Repigmentation of cutaneous scars depends on original wound type
Abstract
Cutaneous scarring is currently an inevitable outcome following skin injury. Abnormal pigmentation within scars makes them more noticeable, causing distress for patients, particularly as there is no reliable and effective treatment available to date. The Duroc pig, known to scar badly, was used to investigate repigmentation of scars resulting from three different wound types: incisional, partial thickness excisional and full thickness excisional. Wounds were created on the backs of Duroc pigs and the resulting scars harvested at days 35, 56, 70 and 90 days post-injury. Scars were processed for histology and immunohistochemistry, quantitatively analysed using image analysis software and subjected to statistical analysis. Photographs of the macroscopic appearance of scars were scored for pigmentation using a visual analogue scale. Results demonstrated temporal and spatial differences in melanocyte repopulation and function within scars from different wound types. The microscopic pigment deposition did not correlate with macroscopic appearances in mature scars. Pigmentation of scars is dependent on the width and depth of wounds. This study has provided important information on which we can base future studies to investigate factors controlling the repigmentation of scars.
Introduction
Abnormally pigmented scars have long been a problem for clinicians and patients alike (Morgan et al. 1975). Despite extensive research, the mechanisms underlying this common problem remain largely unknown and the phenomenon remains difficult to treat (Matsumoto et al. 1996). Cutaneous injury is common, with approximately 175 000 accident and emergency attendances annually in the UK for burn injuries alone (Enoch et al. 2009) and millions more affected by accidental injury and surgical interventions. Another huge economic and clinical burden is chronic wound healing, often complicated by systemic disease such as diabetes mellitus, cardiovascular disease and the ageing process. These wounds often heal with hyperpigmented scars.
Dark (hyperpigmented) or pale (hypopigmented) scars can be highly distressing for patients, even leading to social isolation for some (Zeitlin, 1997). Dyspigmentation makes an otherwise flat, matt and supple scar more visible and distressing for the patient. Aesthetic concerns are even more important to individuals when the affected areas are easily visible, such as on the hands or face (Singer et al. 2000). Furthermore, lack of pigmentation on exposed body surface areas can theoretically cause problems of photoprotection for the patient.
Skin pigmentation plays an important role in many aspects of life; besides the protective function that melanin pigment provides against harmful UV radiation (Eller et al. 1996), the different shades of skin pigmentation between ethnic groups afford it a social and cultural role, too. Fashions also dictate trends in skin colour, making skin tanning and bleaching extremely popular despite the well documented associated health risks (Banerjee et al. 2009).
In human skin, carotene and haemoglobin contribute to pigmentation but the main determinant of skin and hair colour is the pigmented biopolymer, melanin, which is produced within lysosome-related organelles called melanosomes within melanocytes (Lin & Fisher, 2007). Melanocytes are dendritic, neural-crest derived cells which, in skin, reside in the basal layer of the epidermis and distribute formed melanin pigment to neighbouring keratinocytes. Keratinocytes receive and distribute pigment to the upper layers of the epidermis (Yamaguchi & Hearing, 2009). The combination of melanocyte and recipient keratinocytes is commonly known as the ‘epidermal-melanin unit’ (Fitzpatrick & Breathnach, 1963).
Within keratinocytes, melanin sits as a ‘cap’ on the sun-bearing side of the keratinocyte nucleus, acting as a direct barrier to UV radiation (Costin & Hearing, 2007). In addition, melanin acts as a powerful antioxidant, scavenging free radical oxygen species before itself being degraded (An et al. 2009; Randhawa et al. 2009). There are two main types of melanin pigment found in varying ratios in human skin: eumelanin and phaeomelanin. Both are formed by melanogenesis, a process dependent on the amino acid tyrosine being hydroxylated and oxidised by tyrosinase to produce DOPAquinone. An individual's skin colour is dependent on the ratio of the two melanin types, with fairer skinned individuals possessing higher levels of phaeomelanin (Simon et al. 2009).
