: Inferring the hunting behaviour of the hypercarnivorous bush dog (Speothos venaticus) through finite element analysis
Abstract
Cerdocyonina is a clade composed by the South-American canids in which the bush dog (Speothos venaticus) is one of the most elusive species. Known for its unique morphology within the group, this small, bear-like faced canid is the only member of the clade adapted to hypercarnivory, an almost exclusively meat-based diet currently present only in usually large, pack-hunting canids such as the grey wolf (Canis lupus). However, much of the biology of the bush dog is poorly understood, and inferences about its ecology, hunting strategies and diet are usually based on observation of captive individuals and anecdotal records, with reduced quantitative data to offer support. Here, we investigated the craniomandibular functional morphology of the bush dog through finite element analysis (FEA). FEA was employed to model the biting behaviour and to create extrinsic and intrinsic functional scenarios with different loads, corresponding to different bites used to subdue and process the prey. For comparison, the same modelling was applied to the skull of a grey wolf and a grey fox (Urocyon cinereoargenteus). Our analysis showed that the bush dog's responses to loading are more similar to the wolf's than to the fox's in most scenarios, suggesting a convergent craniomandibular functional morphology between these two hypercarnivorous species, despite their distinct phylogenetic positions and body sizes. Differences between the three taxa are noteworthy and suggested to be related to the size of the usual prey. The modelled bite force for the bush dog is relatively strong, about half of that estimated for the wolf and about 40% stronger than the fox's bite. The results strengthen with quantitative data the inferences of the bush dog as a pack-hunting predator with prey size similar to its own, such as large rodents and armadillos, being specialised in subduing and killing its prey using multiple bites. Its similarity to the wolf also confirms anecdotal accounts of predation on mammals that are much larger than itself, such as peccaries and tapirs. These data highlight the ecological specialisation of this small canid in a continent where large, pack-hunting canids are absent.
1 INTRODUCTION
Cerdocyonina is a lineage of South-American endemic canids that include some of the least-known extant canid species (Figure 1, Chavez et al., 2022; DeMatteo & Loiselle, 2008; Pitman & Beisiegel, 2013; Tensen, 2018). This group comprises generalist fox-like morphotypes (Cerdocyon, Atelocynus and Lycalopex), as well as highly specialised species such as the maned wolf (Chrysocyon brachyurus) and the bush dog (Speothos venaticus, Chavez et al., 2022; Lindblad-Toh et al., 2005; Segura et al., 2021; Wozencraft, 2005). Most Cerdocyonina are mesocarnivores (i.e. with a diet comprised of 50%–70% of vertebrate tissues, balanced with non-vertebrate food items such as insects, fruits and seeds; Slater et al., 2009; Van Valkenburgh, 2007), which is the most common condition in living canids. The bush dog, however, is the only living member of Cerdocyonina adapted to hypercarnivory (i.e. diet comprising >70% of vertebrate tissues, Perini et al., 2010; Van Valkenburgh, 2007; Wang & Tedford, 2008). The hypercarnivorous diet is only observed in three other species of living canids, all included in the Canina clade, the sister group of Cerdocyonina (Figure 1; Lindblad-Toh et al., 2005; Zrzavý et al., 2018): the painted wolf (Lycaon pictus) from Africa, the dhole (Cuon alpinus) from Asia and the grey wolf (Canis lupus) with a Holarctic distribution (Van Valkenburgh, 1991).
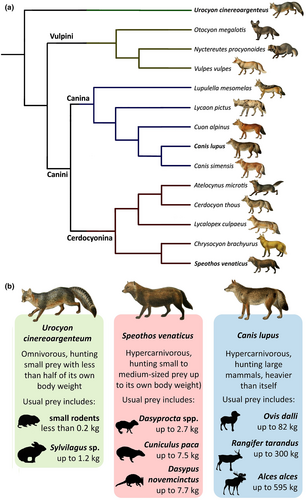
The bush dog is a small, short-legged canid (weighing 4–7 kg, Beisiegel & Zuercher, 2005), and its general biology is poorly studied in its natural habitat, with most of its biological information obtained from observation of captive animals (Beisiegel & Ades, 2002). Anecdotal accounts comprise most of the information about individuals in the wild, including social and hunting behaviours and habitat use patterns (Beisiegel & Zuercher, 2005; Hildebrand, 1954). Most of the known aspects of the functional morphology of the bush dog were made through generic inferences based on its unique morphology. Its massive and deep skull, with short rostrum, reduced dentition and enlarged canines and carnassials points to a specialised diet overwhelmingly based on meat (Damasceno et al., 2013; Van Valkenburgh, 1991). Indeed, the bush dog was historically placed in an exclusively hypercarnivorous subfamily of Canidae called Simocyonina, together with the painted wolf and the dhole, based on the unique traits of its carnassial teeth (Simpson, 1957). The hypercarnivorous diet of the bush dog is verified by observations of captive animals (Macdonald, 1996) and faeces analyses of wild individuals (Lima et al., 2009; Zuercher et al., 2005). However, there are few available quantitative data on the bush dog's predatory behaviours from a functional morphology perspective (Christiansen & Adolfssen, 2005; Christiansen & Wroe, 2007; Penrose, 2019; Penrose et al., 2020).
