Complications in right-sided paraaortic lymphadenectomy: ventral tributaries of the inferior vena cava
Abstract
The purpose of this study was to describe the distribution and structure of ventral tributaries leading into the inferior vena cava where right-sided paraaortic lymphadenectomy is performed. The study examined 21 retroperitoneal specimens by graphic reconstruction, statistical evaluation, and histological examination of ventral tributaries (VTs). Seventy VTs were identified. The average number per specimen was 3.33. There were 20, 40, and 40% of VTs found in Levels I, II, and III, respectively. During the preparation, we observed an unusual arrangement of the IVC wall, into which VTs were led through a preformed sleeve-like channel and anchored near the lumen. This finding is a key mechanism that explains the ease with which VTs are extracted during surgery. Knowledge of the distribution and histological structure of VTs allows proper orientation of the retroperitoneal area of the front wall of inferior vena cava, which is essential for uncomplicated right-sided paraaortic lymphadenectomy. The histological structure of the VT ostium within the wall of the inferior vena cava explains why injury is easy during the procedure.
Introduction
Laparoscopic paraaortic lymphadenectomy requires not only detailed topographic orientation in the retroperitoneum but also intimate familiarity with vascular variations and anomalies.
This operation technique was introduced into gynecological-oncological surgery in 1992, when Childers published the first two cases of laparoscopic paraaortic lymphadenectomy in combination with laparoscopic-assisted vaginal hysterectomy due to endometrial cancer (Childers & Surwit, 1992). A year later he published a set of 52 patients treated by laparoscopic paraaortic lymphadenectomy, in which he mentioned one case of inferior vena cava (IVC) bleeding (Childers et al. 1993). Another case of IVC injury in connection with laparoscopic paraaortic lymphadenectomy was described by Nezhat in 1997 – a small opening in the ventral IVC wall appeared during preparation of a precaval lymph nodes aggregate and it was finally closed by clipping (Nezhat et al. 1997). Injury to IVC during right-sided paraaortic lymphadenectomy is most often caused by extraction of minute veins that lead into the wall. These are minor injuries, although blood loss in the opening of the IVC can cause serious complications in terms of orientation in the bleeding operative field. Only Possover et al. (1998) have described the anatomical distribution of minute veins that lead into the IVC. This retrospective study reviewed 112 systematic right-sided pelvic lymphadenectomies based on video recordings of the surgery. No anatomy handbook or encyclopedia offers a comprehensive description of the structure, number, distribution, and topography of minute veins. No edition of the International Anatomical Terminology discusses them, which is why we have accepted their clinical names, such as fellow's veins, Perforansvenen, small perforators, tributary veins, ventral tributaries, and perforating veins (FCAT, 1998).
The aim of this report was to describe the location, topography, and histological structure of these ventral tributaries of the IVC (hereafter referred to as VTs – ventral tributaries) in detail. We started from the premise that the anatomical dissection is a more exact method of minute vessel identification than a laparascopic procedure and that no sexual difference exists in the blood supply of the lumbar (paraaortic) lymph nodes. Based on our results, we propose a new name for these vessels that better reflects their structure and corresponds to the mechanism by which IVC injuries develop.
Materials and methods
Material from the recently deceased adult patients was collected at the Department of Pathology of the Third Faculty of Medicine (Charles University in Prague, Czech Republic) and processed at the Department of Anatomy of the Third Faculty of Medicine (Charles University in Prague), generating 25 retroperitoneal specimens (Caucasian race, 8–92 years, average age 71.7 years; male average age 71.3 and female average age 72.6), for dissection of large veins and their tributaries. Ultimately, 21 specimens were included in the statistical evaluation of the number and distribution of the venous connections [the male : female ratio was 2 : 1 (n = 14/7)]; four blocks of retroperitoneum were excluded due to difficulties in their dissection (insufficient dispersion of ink into the veins or deformation of the specimen in the fixation solution).