Previous investigations of pigmentation and wound healing have resulted in differing conclusions (Pepper, 1954; Breathnach, 1960; Snell, 1963). Snell & Pepper demonstrated melanocytes migrating across a healing wound just 1–2 mm behind the advancing edge of epithelial cells, both in superficial and deep wounds, in guinea pigs. By contrast, Breathnach could not identify melanocytes in the centre of mid-dermal human scars, proposing instead that melanocytes migrated into a pre-formed sheet of epithelium after the wound had healed. In more recent years, other models such as zebrafish have been used in wound healing research, which has yielded further information about potential roles of melanocytes, other than as pigment-producing cells (Plonka et al. 2009; Lévesque et al. 2012). In particular, these studies have highlighted the potential immune-modulatory function of melanocytes, provoking an inflammatory response in the very early stages of wound healing, and provided further information about melanocyte migration and patterning within skin (Takahashi & Kondo, 2008; Feng et al. 2010; Lévesque et al. 2012). Although most dyspigmentation states may be attributed to problems with melanogenesis, this is not always the case. This is especially true in lipodermatosclerotic lesions, associated with chronic venous insufficiency, in which the hyperpigmentation is due to excess haemosiderin and iron deposition rather than an excess of melanin pigment (Caggiati et al. 2010).
In this porcine study we compared the repigmentation patterns of scars resulting from three different wound types in Duroc pigs: an incisional (surgical) wound (IC), a partial thickness excision (split skin graft donor site) (PTE) and a full thickness excisional (FTE) wound. The Duroc pig, known to scar badly, has a homogeneous skin colour which can vary in shade from red-brown to brown-black between animals but is consistent across the skin of individual animals, making it ideal for this study (Gallant et al. 2004). Pigs provide an excellent model for human skin, possessing high similarity with humans in terms of anatomy, physiology, wound healing response and chromosomal structural homology (Sullivan et al. 2001; Steibel et al. 2009).
Materials and methods
Animal work
All housing and wounding procedures were carried out in accordance with Home Office regulations and approval. Animals were acclimatised for a minimum of 2 weeks prior to entry into the study and housed individually for 1 week before wounding and thereafter. Animals were fed on a standard, antibiotic-free diet (Oakes, Congleton, UK) twice daily and had access to water ad libitum.
Aseptic technique was used throughout all procedures. Animals were fasted overnight prior to general anaesthesia. Four female Duroc pigs were anaesthestised using 2–4% inhalational isoflurane, nitrous oxide and supplemental oxygen via a face mask. The intended wounding sites were shaved and skin cleaned with betadine and aqueous chlorhexidine. Intended wounding sites were marked on the animal using a permanent marker according to a set template. The 2-cm incisional (IC) wounds were made with a number 11 scalpel blade down to, but not including, the subcutaneous fat. Full thickness excisional (FTE) wounds measuring 2.5 cm2 were made using a number 11 scalpel blade; the full thickness of the skin was excised. Partial thickness (PTE) wounds were created using a hand-held motorised dermatome with a 25-mm blade, at a setting of 0.4 mm (Nouvag). Wounds were created in a single row on both flanks of each animal to offer intra-animal replication. Haemostasis was achieved by light manual pressure with sterile gauze. Intramuscular buprenorphine (0.3 mg) was administered at the end of the procedure for postoperative analgesia. Skin wounds were covered with a Release™ (Johnson & Johnson) non-adherent, absorbent dressing and secured with Bioclusive™ (Johnson & Johnson) adhesive dressing and Surgifix™ stocking (Colorline). Dressings were changed at regular intervals and continued until all wounds had re-epithelialised. Wounds healed by secondary intention without any intervention (other than dressing changes) and no wounds became infected.
Scar harvest
Scars were harvested on days 35, 56, 70 and 90 post-wounding. At the designated time point, animals were anaesthetised with inhalational isoflurane and scars excised with a scalpel down to fascia and with a 5-mm rim of surrounding unwounded skin. All samples were snap-frozen, embedded in optimal cutting temperature compound (OCT) and stored at −80 °C until further use. Full thickness normal skin samples from the same animal were harvested at each time point for comparison. Skin removed at day 0 to create the wounds was also similarly preserved and retained for analysis.