Here, we present a functional morphology approach to analyse the skull of the bush dog using finite element analysis (FEA) and compare it to the models of the hypercarnivorous grey wolf (Figure 1), a large, pack-hunting canid (weighing up to 50 kg, Mech, 1974), and the grey fox (Urocyon cinereoargenteus), a similar sized species (weighing 3–7 kg, Fritzell & Haroldson, 1982) that has a mesocarnivorous diet (Figure 1, Allen et al., 2021). We calculated bite forces and simulated biomechanical scenarios to understand the functional morphology of the bush dog and, thus, its possible hunting strategies. By testing previously formulated hypotheses of its hunting and feeding behaviour, we aimed to fill gaps in the limited data from field studies. Our results shed light on biomechanical aspects that may bridge the lack of quantitative data regarding the predation strategies of the bush dog and contribute to a better understanding of this elusive species.
2 METHODS
2.1 Specimens and segmentation
The specimen of bush dog modelled for this study (MVZ 184054—Museum of Vertebrate Zoology, Berkeley, USA) was scanned in the University of Texas High-Resolution X-ray CT Facility, with 0.31 mm slice thickness, voltage of 150 kV and current of 160 μA, and made available on the online platform digimorph.org (Figure 2). For comparison, a specimen of grey wolf (C. lupus pambasileus, LACM(M)23,010—Department of Mammalogy, Natural History Museum of Los Angeles County, Los Angeles, USA) was obtained through published data (Tseng et al., 2016) deposited in the online platform morphosource.org (media ID 000009038), and modelled herein (Figure 2). This specimen was scanned at UCLA Medical Center, with 0.6 mm slice thickness, voltage of 150 kV and current of 198 μA. The grey wolf was chosen for comparison since it is the most studied hypercarnivorous canid (Tensen, 2018), with a solid history of publications about its hunting and dietary habits, which can serve as a model for inferences on the bush dog. Additionally, we also tested a specimen of grey fox (U. cinereoargenteus, UCLA 6928—University of California, Los Angeles, USA), scanned in the University of Texas High-Resolution X-ray CT Facility, with 0.24 slice thickness, voltage of 120 kV and current of 160 μA, also made available on the online platform digimorph.org (Figure 2). The grey fox was tested because of it omnivorous, non-specialised diet (Fritzell & Haroldson, 1982), a condition that could be used as a proxy for the general dietary habits of Canidae. Further information about the computed tomography scans is provided on their respective repositories.
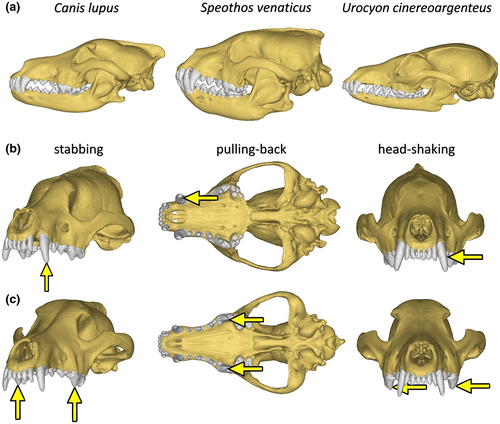
To model the extrinsic (i.e. ‘external’ forces, generated by a struggling prey) and intrinsic (i.e. ‘internal’ forces, generated by the canids' own musculature) scenarios we propose, the skull elements were segmented into osteological (cranium and mandible) and dental (upper and lower dentition) components using the software Amira 5.3.3 (Thermo Fisher Scientific). The segmentation was conducted using automatic and manual tools. The three-dimensional models were exported to the software Hypermesh 13. 0 (Altair Engineering), and were treated as isotropic and homogeneous (solid model consisting of approximately 900,000 tetrahedral elements per model). The material properties of bones and teeth (bone: E = 13.7 GPa, ʋ = 0.3 N; tooth: E = 38,6 GPa, ʋ = 0.4 N) were assigned based on previous works on carnivore mammals, including canids (Attard et al., 2011; Chamoli & Wroe, 2011; Figueirido et al., 2018; Slater et al., 2009; Slater & Van Valkenburgh, 2009). In the absence of direct material testing for each modelled species, material properties obtained for other Carnivora species and applied to all models allowed the functional study of the different skull morphologies in a maximally comparative context. Each modelled scenario was solved in the software Abaqus 6.14-1 (Simulia). The performance of each model was assessed via contour plots of von Mises stress distribution and mean von Mises stress and displacement value of the elements, considering 99% of the values to avoid individual stress singularities on nodes (Figueirido et al., 2018; Montefeltro et al., 2020).
