Serum beta-secretase 1 (BACE1) activity increases in patients with mild cognitive impairment
Abstract
Beta-secretase 1 (BACE1) is considered as the key enzyme in amyloid-β formation. Previous works suggest that high BACE1 activity may be present in brain, cerebrospinal fluid and serum of patients with late-onset Alzheimer's disease (LOAD) as well as mild cognitive impairment (MCI). Therefore, we evaluated whether serum BACE1 activity increases in MCI patients and is associated with the progression from MCI to dementia. BACE1 activity was measured in the serum of 259 MCI patients (162 amnestic—aMCI, 97 non-amnestic—naMCI) and 204 healthy Controls. After a median follow-up of 32 months (range: 10–153), 116 MCI progressed to dementia (87 aMCI and 29 naMCI). Serum BACE1 activity was higher in MCI compared with Controls (p < 0.001), and in aMCI with brain atrophy compared with naMCI without brain atrophy (p = 0.04). No difference in BACE1 activity emerged between converter and non-converter MCI, and this was true for both aMCI and naMCI. However, among aMCI with better cognitive performance (n. 163, MMSE score ≥24/30) those converting to dementia had higher BACE1 activity compared to stable ones (p = 0.05). This was not associated with an increased risk to develop dementia (hazard ratio: 1.65; 95% confidence interval: 0.67–4.01). In conclusion, serum BACE1 activity significantly increased in MCI patients (both amnestic and non-amnestic) compared with Controls. Moreover, higher serum BACE1 activity was observed only among aMCI with a better cognitive performance who progressed to dementia, suggesting that a dysregulation of this enzyme might be an early event primarily associated with neurodegeneration.
Abbreviations
-
- AD
-
- Alzheimer's disease
-
- aMCI
-
- amnestic mild cognitive impairment
-
- ANCOVA
-
- analysis of covariance
-
- APP
-
- amyloid precursor protein
-
- BACE1
-
- beta-secretase 1
-
- BADL
-
- basic activities of daily living
-
- CHD
-
- coronary heart disease
-
- COPD
-
- chronic obstructive pulmonary disease
-
- CSF
-
- cerebrospinal fluid
-
- CT
-
- computed tomography
-
- GDS
-
- Geriatric Depression Scale
-
- HR
-
- Hazard ratio
-
- IADL
-
- instrumental activities of daily living
-
- LOAD
-
- late-onset Alzheimer's disease
-
- MCI
-
- mild cognitive impairment
-
- MIXED
-
- mixed dementia
-
- MMSE
-
- Mini Mental State Examination
-
- MRI
-
- magnetic resonance imaging
-
- naMCI
-
- non-amnestic mild cognitive impairment
-
- NSAIDs
-
- non-steroidal anti-inflammatory drugs
-
- VD
-
- vascular dementia
1 INTRODUCTION
Mild cognitive impairment (MCI) is an intermediate clinical condition in the trajectory from normal cognition to dementia (Roberts & Knopman, 2013). In general, individuals with MCI have a higher rate of progression to dementia over a relatively short period compared to normal subjects (Roberts & Knopman, 2013). After 60 years of age, the prevalence of MCI is approximately 7% to 25%, and it progressively increases with age (Jongsiriyanyong & Limpawattana, 2018). Currently, four subtypes of MCI are described, depending on the presence/absence of a memory deficit, and on the number of affected cognitive domains: amnestic (aMCI) single or multidomain, and non-amnestic (naMCI) single or multidomain (Petersen & Negash, 2008). Most of the studies report a rate of progression to dementia of 20–40%, with an annual conversion rate of 5–17% (Jongsiriyanyong & Limpawattana, 2018; Roberts & Knopman, 2013; Zuliani, Polastri, et al., 2020). While aMCI mainly progresses to Alzheimer's disease (AD), naMCI usually progresses to other types of dementia; however, both naMCI and aMCI have been associated with progression to vascular dementia (VD) (Roberts & Knopman, 2013).