Cryosectioning
Frozen samples were step-sectioned at a thickness of 5 μm (Cryocut 1800, Leica). Sections were cut onto (3-aminopropyl) triethoxysilane (APES)-coated slides, acetone fixed for 10 min, air dried and stored in a sealed plastic box at −20 °C until use. Sections for DOPA oxidase reaction were unfixed and used immediately.
The temporal and spatial distribution of melanocytes within the different scars was studied using immunohistochemistry with the primary antibody HMB45, a monoclonal antibody that detects immature melanosomes in early stage melanocytes and re-activated adult melanocytes (e.g. those within or adjacent to a surgical scar) (ab787, Abcam) (Smoller et al. 1989, 1991). Melanogenesis and activity of melanocytes was studied by immunodetection of tyrosinase-related protein 1 (TRP1), an essential enzyme in the melanogenesis pathway (TA99, Abcam) and by the DOPA oxidase stain, which detects the oxidation of DOPA into an insoluble brown-black pigment by tyrosinase-producing melanocytes. Melanin pigment was detected using Warkel–Luna Helwig stain, a modified silver nitrate stain.
DOPA oxidase staining
Unfixed, frozen sections were allowed to reach room temperature prior to incubation in 4% formaldehyde fixative at 4 °C for 1 h. After incubation, slides were washed in cold 0.2 m sodium cacodylate buffer pH 7.4 prior to being incubated in either 0.2% DOPA solution or warmed 0.2 m sodium cacodylate buffer for 4 h at 37 °C (control). Following incubation, slides were rinsed in two changes of deionised water for 5 min each, counterstained with nuclear fast red, rinsed quickly in deionised water and dehydrated through graded alcohols, and then two changes of xylene. Slides were mounted with Pertex and coverslipped.
Warkel–Luna Helwig stain for melanin
Frozen sections were allowed to reach room temperature, rinsed briefly in deionised water and incubated in 1% silver nitrate solution for 30 min at 43 °C. Slides were flooded with developer solution (2% silver nitrate, 1% gelatin and 0.15% hydroquinone) and incubated, protected from light, in a humid staining trough for 8 min at room temperature. The developer was rinsed off with hot running tap water prior to a second rinse in deionised water. Sections were counterstained with nuclear fast red, rinsed in tap water, dehydrated through graded alcohols and two changes of xylene, before being mounted with Pertex and coverslipped.
Immunohistochemistry (TRP1 and HMB45)
Sections were allowed to reach room temperature, rehydrated in phosphate-buffered saline (PBS) (15 min) and placed in 0.3% hydrogen peroxide and methanol for 30 min to block endogenous peroxidases. Sections were rinsed in two changes of PBS-TritonX100 (15 min each) before all sections were blocked with 1.5% donkey serum in PBS containing 0.1% bovine serum albumin for 30 min. The serum was removed and the primary antibody applied (TRP1 1/250, HMB45 1/20 dilutions). A control section received further blocking serum instead of the primary antibody. Following incubation overnight at 4 °C, sections were rinsed twice in PBS-TritonX100 (15 min each) and the secondary biotinylated antibody applied (biotinylated donkey anti-mouse 1/200 dilution, Jackson Laboratories) for 30 min incubation at room temperature. Sections were rinsed for 15 min in PBS-TritonX100 and then plain PBS, prior to incubation for 30 min in Vectastain ABC reagent (made following the manufacturer's instructions). Two further rinses in PBS and Tris-buffered saline (TBS) (15 min each) were done prior to application of Vectastain NovaRed. The reaction was stopped by dipping sections in deionised water. Sections were counterstained in Harris haematoxylin, rinsed in running tap water for 5 min, dehydrated through graded alcohols and two changes of xylene, mounted with Pertex and coverslipped.