2.2 Extrinsic scenarios
Twelve extrinsic scenarios with different load assignments were tested to understand the craniomandibular functional properties in different conditions, based on the application of an extrinsic load of 500 N on each tested tooth (Figure 2), a magnitude which concerns the bite force recovered for both species (see Section 3.2). The scenarios follow Figueirido et al. (2018) and consist of: (i) stabbing, in which a dorsally direct load was placed at the tip of the tooth; (ii) pulling back, in which an anteriorly direct extrinsic load was placed at the posterior surface of the tooth; and (iii) head shaking, in which a laterally direct load was placed at the mid-left surface of the tooth (Figure 2). Both canines and carnassials were tested in unilateral and bilateral extrinsic scenarios (Figure 2). The tests aim to investigate cranial properties in situations that simulate possible behaviours during prey capture and consumption, including bite (stabbing) and postcranial guided head movements (pulling back and head shaking, Montefeltro et al., 2020). For comparison, the same scenarios were applied to the corresponding teeth on the lower jaw, that is, the lower canines and carnassials, of the three species in mandible-driven bilateral bites. The wolf and the grey fox models were scaled to the same surface area of the bush dog model to avoid incongruities due to the difference size between the specimens (Dumont et al., 2009). In the cranium models, constraints were applied on the occipital condyle (10 in total) and the temporomandibular joint (five on each side); in the mandible models, they were applied at the condyloid process (five on each side), with each node constrained in all directions (x, y and z).
2.3 Intrinsic scenarios and bite force
To calculate bite force, intrinsic scenarios were simulated for the mandible models, using a simplified jaw adductor muscle-driven biting (following Figueirido et al., 2018; Montefeltro et al., 2020). The m. temporalis, m. masseter and m. pterygoideus (including the medialis and lateralis) were reconstructed based on the dry-skull method and without considering their subdivisions, following previous studies (Penrose et al., 2020; Tseng et al., 2016, 2017). The adductor force of each muscle (Table 1) was estimated by considering its attachment area, as in previous works on canids and other Carnivora (Penrose et al., 2020; Tseng et al., 2017). The attachment areas were used as a proxy for physiological cross-sectional area, which was then multiplied by an isometric muscle stress value of 37.0 N cm−2 (Christiansen & Adolfssen, 2005; Christiansen & Wroe, 2007; Koolstra et al., 1988; Penrose et al., 2020; Weijs & Hillen, 1985).
| Temporalis (N) | Pterygoideus (N) | Masseter (N) | |
|---|---|---|---|
| Speothos venaticus | 833.61 | 422.54 | 152.81 |
| Canis lupus | 2205.2 | 959.52 | 468.79 |
| Urocyon cinereoargenteus | 608.65 | 216.93 | 114.7 |
Four intrinsic scenarios were simulated in the mandibles: (i) unilateral bite with the canine; (ii) bilateral bite with both canines; (iii) unilateral bite with the carnassial; and (iv) bilateral bite with both carnassials. For each test, constraints were placed on the condyloid process (five on each side) and also on the tip of each tested tooth (for the unilateral scenarios, on the left tooth; for bilateral scenarios, on the teeth of both sides); each node was constrained in all directions (x, y and z).
3 RESULTS
3.1 Extrinsic scenarios
In general, the extrinsic scenarios in the cranium do not show great differences in mean von Mises stress per element between the three species, being similar in the all tested taxa (Figure 3). In most tests, the bilateral scenarios recorded higher stresses than the unilateral ones, and the carnassial bites proved to be less prone to stress than the canine bites in all but the pullback unilateral scenarios of the hypercarnivorous taxa (even in these, the differences were minimal). Regarding how the crania reacted to the different scenarios, the stabbing and head shaking behaved relatively similar to each other, while the pulling back stood out from the others with the lowest mean von Mises stress per element. The contour plots and mean von Mises stress per element of each cranial scenario is shown in Figures 4-6.