β-secretase (BACE1) is a key enzyme in the formation of amyloid-β (Aβ), which plays a central role in AD pathogenesis (Hardy & Higgins, 1992; Sanabria-Castro et al., 2017). This protease actively participates in the formation of Aβ peptide, generated from the sequential enzymatic cleavage of amyloid precursor protein (APP) by enzymes with β- and γ-secretase activities. It has been demonstrated that BACE1 activity is increased in both brain/cerebrospinal fluid (CSF) of patients with late-onset Alzheimer's disease (LOAD) (Fukumoto et al., 2002) and MCI (Cheng et al., 2014; Zhong et al., 2007). BACE1 is also present in plasma, as demonstrated by Shen et al. (Shen et al., 2018). Interestingly, these authors showed that plasma BACE1 activity was higher in patients with AD and MCI compared to controls; moreover, they reported that BACE1 was higher in MCI progressing to dementia compared with stable MCI (Shen et al., 2018). By investigating a much larger sample of older individuals, we confirmed the finding of higher levels of serum BACE1 activity LOAD compared to controls, showing that this difference is independent of possible confounders including age, gender, and other risk factors for dementia (Cervellati et al., 2020). More recently, we found that BACE1 serum activity is significantly higher also in VD and MIXED (LOAD + VD), but not in other types of dementia, compared with controls (Zuliani, Trentini, et al., 2020).
In the present study we analyzed a large sample of elderly patients affected by MCI, with the aim of verifying whether serum BACE1 activity increased in this specific condition, and it might be useful in predicting the patients’ possible progression to dementia.
2 MATERIALS AND METHODS
2.1 Subjects
Two hundred and fifty-nine elderly (≥65 years) outpatients with diagnosis of MCI, referred to the Memory Clinic of the Department of Internal Medicine, S. Anna University Hospital, Ferrara (Italy) or to Casa Sollievo della Sofferenza, San Giovanni Rotondo (Italy) were enrolled into the study from 2006 to 2018. The study was not pre-registered to any of publicly available database and it was exploratory. No randomization methods were applied in the study, no blinding was performed. MCI was defined as the presence of a documented deficit in memory and/or other cognitive domain, without (single domain) or with (multiple domain) impairment in other cognitive domains, in an individual who did not meet the clinical criteria for dementia (Petersen & Negash, 2008). These patients underwent a regular follow-up for a median period of 32 months (range: 10–153). The Mini Mental State Examination (MMSE) ranged from 18/30 to 28/30 (median: 24.6/30). One hundred sixty-two subjects were affected by amnestic MCI (aMCI); of these, 87 progressed to dementia (53%). Ninety-seven patients had non-amnestic MCI (naMCI); of these, 29 progressed to dementia (30%). MCI patients were compared with 204 older individuals with no evidence of cognitive and/or functional impairment (Controls).
All subjects underwent structural magnetic resonance imaging (MRI) using a 64 volumetric scanner. Radiograms were evaluated by trained radiologists, uninformed about the clinical characteristics of the patient. Selected patients underwent F-fluorodeoxyglucose-positron emission tomography (FDG-PET) instead of MRI (e.g., foreign bodies, mechanical heart valves, pacemakers, cochlear implants, etc.) or besides MRI (uncertain diagnosis). Magnetic resonance imaging readings were dichotomized as follows: Leukoaraiosis: 0 = absent, 1 = present; cortical lesions: 0 = absent, 1 = present; lacunar lesions: 0 = absent, 1 = present; medial temporal lobe atrophy (MTA—as assessed using oblique coronal reconstructions of T1-weighted gradient-echo volume sequences perpendicular to the long axis of the hippocampus): 0 = absent, 1 = present.
LOAD diagnosis was based on the National Institute on Aging-Alzheimer's Association (NIA-AA) workgroups criteria (McKhann et al., 2011); since the vast majority of patients underwent structural MRI and/or FDG-PET, the most frequent diagnosis was “probable AD dementia with intermediate level of evidence.” The diagnosis of VD was carried out following the NINDS-AIREN criteria (Román et al., 1993).