Image capture and analysis
Stained slides were quantitatively analysed using Leica qwin software. Six stepped sections were analysed from each wound for each of the analyses. Each section was image captured along its length in 650-μm fields and within each field, data were collected for number of positively stained cells or area of melanin deposition (Supporting Information Fig. S1). For DOPA oxidase and immunohistochemistry, cell counts were measured. For pigment assessment, the area of the epidermis that was black (i.e. stained with WLH stain) was measured along with the entire area of the epidermis. The area of pigmentation was then expressed as a percentage of the whole area of the epidermis. Data were exported and subjected to statistical analyses.
Macroscopic re-pigmentation of scars
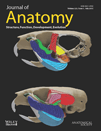
Re-pigmentation of scars was macroscopically assessed from standardised digital photographic images using a visual analogue scar (VAS) scoring system (Duncan et al. 2006). Scars were divided into three zones (within Fig. 1) to facilitate assessment of each scar's centre and periphery. Each zone was awarded a score by drawing a perpendicular line along a 20 cm scoring bar, on which −10 denotes complete hypopigmentation, 0 is ‘normal’ and +10 denotes hyperpigmentation. This point was then measured to give the VAS score. The data were collated in an excel file and statistically analysed. As full thickness scars matured, they underwent some contracture but were still scored in three zones. Intra-rater validation was performed and after confirmation of consistency, the images were coded and scored blindly.
Statistical analysis
Data were analysed by SPSS using the non-parametric Kruskal–Wallis test for comparisons between time points (unrelated data) and the Wilcoxon test for comparisons within the same animal (related data). A P value of < 0.05 was considered statistically significant.
Results
To summarise the methodology, two each of incisional, partial thickness excisional and full thickness excisional wounds were made on both flanks of four female Duroc pigs (total 12 wounds per animal). Prior to harvesting the resultant scars at varying time points post-injury, scars were photographed and macroscopically assessed using a VAS scale for pigmentation. Melanocytes were detected immunohistochemically using HMB45 antibody; active melanocytes were detected using the DOPA oxidase reaction and TRP1 immunohistochemistry. Melanin pigment was detected using WLH stain. Normal skin samples from day 0 (e.g. skin removed to create wounds) and at harvest time points were also collected and processed similarly. Stepped cryosectioned sections from the processed scars and normal skin were analysed across their width in 650-μm fields to allow data to be collected and compared across the scars, at the scar edge and scar centre.
Is there a macroscopic difference in the repig-mentation of scars resulting from different wound types?
Prior to harvest, scars were assessed every 4 days from day of wounding until day of harvest. Observations were made at these time points regarding re-epithelialisation, vascularity and pigmentation. However, as many scars were obscured by keratin scabs until approximately day 21–28, the scar itself was not well visualised until day 35.
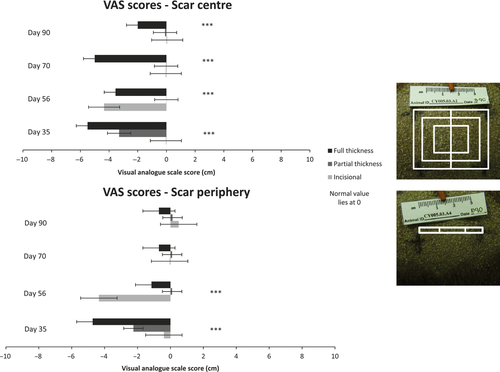
Partial thickness scars were macroscopically repigmented at day 35 post-wounding and by day 90 were completely homogeneous with the surrounding skin (Fig. 2) Incisional scars were difficult to distinguish from surrounding skin at day 35, predominantly due to their fine linear appearance. However, there was a fine pale line at the site of the scar which persisted until the final time point studied (Fig. 2).
At day 35 full thickness scars were hypopigmented centrally. Over time the central hypopigmented area decreased in size, and the scar periphery became less pale, but the scars were still hypopigmented at 90 days post-wounding, making them distinct from the surrounding skin (Fig. 2).
To semi-quantitate the macroscopic pigmentation within scars, scars were scored in three areas (Fig. 1). Visual analogue scores of pigmentation (VAS) (Fig. 1) demonstrated significant differences in macroscopic pigmentation both within scars (i.e. scar centre compared with scar periphery) and between scars resulting from different wound types. These differences were particularly noticeable within full thickness scars (P < 0.001) and between full and partial thickness scar centres at each time point (P < 0.05).