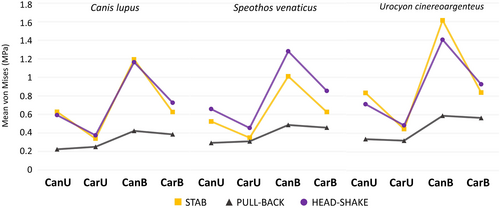
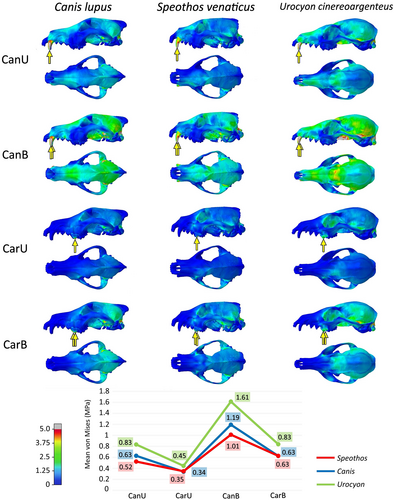
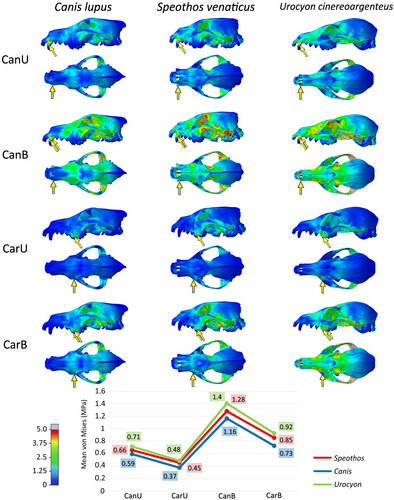
The stabbing and head-shaking scenarios not only showed more stress per element than the pulling back scenario, but also had more widespread stress across the crania (Figures 4 and 5), while in the pulling back scenario most of the stress was limited to the ventral region of the cranium (Figure 6). Between the three taxa, the stress varied more in terms of magnitude (i.e. the mean von Mises stress per element) than spatial distribution (i.e. the regions more or less stressed in the cranium). This suggests the same pathway, as stress is distributed in similar areas of the cranium of these canids, as represented in the contour plots of von Mises stress (Figures 4-6). The most affected areas in the stabbing and head-shaking scenarios are the attachment region of the temporalis muscle, including most of the temporal, parietal and the orbital region of the frontal, the zygomatic arch (especially in the head-shaking scenarios), and the maxilla and nasal (Figures 4 and 5). In the grey fox, the canine scenarios also presented higher stress levels in the anterior portion of the rostrum when compared to the hypercarnivorous taxa, especially in the stabbing and pulling back scenarios (Figures 4 and 6). In the pulling- back scenarios, most of the stress is restricted to the palatines and pterygoids in all species (Figure 6).
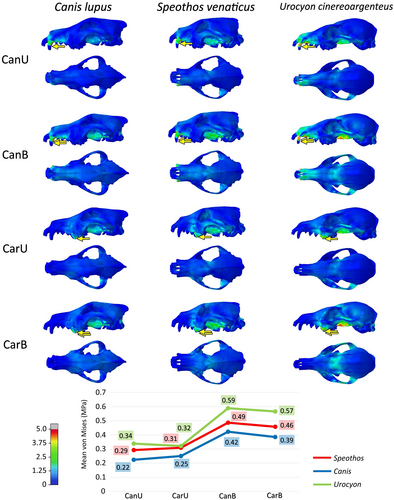
The bilateral extrinsic scenarios of the mandibles in the hypercarnivorous species showed similar results to the cranial scenarios in both mean stress per element and stress spatial distribution, while in the grey fox tests the mandible was significantly more stressed in the stabbing and head shaking scenarios (Figure 7). The main difference noted in the tests performed with the mandibles is that the stabbing scenario with the canines presented a much higher mean stress than any other scenario, both in the mandibles and in the cranium in the modelled species.
The bush dog cranium proved to be more stressed in most of the scenarios than the grey wolf. When comparing these two hypercarnivorous species, the head shaking and the pulling back tests presented similar differences between the mean stress per element. Although the bush dog cranium showed greater stress in most tests (Figures 5 and 6), the stabbing scenarios deviate from the pattern (Figure 4). In the latter, the bush dog cranium was less stressed than the wolf during the canine bites (in both unilateral and bilateral scenarios) and similarly during the carnassial bite (in the bilateral scenario). The only other scenarios where a bush dog bite was less or as stressed as the wolf bite is during the head shaking bilateral bites with the mandibles (Figure 7). In these scenarios, the canine bite showed less stress in the bush dog, while the carnassial bite had the same mean stress as the wolf's bite.
The grey fox is more stressed in all tested scenarios when compared to the hypercarnivorous species, especially in the stabbing of both cranium (Figure 4) and mandible (Figure 7). Both bush dog and grey fox showed very similar results in the carnassial unilateral bites in the head shaking and pulling back scenarios (Figures 5 and 6) and, in general, the head shaking results as a whole are more similar between these two small species than either is to the wolf. The stress in the mandible, however, is far greater in the grey fox than in the other two species in every scenario, but the enormous discrepancy of the results for this species in the head shaking and, above all, the stabbing scenario, is noteworthy, showing virtually double the stress when compared to the hypercarnivorous species.
Comparing the different scenarios within the same species, the stabbing scenarios of the cranium have a lower mean stress than head shaking in the bush dog (Figure 3), suggesting a cranium less suited to lateral shaking movements. This condition is dissimilar to the wolf, which presents mean stress values that are similar in these different scenarios (Figure 3). The mean stress of the carnassial bites in the stabbing and head shaking scenarios of the grey fox is very similar to each other, resembling the condition seen in the wolf (Figure 3), but when taking into account the canine bites in these same scenarios, the stabbing bites evidently generate more stress, unlike in the other two species.