There was no evidence of acute illness at the time of clinical observation and blood sampling. No subject was taking non-steroidal anti-inflammatory drugs (NSAIDs), antibiotics, or steroids at the time of recruitment.
For neuropsychological assessment, all subjects were given a battery of tests. The cognitive functions evaluated were the following: verbal memory (Rey's 15 words test, digit span forward–backward), episodic memory (Babcock story recall test), space/time orientation (items from MMSE), attention (Trail Making test, backward calculation from MMSE), executive functions (Trail Making test, Frontal Assessment Battery), constructional and visuospatial functions (Clock Drawing test), abstract reasoning (Raven's progressive matrices), language and comprehension (Token test, items from MMSE), verbal fluency (for letters and categories), and routine clinical tests for the evaluation of agnosia, apraxia, and aphasia. All tests were corrected according to age and education and the equivalent score (ES: from 0 to 4) for the Italian population was obtained (Spinnler & Tognoni, 1987).
Basic activities of daily living (BADLs) (Katz et al., 1963), instrumental activities of daily living (IADLs) (Lawton & Brody, 1969), and 15-items Geriatric Depression Scale (GDS) (Sheikh & Yesavage, 1986) were carried as previously described (Cervellati et al., 2020). Personal data and medical history (e.g., hypertension, coronary heart disease—CHD, diabetes, chronic obstructive pulmonary disease—COPD) were collected by trained personnel. Clinical chemistry analyses (serum B-12 vitamin and folate, liver, kidney and thyroid function tests, blood cell count) were routinely performed to exclude causes of secondary cognitive impairment.
The study was approved by the Local Ethic Committee of "Casa Sollievo della Sofferenza," San Giovanni Rotondo (protocol n. 3877/DS) and Local Ethic Committee of "Azienda Arcispedale S. Anna," Ferrara (protocol n. 170579). This study conforms to The Code of Ethics of the World Medical Association (Declaration of Helsinky, 1975) and was conducted according to guidelines for Good Clinical Practice (European Medicines Agency). Patients were informed and a written consent was obtained.
2.2 BACE1 activity assay
Peripheral blood samples were collected by venipuncture into Vacutainer® tubes without anticoagulant after overnight fasting. After 30 min of incubation at room temperature, the blood samples were centrifuged at 4650 g for 20 min and sera were collected and stored in single-use aliquots at −80℃ until analysis.
2.2.1 Substrate synthesis
The substrate was designed following Grüninger-Leitch and co-workers (Grüninger-Leitch et al., 2002) with chemical modification of the C-terminus, and essentially as described before (Cervellati et al., 2020). Briefly, the BACE1 substrate (sequence: SEVNLDAEFR) was C-terminally labeled with the fluorescent group Luciferase Yellow (LY) and N-terminally labeled with the quenching group Dabsyl, forming a FRET donor–acceptor pair. The Dabsyl moiety was inserted as a lysine side chain whereas the light-sensitive LY was added to the C-terminus during the last step of the substrate synthesis. To do this, the peptide sequence H-K(Dabsyl)SEVNLDAEFRC-NH2 and the maleimido-functionalized LY were synthesized and purified separately. Finally, the Thiol-Michael reaction between the sulfhydryl group of the C-terminal Cys and the maleimide group of the LY provided the desired product with a quantitative yield and high purity. Detailed information regarding the substrate synthesis can be found in the supplementary material from Cervellati C. and co-workers (Cervellati et al., 2020). Substrate may be available upon request.