Does the spatial distribution of melanocytes within scars differ between scar types over time?
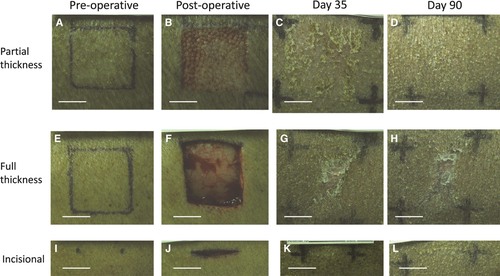
In all three scar types, the epidermal layer had reformed over the entire scar surface when examined microscopically at day 35 post-wounding (Fig. 2). Melanocytes detected by HMB45 positivity were distributed amongst basal keratinocytes, throughout the partial thickness and incisional scars, at all time points examined (Fig. 3). By contrast, melanocytes were only present at scar margins of the full thickness excisional scars at day 35 post-wounding and absent from the scars' centres (Figs 4 and 5). However, the length of neo-epidermis of full thickness scars that repopulated with melanocytes increased with time, and by day 90 the whole scar was repopulated with melanocytes along its width (Fig. 4).
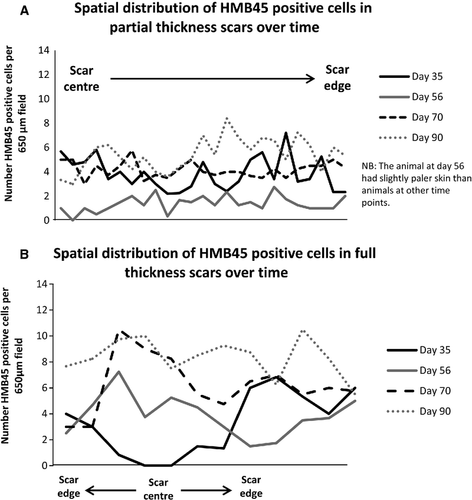
Does the activity of melanocytes differ between scar types?
At day 35, melanocytes positive for DOPA oxidase activity and TRP1 were seen across the incisional and partial thickness scars, indicating melanocytes were present and enzymatically active within the neo-epidermis of the scar (Figs 3 and 5). By contrast, in full thickness scars, no DOPA oxidase or TRP1-positive melanocytes were present in the centre of these scars at day 35 post-wounding, in keeping with the absence of HMB45 positive cells (Figs 3 and 5).
Interestingly, the DOPA oxidase staining in the centre of both full thickness and partial thickness excisional scars increased with time. Within full thickness excisional scars, DOPA oxidase staining was higher than in normal skin at later time points (Fig. 3).
Does macroscopic scar appearance relate to micro-scopic melanin deposition?
Over time, the percentage area of the epidermis with melanin deposition essentially increased within partial thickness and full thickness scars, but followed different patterns of repigmentation (Fig. 6). In partial thickness scars a fine layer of melanin pigment was present throughout the entire basal neo-epidermis at day 35 post-wounding; over time, this area of distribution of pigment increased throughout the scars (Fig. 3). By contrast, at day 35 post-wounding, melanin pigment was absent from the centre of full thickness scars and pigment gradually crept in from the periphery to the centre of the scar over time (Fig. 3). Interestingly, despite the fine layer of the melanin pigment present throughout the incisional scar the macroscopic appearance of a fine hypopigmented linear scar persisted (Fig. 2).