Bibliographic search
We performed a literature search using the Medline and Embase databases by title, abstract, keywords, and MeSH. The keywords were: tributaries, fellow veins, small perforators, lymphadenectomy, lymph node dissection, and lymph node excision.
Dissection
After loops and their folds were removed from the small and large intestine, large veins in the retroperitoneum were identified. Between the L1 and L5 vertebrae, with the renal vessels, superior mesenteric artery, and common iliac vessels having been ligated, India ink (diluted 1 : 1 in a physiological solution) was injected via the terminal portion of the inferior mesenteric artery with a glass cannule. Then, a broad strip of retroperitoneal tissue was removed en bloc and fixed in 10% formaldehyde. The specimens were dissected under Zeiss dissection microscope at 3.9× to 10× magnification. Ventral tributaries (VTs) were dissected and examined to their openings into the IVC, left renal vein, and common iliac veins (Fig. 1). All VTs were photographed and registered into a prepared schema, based on the until now only published classification proposed by Possover (Possover et al. 1998), and we applied the same descriptive systematization. We divided the retroperitoneal block into three levels, as follows:
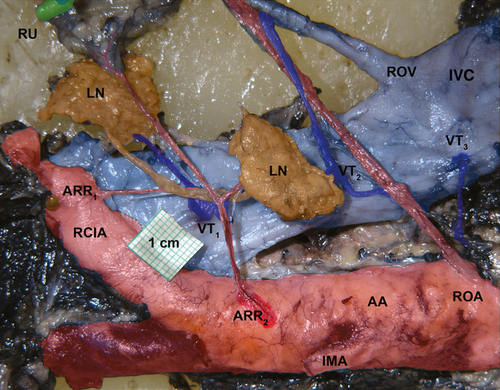
- Level I: between the right common iliac artery, where the right common iliac vein disappears under the common iliac artery, and the aortic bifurcation.
- Level II: between the aortic bifurcation and the origin of the inferior mesenteric artery.
- Level III: between the origin of the inferior mesenteric artery to the ostium of the right ovarian/testicular vein (Fig. 2).
Histological sections were treated by a single pathologist. A sector of the IVC wall with the initial section of the VTs was dissected and examined by haematoxylin-eosin staining by light microscopy.
Each prepared specimen was photographed, and the identified VTs were registered in a grid that was marked by the specimen number. The filled-in VT grids were used to process the results statistically.
Our aim was to recreate the VT topography in relation to the surrounding structures (nerves, veins, and AA or IVC covers). Ultimately, a graphic reconstruction of the histological and dissection findings of VTs that lead into the IVC was made.
In three specimens, a VT was experimentally torn out of the IVC wall, effecting a perforation (simulating the peroperational situation). The diameter of the perforation opening was measured with a digital caliper rule.
Statistical analysis
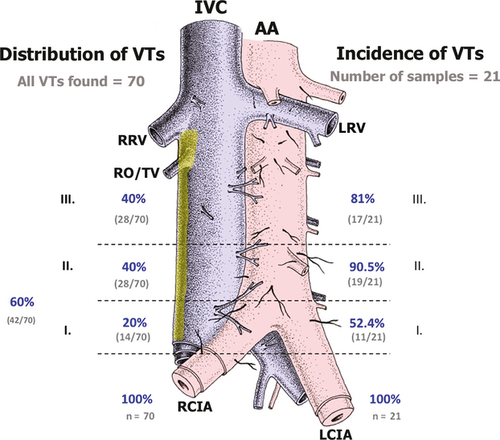
Our aim was to determine the frequency of VTs in the three levels of the retroperitoneum (I–III). The statistical analysis was performed using statistica 9. First, we calculated the basic descriptive statistics (the average, median, mode, minimum and maximum value, 95% reliability interval, dispersion, and standard deviation) and included in Fig. 2 the percentage of VTs in each section relative to the total number of VTs in the IVC. Next, we calculated the incidence of VT in patients within individual sections, in combined sections (I+II, I+III, II+III), and in all three sections. We created incidence tables of the number of VTs and their percentage in each section. Due to a small number of male and female samples, gender analysis was not performed.