3.2 Intrinsic scenarios and bite force
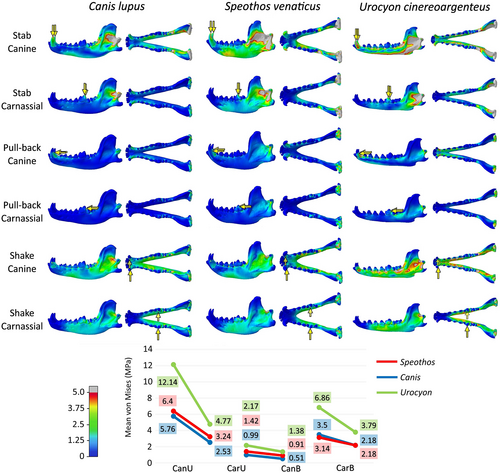
The magnitude of von Mises stress per element varies between the species, with higher mean stress in the mandible of the grey fox and lower mean stress in the mandible of the grey wolf, with the bush dog presenting results more similar to the wolf than to the fox in all tested intrinsic scenarios (Figure 8). The differences between stress mean in each scenario remain relatively constant, with the bush dog's mandible exhibiting approximately 30% more stress than the wolf's mandible and about 60% less stress than the fox's mandible. In the three species, the bilateral bite presents less stress per element than the unilateral ones, a subtle difference that is more evident in the fox results.
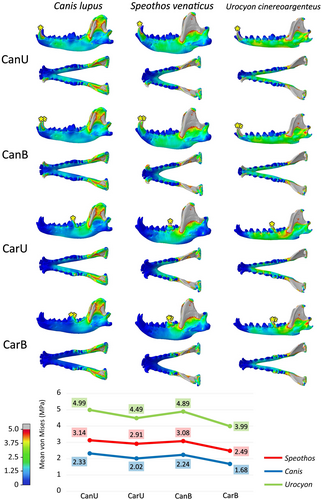
The mandibles reacted similarly (Figure 8) in terms of von Mises stress distribution, with the same stress pathways, as observed in the extrinsic scenarios (Figures 4-8). As expected, in all models, the greatest von Mises stress was observed in the region of attachment of the adductor muscles, especially in the coronoid process, corresponding to the area of attachment of the m. temporalis, the largest adductor muscle in Canidae, but also in the masseteric fossa, where the m. masseter is attached. The mandibular rami also show more stress posteriorly according to the tested tooth.
The bite forces modelled in all intrinsic scenarios are expressed in Table 2. The relationship between the forces exerted by the canines and the carnassials proved to be roughly the same with each other in both the unilateral and bilateral scenarios. When comparing the hypercarnivorous species, the raw bush dog's bite force was about half of the wolf's bite force in all tested scenarios (48% weaker in unilateral bites scenarios and 45%–50% in the bilateral bites scenarios). Compared to the grey fox, the raw bush dog's bite was significantly stronger (36%–43% stronger in the unilateral bites and about 50% in the bilateral bites scenarios).
| Speothos venaticus (N) | Canis lupus (N) | Urocyon cinereoargenteus (N) | |
|---|---|---|---|
| Canine unilateral | 591.74 | 1210.14 | 337.74 |
| Canine bilateral (left + right) | 342.41 + 286.85 | 712.87 + 686.39 | 188.35 + 214.29 |
| Carnassial unilateral | 1051.94 | 2153.54 | 502.31 |
| Carnassial bilateral (left + right) | 570.49 + 497.16 | 1112.51 + 1010.86 | 292.09 + 279.98 |
In general, the canine bites resulted in lower bite forces when compared to the carnassial bites (56% weaker in the unilateral scenarios of both hypercanivorous species and 33% weaker in the fox's bites). In all species, the total force of the carnassial bite was similar in the unilateral and bilateral scenarios, but the canine bilateral bite proved to be slightly stronger than the unilateral bites, with the estimated force being about 106%, 115% and 119% higher in the bush dog, wolf and fox respectively.
4 DISCUSSION
Regarding the von Mises stress, the differences between all species are restricted to specific regions of the skull and variation is seen more in terms of magnitude of stress than in the distribution of affected areas. Slater et al. (2009) performed similar extrinsic tests (stabbing, pulling back and head shaking scenarios) to the ones presented here, with different Canina species, including hypercarnivorous (painted wolf Lycaon pictus) and non-hypercarnivorous (Ethiopian wolf Canis simensis, black-backed jackal Lupulella mesomelas). Their substantially similar results indicate that Canina present a similar stress distribution in the cranium. Our results reinforce that of Slater et al. (2009) in showing that the specialisation on diet and prey size will be represented more as a variation in the stress gradient than in different stress areas in the von Mises stress plot (Figures 4-8).
The extrinsic and intrinsic tests modelled lead to a complex panorama since the bush dog's results do not entirely mirror the results of the grey wolf, a much larger species with an equivalent, hypercarnivorous diet, nor the results of the grey fox, a similar-sized species but with a distinct, more generalist diet. Still, the mean element von Mises stress of the bush dog is similar to that of the wolf in most scenarios (with the exception of cranial head shaking), except in few particular cases in which they are closer to the fox (Figures 4-8). Next, we discuss the similarities and differences found in each species considering (i) the selection and capture of prey, and (ii) the bite force.