2.2.2 Assay procedure
The assay was carried out essentially as previously described (Cervellati et al., 2020). Briefly, a stock solution of the substrate (392 µM in Dimethyl sulfoxide, DMSO, Sigma-Aldrich, Cat. No. D8418) was diluted to a final concentration of 30 µM in 50 mM acetate buffer, pH 4.5, 0.1 M NaCl and 100 µL were dispensed in triplicate to the wells of a black, flat-bottom microplate (Nunc Cat. No. 237108). After pre-incubation for 10 min at 37℃, the reaction was started by the addition of 5 µL of undiluted serum and the fluorescence was read every 30 s for 20 min using excitation and emission wavelengths of 430 and 520 nm, respectively, in a Tecan Infinite M200 (Tecan Group, Switzerland) microplate reader. The reaction rates were converted from relative fluorescence units (RFU) per minute to enzyme units (U) by interpolation with a standard curve constructed using known concentrations of the wild-type enzyme (beta-secretase human, Sigma-Aldrich, Cat. No. S4195). Intra-assay and inter-assay percentage coefficient of variation (%CV) were 6.5% (min–max: 2.6–10.9%) and 11.4% (min–max: 9.9–13%), respectively. Intermediate precision was 10.5% (min–max: 6.3–13.5%). For detailed data see Table S1.
2.3 Statistical analysis
All continuous variables considered in the study were first analyzed for normal distribution using Kolmogorov–Smirnov and Shapiro–Wilk tests. Variables with significant deviation from normal distribution were log transformed before entering statistical analyses. Parametric tests were used for the analysis of variables (such as BACE1 activity) normally distributed after log-transformation. Continuous variables were expressed as mean (standard deviation—SD) and median (interquartile range) when they were normally and non-normally distributed, respectively. The mean of the variables that were normal upon log-transformation were expressed as antilog (geometric mean, 95% confidence interval, CI). Difference between two groups was determined by using unpaired t-test and Mann–Whitney U-test for normal and non-normal variables, respectively. Comparison of multiple groups were performed by ANOVA (pairwise comparison by Sidak post-hoc) and Kruskal–Wallis (pairwise comparison by Mann–Whitney tests with Bonferroni correction) for normal and non-normal variables, respectively. Chi-square test was used to compare categorical variables. The outliers were detected first by converting the data to z-scores, and then by evaluating whether the absolute z-value was higher than 3. Analysis of covariance (ANCOVA) was performed to test whether the differences revealed at univariate analysis were independent of potential confounders. Association between continuous normal and non-normal variables were tested by assessing the coefficient of Pearson's (rp) and Spearman's (rs) correlation, respectively. Hazard ratios (HR) were estimated by Cox proportional hazard multivariate regression analysis, including age, sex, baseline MMSE, education, and hypertension as covariates to correct for possible confounding factors. The assumption of proportionality of all variables introduced in the models was assessed through the analysis of Schoenfeld residuals. Analyses were performed by SPSS for Windows statistical package, version 13.0. No sample calculation was performed and the number of subjects was determined on previous studies (Cervellati et al., 2020; Shen et al., 2018; Zuliani, Trentini, et al., 2020).
3 RESULTS
3.1 Cross-sectional findings
3.1.1 Serum BACE 1 activity in Controls vs MCIs
Table 1 summarizes the principal characteristics of Controls and MCI patients stratified according to amnesic and non-amnestic subtype. Compared with Controls, MCI patients were older and had less years of education and lower MMSE score (ANOVA: all p < 0.001). Compared with Controls, aMCI presented lower total cholesterol and glycemia (ANOVA: p = 0.02), while naMCI had lower low-density lipoprotein cholesterol (LDL-C) (ANOVA: p = 0.02) and higher high-sensitivity C-reactive protein (hs-CRP) (Kruskal–Wallis, p = 0.001). As regards comorbidities, MCI patients presented a higher prevalence of CHD (ANOVA: p < 0.001) and hypertension (p = 0.003) than Controls.