Ethical considerations
Institutional review board
The study was approved by the Ethics Commission of the Third Faculty of Medicine, Charles University in Prague, Czech Republic.
Results
We found only one scientific work that has examined the number and influence of VTs in laparoscopic surgeries (Possover et al. 1998). We failed to identify any anatomical studies that have focused on these venous tributaries to the IVC using material from fixed or recently deceased patients. No updated findings have been reported in ancient, classical or modern anatomy encyclopedias (Williams, 1995).
Twenty-one specimens were chosen for statistical processing. The male : female ratio was 2 : 1 (n = 14/7). The age span was 38–92 years, with an average age of 71.7 years. In all, 73 ventral tributaries were extracted and identified. Seventy VTs were found within Level I–III, but three VTs were situated above Level III, in the area of the renal arteries origin and these were therefore excluded from the statistical analysis. The average number of VTs per specimen was 3.33. The maximum number of VTs in a single patient was eight and the minimum was one.
Twenty percent of all VTs were observed in Level I (n = 14/70) vs. 40% in Level II (n = 28/70), and 40% in Level III (n = 28/70) (Fig. 2). Ventral tributaries were also common in the ventral wall of the common iliac vein, located in the same level (Level I) in the caudal part of the IVC, immediately before its origin at the confluence of both common pelvic veins. Other VTs were also observed above the ostium of the right ovarian/testicular vein at the level of the renal veins ostia.
The ventral tributaries led into the IVC primarily on the medial half, facing the abdominal aorta. Few VTs were observed on the medial half of the IVC and none on the lateral (right) side of the IVC. No VTs were found in any sample (Fig. 3). Before opening into the IVC, VTs often branched dichotomously (Fig. 3).
Three specimens were also treated to simulate a peroperational situation, in which a VT was experimentally torn out of the IVC wall, effecting a perforation 4 mm in diameter. The produced perforation was an oval opening, preformed by the sleeve-like arrangement of the TV course within the wall of the IVC and no laceration of the IVC wall occurred (Fig. 3).
Histological examination
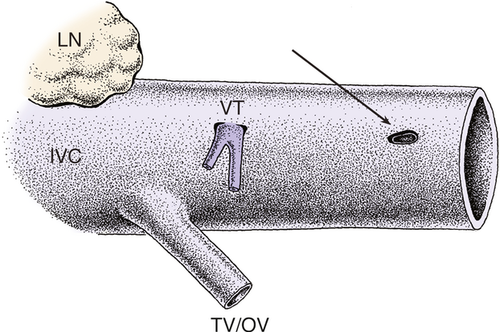
During the preparation of a venous bloodstream, an unusual arrangement of the IVC wall was observed, into which VTs were led through a preformed sleeve-like channel and in which VTs were anchored near its lumen (Fig. 4).
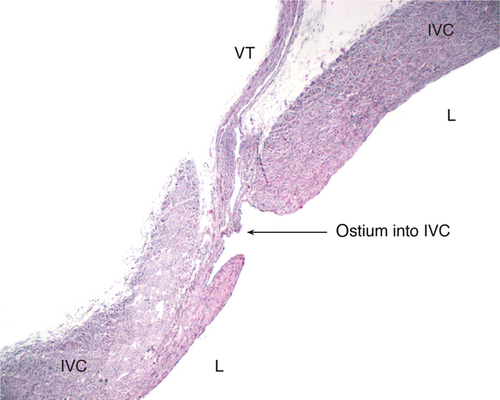
This architecture of venous entry is unique compared with the entries of other veins (e.g. ovarian/testicular veins and lumbar veins). To verify the preparational findings, IVC specimens with VTs were extracted. Based on the dissections, a graphic reconstruction of the sleeve-like foramen of VTs into the IVC was generated (Fig. 5).