4.1 Prey selection and capture
In general, differences in functional morphology in the tested species can be attributed to the relative size of the usual prey. A skull better suited to withstand high amounts of stress can be crucial to deal with larger prey since, unlike the grey fox, bush dogs can occasionally hunt animals much larger than themselves, even in a prey–predator proportion similar to the wolf and its usual large prey (see Supplemental Material). The mechanical efficiency of the skull in the stabbing and pulling back scenarios and the powerful bite force (see Section 4.2) makes the inferences and anecdotal records of bush dogs hunting large mammals such as Mazama deer, peccaries and capybaras (Beisiegel, 1999; Beisiegel & Zuercher, 2005; see Supplemental Material) quite plausible.
Perhaps the most notable report of large-prey hunting by bush dogs was made by Wallace et al. (2002) of a pack of six individuals chasing and badly injuring an adult tapir (Tapirus terrestris) in Bolivia. The chase was watched for 3 h and after the dogs fled as the witnesses approached, the tapir was found lying down, exhausted and with numerous bite wounds on the lower parts of all four legs. The record of a group of bush dogs chasing and exhausting an adult tapir, weighing between 150 and 250 kg (Padilla & Dowler, 1994), is impressive. This implies a predator–prey size relation of at least 1:21, which is possible unique among Canidae and even Carnivora as a whole. Even populations of lions (Panthera leo) specialised in hunting bush elephants (Loxodonta africana) in Botswana and Zimbabwe do not deal with a body size difference of this magnitude, with a predator–prey size relation of 1:10–15 (Joubert, 2006; Loveridge et al., 2006; Power & Compion, 2009).
A trade-off of hunting big, dangerous prey is that it poses a greater risk to the predators' lives. Reports of wolves severely and even fatally injured during hunting are common (Mech & Peterson, 2003 and references therein; Lazar et al., 2008; Severtsov et al., 2016). To date, the only occurrence of injuries resulting from hunting in the bush dog is the record of a broken canine when a solitary individual attempted to subdue a paca (Deutsch, 1983). The scarcity of records like this may be related to the lower volume of published data on the bush dog when compared to the wolf. The results presented here, however, also suggest that these types of injuries may be less common in the bush dog, given the preference for smaller, less demanding prey. As expected, traumas and injuries derived from struggling prey are uncommon in the grey fox; in an analysis with 569 specimens, Evenhuis et al. (2018) found only terminal or post-mortem fractures in 56 (9.8%) of the specimens and concluded that skull traumas are most likely to be caused by interspecific aggression or predation and anthropogenic causes.
Besides prey size, another factor that may allow the bush dog to deal with less stress than the wolf during prey subjugation is related to habitat use. As the short legs of the bush dog reduce its speed (Hildebrand, 1954), it is unlike that it hunts in long chases as other hypercarnivorous canids do, relying instead on environmental factors to subdue its prey. This species is usually associated with rivers and watercourses (Barnett et al., 2001; Deutsch, 1983; Linares, 1967; Peres, 1991; Wallace et al., 2002); indeed, it presents interdigital membranes (Beisiegel & Zuercher, 2005) and is considered semi-aquatic by some authors (Langguth, 1975; Nowak, 1999). Contrary to the wolf, whose prey tends to enter the water as a defence mechanism (Mech et al., 2015), some records and anecdotes point out that the bush dog tends to take its prey into water bodies (Strahl et al., 1992; Tate, 1931). Another hypercarnivorous canid, the dhole, has a similar behaviour (Fox, 1984).
The short legs of the bush dog also allow it to hunt in narrow spaces, where the movements of its prey are also limited. It is known by the Matse people of north-eastern Peru that the bush dog actively hunts the long-nosed armadillo (Dasypus kappleri) by entering the armadillo's burrow, capturing it in the tunnels and dragging it to the entrance of the burrow, where it is finally consumed (Fleck & Voss, 2016). Some authors suggest that its interdigital membranes are also helpful while digging (Chavez et al., 2022), and its robust forelimbs are distinct to the slender bones of cursorial Carnivora, being more similar—although not as robust—to those of the Canadian river otter (Lontra canadensis) and European badger (Meles meles), mustelids of aquatic and burrowing habits respectively (Martín-Serra et al., 2014). These behaviour and anatomic adaptations could help the bush dogs since, presumably, prey movements become reduced in the water and in narrow space, and also may be related to the bush dog's cranium being less suited to deal with lateral movements, as seen in the head shaking scenarios (Figure 5). Once in the water or inside a burrow, the bush dog is much more apt to apply its powerful bite (see Section 4.2) in conditions where the prey would be slower and struggling less than if it were on land surface.