| Controls (n = 204) |
Non-amnestic MCI (n = 97) |
Amnestic MCI (n = 162) |
p-value* | |
|---|---|---|---|---|
| Age (years) | 74 ± 5 | 77 ± 5a | 78 ± 5a | <0.001 |
| Female gender (%) | 49 | 61 | 54 | 0.06 |
| Education (years) | 11 (8–13) | 5 (5–8)a | 5 (4–6)a | <0.001 |
| Active smoking | 9 | 7 | 7 | 0.65 |
| MMSE (/30) | 27 (26–29) | 26 (24–27)a | 25 (23–26)a, b | <0.001 |
| GDS (/15) | 4 (2–7) | 5 (3–8)a | 3 (2–6)a, b | 0.18 |
| Hemoglobin (g/dl) | 13.5 ± 1.4 | 13.1 ± 1.4 | 13.1 ± 1.5 | 0.17 |
| Total Cholesterol (mg/dl) | 216 ± 42 | 213 ± 49 | 202 ± 37a | 0.02 |
| Triglycerides (mg/dl) | 106 ± 43 | 119 ± 69 | 106 ± 49 | 0.10 |
| HDL cholesterol | 65 ± 18 | 59 ± 17 | 64 ± 39 | 0.27 |
| LDL Cholesterol | 131 ± 37 | 119 ± 32a | 130 ± 42b | 0.02 |
| Glycemia (mg/dl) | 103 ± 24 | 98 ± 25 | 95 ± 14a | 0.02 |
| hs-CRP (mg/dl) | 0.12 (0.06–0.24) | 0.19 (0.09–0.38)a | 0.11 (0.07–0.33)b | 0.001 |
| Hypertension (%) | 42 | 60a | 58a | 0.003 |
| Coronary heart disease (%) | 3 | 15a | 16a | <0.001 |
| Diabetes (%) | 8 | 11 | 16 | 0.08 |
| Stroke (%) | 3 | 2 | 3 | 0.29 |
| Cortical lesionsc (%) | 12 | 14 | 13 | 0.84 |
| Multiple lacunar lesions (%) | 29 | 40 | 43 | 0.34 |
| Leukoaraiosis (%) | 53 | 41 | 36 | 0.24 |
| Atrophy (%) | 31 | 54 | 54 | 0.06 |
- Normal continuous variables are expressed as mean ±standard deviation, while non-normal variables as Median and InterQuartile Range. Categorical variables are expressed as percentage within group.
- Abbreviations: GDS: geriatric depression scale; HDL: high-density lipoprotein; hs-PCR: high-sensitivity C-reactive protein; MMSE: mini mental state examination.
- a p < 0.05 vs controls.
- b p < 0.05 vs non-amnestic MCI.
- c MRI parameters were reported only for a fraction of the sample subjects
- * Statistical analysis: ANOVA (Sidak Post-hoc test) if the variables were normally distributed; Kruskal–Wallis (pairwise Mann–Whitney tests with Bonferroni correction) if the variables were not normally distributed; Chi-squared test if the variables were categorical.
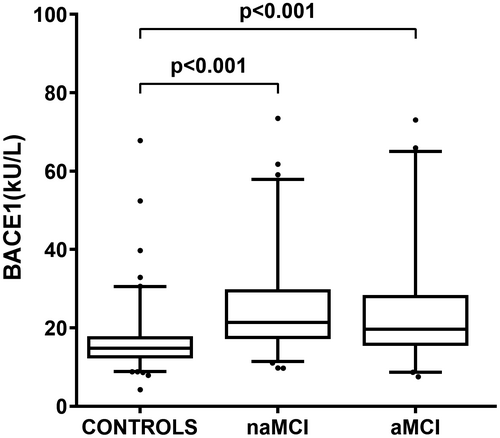
BACE1 activity was significantly higher (more than 30%) in both MCI subtypes compared with Controls (Figure 1) (ANOVA post-hoc: p < 0.001 for both pairwise comparison; effect size r = 0.333 and r = 0.412, respectively).