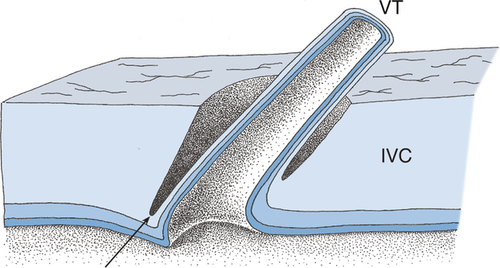
Discussion
This report is to our knowledge the first anatomically based study of the minute veins that lead into the ventral wall of the IVC, providing not only a detailed description of the location and number of these branches but also a histological snapshot of the IVC wall foramen. These findings can be used to address the following issues.
Hemorrhage in the IVC area during paraaortic lymphadenectomy can be caused by direct injury to the IVC wall, such as the rupture or extraction of a VT from the IVC wall. The anchoring of the VT near the IVC intima is the locus minoris resistentiae, which explains the ease with which a VT is extracted from the IVC wall. Based on our clinical experience, VTs are extracted most frequently from the caudal part of the IVC, above the right common iliac artery.
The sleeve-like course of minute veins through the IVC wall represents an entirely different arrangement of the termination of a smaller vein in a larger one, unlike the nearby lumbar veins or ovarian/testicular veins. VTs are led through the thick adventitia (containing both collagen fibers and smooth muscle cells) and media, entering along the fibers of these two layers. In this model, the IVC wall accommodates the hydrostatic pressure in the IVC below the heart by the muscle/ligament ratio. The sleeve-like arrangement of minute tributaries thus protects their thin lumina from compression.
Berek stated in his ‘Practical gynecologic oncology’ that Possover's work is both a significant contribution to the clinical anatomy of IVC and a useful manual for young laparoscopists (Berek & Hacker, 2004).
The differences between our results and Possover's study lie primarily in the imprecise delineation of individual levels in live vs. fixed material. Possover worked with women only, whereas in our study, both genders were represented. Although our study dealt with a relatively small number of specimens (due to the time-consuming dissection, which took approximately 8–10 h of work per specimen), we assume that there are no gender-based influences on the number of VTs. In our study, the male/female ratio was 2 : 1 and the only little difference was found in the average number of VTs per specimen (male/female 3.43 : 3.14).
The distribution of VTs in our material was relatively equal. Level III harboured 40% of VTs, and Levels I and II 60%. Possover observed only 0.4% of VTs in Level III. Three VTs were observed above the level of the ostium of the right ovarian vein, but no VT was recorded between the level of the ostium of the right testicular/ovarian vein and caudal margin of the ostium of the renal veins (identical to Possover). VTs were found in all three levels in one-third of specimens, unlike Possover, who detected none. No specimen lacked a VT, unlike Possover, who documented 21.43% patients with no VTs (Possover et al. 1998). Results of both studies are summarized in Table 1. Unfortunately, the studies are each defined rather differently and not all their data can be compared statistically. There is no hint that the gender bias should be relevant in the branching of the abdominal aorta and inferior vena cava as no such information has been found in the literature concerning arterial and venous variations. As for the selection bias – there is no hint that female patients with tumours treated by the paraaortic lymphadenectomy should possess different anatomy than any random selection of patients. And finally, related to the age bias, although not stated by Possover, there is no suggestion that an older patient should possess more or fewer branches from the abdominal aorta. Patients with severe atherosclerosis were not included in the study.