In the three tested species, the pulling back scenarios are pointed out as the less stressful of all scenarios. Smaller mean stress in pulling back scenarios was also detected in previous analyses of canids (Slater et al., 2009). Unlike felids, which rely on the combination of bite and forelimbs action to capture their prey (Figueirido et al., 2018; Montuelle & Kane, 2019), canids are usually cursorial predators that do not use their legs in prey capturing, but only their heads (Slater et al., 2009). Hypercarnivorous canids usually hunt in groups using successive bites, subduing and tearing the prey in the process (Creel, 2001; Creel & Creel, 2002; Grassman et al., 2005; Mech et al., 2015). The pulling back scenario is also supported by the common ‘tug-of-war’ behaviour when a hypercarnivorous canid constrains large prey through a bite (usually in the face) while other members of the pack simultaneously pull in opposite directions (Buskirk & Gipson, 1978; Creel, 2001; Grassman et al., 2005; Mech et al., 2015). The act of taking down an animal through the combination of bite and movement of the postcranium during the capture could explain why the pulling back scenarios are less stressful in all tested taxa, but especially in the bush dog and the wolf, which need to apply several bites to subdue its medium to large-sized prey.
Pull-back movements in hunting are extensively documented in the wolf (Mech et al., 2015). In the bush dog, there are observations in captivity and in the wild. Macdonald (1996) describes six captive bush dogs disembowelling a duck by biting and pulling their head back. In central Brazil, Deutsch (1983) recorded photos and described a single bush dog struggling with a paca after dragging it about 200 metres across a pasture; the photo record on this reference clearly shows the pull-back movement.
4.2 Bite force
Due to the many ways in which it can be measured, estimates of bite force from biomechanical models serve more as a comparative tool than as absolute values (Ellis et al., 2008). One of the most used ways to calculate bite force is the dry-skull method developed by Thomason (1991), which reconstructs the adductor musculature to estimate the muscle forces solely using the skull morphology, modelling the jaws as simple levers. It has been applied in analyses of muscular and bite force in many organisms, including canids (Christiansen & Wroe, 2007; Ellis et al., 2008; Nanova et al., 2017; Penrose et al., 2020; Slater et al., 2009; Wroe et al., 2005).
Our analyses show a strong bite for the bush dog when compared to the other tested species (Table 3). In all tested scenarios, the bush dog exerted a bite force of about half the strength of that of the wolf, a canid about seven times heavier (see Section 4.2). This is likely directly related to the skull morphology of the bush dog, as its short rostrum and deep cranium is linked to a strong bite (Penrose et al., 2020; Slater et al., 2009). In other analyses using the dry-skull methods to calculate bite force in both bush dog and wolf, the results were not so extreme (Table 3). A more similar result was obtained by Penrose et al. (2020), who tested the bite force in canids using reduced physiological cross-sectional area (a methodology that considers the muscle mass and angle of pinnation of the fibres to estimate muscular force; Anapol et al., 2008) instead of the dry-skull method and, in near occlusion scenarios, obtained bite force estimates for the bush dog about half of those of the wolf.
| Canine | Carnassial | |||||
|---|---|---|---|---|---|---|
| Speothos (N) | Canis (N) | Proportion (%) | Speothos (N) | Canis (N) | Proportion (%) | |
| Present study | 591.74 | 1210.14 | 48.89 | 1051.94 | 2153.54 | 48.84 |
| Christiansen and Adolfssen (2005) | 170.1 | 743 | 22.89 | 272 | 1262.3 | 21.54 |
| Christiansen and Wroe (2007) | 150 | 493.5 | 30.39 | 233.5 | 773.9 | 30.17 |
| Penrose et al. (2020) | 222 | 508 | 43.7 | 319 | 715 | 44.61 |
- Note: Proportion columns indicate the value of the bite force of the bush dog relative to that of the wolf. The grey fox (Urocyon cinereoargenteus) is not represented here since it was not tested in the referred studies.
The increase in the bite forces during the evolution of Carnivora occurs as an adaptation to two specialised diets: as a specialisation to herbivory (e.g. giant panda Ailuropoda melanoleuca, red panda Ailurus fulgens) and in carnivores that feed on large preys (Christiansen & Wroe, 2007). Hypercarnivorous canids tend to have stronger bites than mesocarnivorous taxa (Slater et al., 2009), as a functional adaptation both for subduing and killing larger prey, as for dismembering and processing it (Christiansen & Wroe, 2007). Our results reinforce this hypothesis since the grey fox not only presented weaker bites (Table 3) as its mandible stressed notably more than the other two species in all tested bites (Figure 8).
In canids, the canines and incisors teeth are used to grab and cut the prey's tissues (Buskirk & Gipson, 1978; Peterson & Ciucci, 2003; Severtsov et al., 2016). In the hypercarnivorous taxa, the canine's robust shape and size allow it to slash the skin and muscles during the bite, promoting laceration and extensive bleeding (Peterson & Ciucci, 2003). The records of paca (Deutsch, 1983) and tapir (Wallace et al., 2002) predation by the bush dog attest to the importance of these teeth to subdue prey in this species, including, presumably, in long-lasting battles, as in wolves. Bilateral bites with canines proved to be slightly stronger than unilateral ones in all tested species, a condition which can guarantee more effective, stable bites during the submission of prey and, allied with high bite forces, are of extreme importance in the capture process, especially for the hypercarnivorous species.