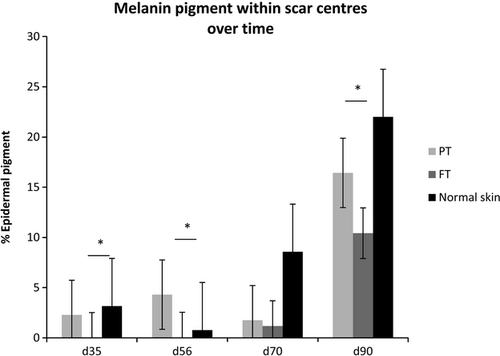
The relationship between macroscopic appearance of full thickness scars and melanin pigment deposition appeared to change with time. At day 35, the scars were centrally devoid of melanin pigment and this was in keeping with their hypopigmented macroscopic appearance (Figs 2 and 3). However, at day 90 post-wounding, the full thickness scars had a dense layer of melanin pigment within the rete ridges of the scar epidermis, which was not reflected in their macroscopic appearance where they appeared centrally hypopigmented. Microscopically, in all scar types, the percentage area of epidermis containing melanin pigment remained less than that of surrounding normal skin (Fig. 6); despite this, partial thickness scars were practically indistinguishable from the normal skin after day 56 post-wounding.
Discussion
Historically, clinicians have recognised pattern differences in scar repigmentation between different types of wounds. Morgan et al. (1975) reported differences in pigmentation between full and split thickness grafts, noting in particular the tendency for split thickness grafts to hyperpigment and for these changes to be more noticeable in individuals with deeply pigmented skin. Successful repigmentation of a scar requires migration of functioning melanocytes into scar tissue, production of melanin within melanosomes and transfer of melanin to neighbouring keratinocytes. A failing at any point in this process will lead to an abnormally pigmented scar. Our study has sought to investigate differences in repigmentation of scars resulting from donor sites of split and full thickness skin grafts, as well as incisional wounds, using the Duroc pig model.
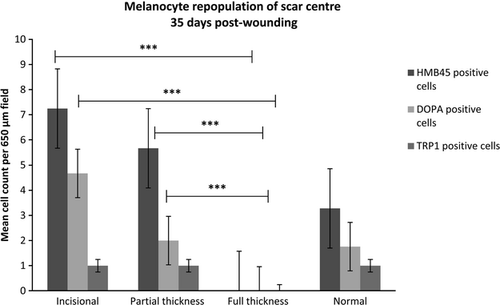
At the first time point in our study (day 35 post-wounding), all wounds were completely re-epithelialised. Incisional and partial thickness excisional scars had melanocytes distributed throughout their neo-epidermis but, by contrast, melanocytes were only present at the scar margins of full thickness excisional scars. Over time, however, the length of neo-epidermis repopulated by melanocytes in full thickness excisional scars increased, such that by day 56 the entire full thickness scar was repopulated along its length and width with melanocytes (Fig. 4).
There are two potential sources from which melanocytes can be recruited to repopulate scars. Melanocytes from surrounding unwounded skin can migrate into the wound at the wound edges, and adnexal elements such as hair follicles and sebaceous glands in the wound bed can also provide a rich source of melanocytes (Tobin, 2011). The differences in repopulation patterns of incisional, partial thickness and full thickness excisional scars suggests a relationship with the depth and width of the original wounds, as this will influence potential sources of melanocytes for scar repopulation. During the wounding process, adnexal elements were preserved in the partial thickness and incisional scars. By contrast, to create full thickness excisional scars, the entire epidermis and dermis were removed, leaving no adnexal elements in the wound bed. This suggests that in partial thickness excisional scars, intrafollicular melanocytes from residual hair follicles in the wound centre spread centrifugally towards the wound periphery, whereas interfollicular melanocytes spread centripetally from the wound edges. By contrast, in full thickness scars, only melanocytes from the wound edges contribute to the repopulation of the scars. In the incisional wound, the melanocytes may well have repopulated from the surrounding wound edge, but due to the narrow width of the neoepidermis of this scar type, by day 35 melanocytes were already present throughout the scar.