| Possover (1996) | Turyna (2013) | |||
|---|---|---|---|---|
| Number of patients/samples | 100% | 112 | 100% | 21 |
| Level I | 58% | 65/112 | 52.4% | 11/21 |
| Level II | 19% | 22/112 | 90.5% | 19/21 |
| Level III | 0.9% | 1/112 | 81% | 17/21 |
| No VT | 21.4% | 24/112 | 0% | 0/21 |
| Total number of VTs | 100% | 237 | 100% | 70 |
| Level I | 82.3% | 195/237 | 20% | 14/70 |
| Level II | 17.3% | 41/237 | 40% | 28/70 |
| Level III | 0.4% | 1/237 | 40% | 28/70 |
| Number of VTs per patient per sample | 2.11 | 237/112 | 3.33 | 70/21 |
Ventral tributaries are minute veins in the retroperitoneum, whose foramina were observed not only on the ventral wall of the IVC but also on renal veins and common iliac veins. These veins drain the retroperitoneal lymph nodes and the scarce connective tissue around the IVC and AA, and receive their venae vasorum, conducting blood away from both ureters and the neural abdominal aortic plexus. They form a broad venous network of mutual anastomoses and collaterals.
The ventral tributaries are mostly on the medial half, facing the abdominal aorta. Conversely, the lateral (right) side of the IVC, where no VTs were found, could be a safe zone for the surgery (see yellow area in Fig. 2).
Traction of the VT perpendicular to the IVC wall can probably result in its mechanical extraction, based on its histological arrangement. During the surgery, the VT should be actively sought for, visualized, dissected and coagulated.
The course of these veins does not follow or correspond in number to the minute arteries that run from the AA. Due to the variability in their number and location, these arteries are not discussed in International Anatomical Terminology (FCAT, 1998). Generally, they form branches that service the retroperitoneal structures (retroperitoneal branches). Their number and distribution have been described by Sasaki (1990), but he fails to assign them a name, calling them ‘filamentous arteries’ or ‘unnamed arteries’. Borovanský (1927) called them ‘tiny arteries, ramifying in larger amount from aorta and common iliac artery, and heading to the ureter’ and Clarke (1965) called them ‘long sinuous arterioles’. We propose the descriptive term ‘anterior retroperitoneal rami (ARR)’.
The venous drainage of the retroperitoneum has not been described. One can assume, however, that the venous counterpart of the so-called retroperitoneal arterial plexus of Turner lies underneath the peritoneum and stretches from the pelvis via the lumbar area toward the diaphragm. It forms a fine web of thin, mutual anastomoses that communicate with renal and suprarenal arteries, as well as with pancreatic, superior mesenteric, and splenic arteries. In the pelvis, Turner's plexus is located in the mesorectum, anastomosing with arteries of the rectum. In women, it forms anastomoses in the broad ligament with uterine, ovarian, and vesical arteries. Branches toward lymphatic nodes near the large arteries and their vasa vasorum also constitute part of Turner's plexus (Turner, 1863).
Due to the lack of uniformity concerning the moniker for VTs in the literature, and due to the typical anatomical position and distribution of VTs within retroperitoneum, we propose that these veins should be called venae retroperitoneales anteriores, or ‘anterior retroperitoneal veins’ (Kachlik et al. 2008).
Concluding remarks
Knowledge of the distribution and histological structure of VTs enables proper orientation within the retroperitoneal area of the anterior wall of inferior vena cava, which is essential for uncomplicated right-sided paraaortic lymphadenectomy. Proper anatomical terminology also helps to assign correct descriptions when exploring certain structures.
Author contributions
R.T.: Personal experience with the procedure (lymphadenectomy), substantial contributions to conception and design, preparation of the specimens, acquisition of data, or analysis and interpretation of data.
D.K.: Substantial contributions to conception and design, performance of the injections, corrosion casting, acquisition of data, or analysis and interpretation of data. Substantial contributions to conception and design, performance of the injections, corrosion casting, acquisition of data, or analysis and interpretation of data.
E.K.: Preparation of the specimens, statistical analysis.
P.K.: Histological processing of specimens.
J.F.: Senior author in gynaecology, clinical experience with lymphadenectomy and confirmation of the results obtained, critical draft and revision of the article.
V.B.: Senior author in anatomy, giving final approval of the version to be published.
Acknowledgements
Supported by the Charles University Grant Agency, Project no. 126107 and by the Charles University Prvouk, Project no. 33.