For all tested species, the greatest forces occurred during carnassial bites. In the unilateral scenarios, the canine bite corresponded to about 56% of the force of the carnassial bite in both bush dog and wolf, suggesting a similar importance of these teeth in both species; this relation could be directly linked to the role of the carnassials in the disintegration of the food, including processing skin, muscles and hard tissues as tendons and bones of large prey. In the grey fox, the differences between the bite forces are less pronounced, with the canine bites being around 70% of the carnassial bites. Given the importance of insects and fruits for this species, a less powerful mastication was expected.
The typical traits of the skull and teeth of a hypercarnivorous canid, such as short rostrum, reduced dentition, robustness of the skull elements and teeth and, of course, higher bite forces, are even more marked in the extinct bone-crushing forms (Tseng & Wang, 2010; Wang & Tedford, 2008). Bone elements do not comprise the majority of the biomass found in faeces (Lima et al., 2009; Zuercher et al., 2005) to consider the bush dog a bone-crushing species. However, the high predominance of dermal plates of nine-banded armadillos in the diet of northern Pantanal bush dogs described by Lima et al. (2009) suggests an ability to process hard materials. The carapace of this armadillo species has a complex architecture that can handle forces up to 500 MPa in the banded part of the shell and 1500 MPa in the forward and rear shells (Rhee et al., 2011); the bush dog is not only able to deal with the banded carapace, but seems to prefer armadillos over medium-sized rodents in some locations (Lima et al., 2009).
5 CONCLUSIONS
The in silico tests align with previous assumptions and anecdotes about the bush dog and we could infer hunting behaviours by comparing it with the better-known grey wolf and grey fox. The results indicate that the unusual morphology of the bush dog is also reflected in its biomechanical performance. The bush dog's functional morphology holds many similarities, but also important differences to both the grey wolf—a much larger species with a similar diet and hunting strategies—and the grey fox—a similar-sized species, but with a very distinct, generalist diet. The bush dog's cranium performs more similarly to the grey fox's cranium in lateral head movements, presumably related to the habit of subdue prey constrained in limited spaces. On the other hand, it has a robust skull and teeth that deal much better with stress overall, and generates a relatively strong bite force, being more similar to the grey wolf in these aspects, which supports the conjectures of the bush dog being, as the wolf, a pack hunter. We propose that these results can be explained by the preferred prey size of the different canid species analysed. While wolf prey on larger and potentially dangerous mammals, and grey fox hunt animals usually half its own body weight, the bush dog generally prefers rodents and armadillos that are similar or slightly larger than its own size. This may explain the bush dog stress distribution and magnitude fitting in between the other two species, albeit slightly more similar to the wolf. Our results, when combined with morphological and behavioural characteristics, for example, robustness of forelimbs, webbed digits and preference to live near waterbodies, suggest that bush dogs typically pursue prey in conditions where its movements are limited, like in the water and inside burrows, unlike most of other hypercarnivorous, pack-hunting canids, as the grey and painted wolves, which chase their prey for long distances. Our results also stress the possibility of using FEA as a tool to infer hunting behaviour in species that would otherwise be difficult or even impossible to study in vivo, and provide new insights about the biology of the bush dog. One of the main threats to this species is habitat transformation (DeMatteo & Loiselle, 2008); thus, a better understanding of its hunting strategies (e.g. preferred type of prey, habitat selection) can be useful to propose conservation actions.
AUTHOR CONTRIBUTIONS
Juan V. Ruiz, Felipe C. Montefeltro and Mariela C. de Castro conceived and designed the experiments. Juan V. Ruiz and Felipe C. Montefeltro wrote the paper. All authors analysed the data, and read and commented on the final version of the article and approved it.
ACKNOWLEDGEMENTS
We are grateful to the Digimorph team, especially Jessie Maisano and Dr. Pamela Owen, for kindly providing the CT dataset of the bush dog (available at http://www.digimorph.org/specimens/Speothos_venaticus/) and the grey fox (available at http://www.digimorph.org/specimens/Urocyon_cinereoargenteus/male/) analysed in this study. We also thank Zhijie J. Tseng, Camille Grohé and John J. Flynn for sharing the wolf's data through Morphosource (available at http://n2t.net/ark:/87602/m4/M9038). Fay Penrose kindly supported us with her data. We also appreciate the insights of two anonymous reviewers and the journal's editor, Dr. Philip Cox, whose advises greatly improved our manuscript. This study was partly financed by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001, for JVR. JVR, FCM and MCC are funded by FAPESP (process no. 2020/12786-2) and FAPEG (process no. 202110267000072). JVR, GSF, FCM and MCC are funded by PROBRAL (process no. 88887.628046/2021-00).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