Melanocyte precursor cells/melanocyte stem cells are another alternative source of melanocytes for scar repopulation. These melanocytes, originating from the bulge region of the hair follicle, contain little or no pigment and travel with the advancing neoepithelium, only becoming visible when they undergo melanogenesis or the amount of pigment they produce reaches a detectable level (Staricco & Pinkus, 1957). We observed a delay in repigmentation which may be explained by this phenomenon and this may also explain why scars at days 70 and 90 post-wounding possessed higher than normal numbers of melanocytes but lower levels of melanin pigment. Recent literature has also reported the presence of dermally derived stem cells, capable of migrating into the basal epidermis, where they are able to differentiate into melanocytes with the ability to produce pigment (Zabierowski et al. 2011). Again, the time taken for cells arising from the dermis to migrate to the basal epidermis and differentiate into functional melanocytes may explain the delay in scar repigmentation. Our current study does not address the dilemma of whether melanocytes migrate alongside keratinocytes (Pepper, 1954; Snell, 1963) or after the wound has completely re-epithelialised (Breathnach, 1960). However, findings from re-population of full-thickness scars with melanocytes would favour the latter.
Active melanocytes and pigment were present throughout the incisional and partial thickness scars at 35 days post-wounding, but were absent from the scar centre in full thickness scars at that time (Fig. 3). However, from day 56 post-wounding, active melanocytes and melanin pigment were seen at increasing proximity to the centre of the full thickness scar, so by day 90 active melanocytes and a dense layer of melanin pigment were seen throughout the length of the full thickness scar (Fig. 3).
It is interesting to note that at day 90 post-wounding, full thickness scars had significantly more active melanocytes than all other scars and normal skin (Fig. 3). However, the percentage area of the epidermis with melanin deposition within full thickness scars did not reach that of the normal skin (Fig. 6) and the full thickness scars macroscopically appeared hypopigmented centrally (Figs 1 and 2). It was also interesting to note that the pigment in the neo-epidermis of the scars was confined mainly to the basal layers, whereas in the epidermis of normal skin, pigment was distributed throughout all layers of the epidermis, which could perhaps explain the variance in appearance of scars (Fig. 3).
At day 90 post-wounding, partial thickness and full thickness scars had similar percentage areas of epidermis containing melanin (Fig. 6) despite their very different macroscopic appearances (Fig. 2). These differences could perhaps be explained by the density of melanin: in partial thickness scars melanin appeared as a fine dusting compared with the compact dense melanin deposition seen in full thickness scars (Fig. 3). Furthermore, the thick hyperkeratotic layer found in full thickness scars could also contribute to the difficulty in visualising the dense pigment, potentially making the scars macroscopically paler.
The relationship between inflammation and skin pigmentation has been investigated previously but is still not fully understood (Ortonne & Bissett, 2008). It is possible that ongoing inflammation in scars has an adverse effect on successful repigmentation, either inhibiting melanocyte migration or leading to hyperpigmentation, as seen in post-inflammatory states (Nordlund & Abdel-Malek, 1988; Ortonne & Bissett, 2008).
An important consideration when interpreting these results is the unavoidable inter-animal variation in skin colour; in this study the animal whose scars were harvested at day 56 post-wounding had red-brown skin, in contrast to the other three animals, who had brown-black skin. Despite this, the paler-skinned animal's scars repigmented in the same pattern as the darker-skinned animals, indicating the reliability of the scar model. The normal skin analyses for each animal at the harvest time points also allowed normalisation for each animal at each time point.
In summary, our study has demonstrated differences in the temporal and spatial patterns of melanocyte repopulation and repigmentation of scars dependent on the width and depth of the original wounds.
Concluding remarks
The mechanisms underpinning abnormal pigmentation are an area for further work, but this study provides information about melanocyte repopulation of scars resulting from different wound types, the activity of melanocytes within those scars, and the pattern of melanin distribution between scars resulting from different wound types.
Authors' contributions
Sarah L. Chadwick is the primary author of this article and conducted all laboratory work and results analysis. Christina Yip conducted the animal work within this study, from wound creation to scar harvest. Yip reviewed the article prior to submission. Mark W. J. Ferguson supervised both Chadwick and Yip for the duration of this work and edited the manuscript prior to submission. Mamta Shah supervised both Chadwick and Yip for the duration of this work. Shah also edited the manuscript prior to submission.
Acknowledgements
Mrs Alison Thomlinson, Mr John Denton, Mr Brian Landamore.
Conflict of interest
None.