3.1.2 Effect of age and other potential confounders on serum BACE1 activity
We evaluated the influence of potential confounders on the relationship between BACE1 serum activity and MCI diagnosis. Age was the most important factor to be considered since it is the foremost risk factor for cognitive decline and is negatively correlated with BACE1 activity (rp = −0.189, p < 0.001). In order to make negligible the effect of age on BACE1 activity, a further comparison between the groups was performed after the exclusion of subjects aged over 76 years; the generated three subsets presented identical age (Controls, n:121, age: 71 ± 4 years; naMCI, n.37, 71 ± 4 years; aMCI, n:45, 72 ± 3 years; ANOVA: p = 0.21). Serum BACE1 activity was still significantly higher in naMCI (mean: 20 KU/L, 95% CI 18–24) and aMCI (mean: 24 KU/L, 95% CI 21–25) compared with Controls (mean: 14 KU/L, 95% CI 16–19) (ANOVA post-hoc: p = 0.001 for both pairwise comparisons; effect size r = 0.380 and r = 0.395, respectively).
Correlation analysis between BACE1 and the demographic/clinical variables identified the following potential confounders: gender (unpaired t-test: p = 0.001), MMSE (rs: −0.193, p < 0.001), education (rs: −0.350, p < 0.001), and arterial hypertension (unpaired t-test: p = 0.02). The difference in BACE1 activity between Controls and both MCI subgroups remained significant after adjustment for age and these covariates (ANCOVA p < 0.001 for both comparisons; effect size ω = 0.553).
3.1.3 BACE1 activity in association with presence/absence of MRI abnormalities
We evaluated the possible association between BACE1 activity and the presence/absence of brain atrophy, multiple lacunes, leukoaraiosis, or cortical lesions in patients with MCI and the two principal subtypes (naMCI and aMCI). Serum BACE1 activity was higher in MCI patients with documented brain atrophy compared with those without (unpaired t-test, p = 0.05, effect size r = 0.195); no differences emerged with regard to multiple lacunes, leukoaraiosis, or cortical lesions on MRI (data not shown). No differences in serum BACE 1 activity emerged according to the presence/absence of brain atrophy within aMCI and naMCI subgroups; however, a significant difference was found between aMCI with brain atrophy and naMCI without brain atrophy (ANOVA post-hoc: p = 0.04, effect size r = 0.194) (Figure S1).
3.2 Longitudinal findings
3.2.1 MCIs evolution and serum BACE 1 activity
We checked whether BACE1 activity might be associated with the progression from MCI to dementia. Compared with stable MCIs, patients who progressed to dementia were older (t-test: p = 0.002), had lower MMSE score (Mann–Whitney: p < 0.001), GDS score (Mann–Whitney: p = 0.05) and triglycerides (t-test: p = 0.04), were more often of amnestic type (Chi-square, p < 0.001), and had a lower prevalence of multiple lacunes on MRI (Chi-square, p = 0.04). BACE1 serum activity did not differ between stable MCIs and MCIs converted to dementia (Table S2); moreover, BACE1 serum activity did not differ between MCIs converting to LOAD, VD, or MIXED dementia, respectively (Table S3).
No difference in BACE1 activity emerged between converter and non-converter MCIs even after considering the two MCI subtypes separately (Figure 2a). However, when we focused our analysis on MCI patients with a better cognitive performance (n.163; MMSE score ≥24/30; median MMSE: 26/30), serum BACE1 activity was significantly higher (+20%) in aMCI progressing to dementia compared to stable aMCI (Figure 2b, p = 0.05, effect size: r = 0.171).

3.2.2 Multivariate Cox regression analysis
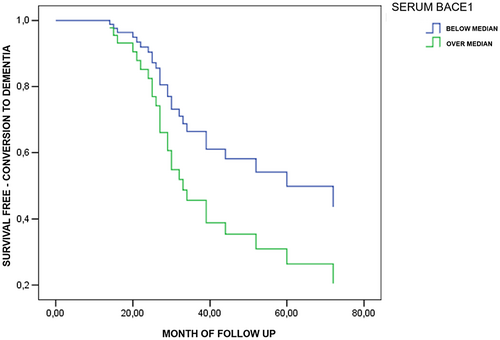
The survival curves for progression to dementia, obtained by Cox multivariate regression analysis in MCI dichotomized by levels of serum BACE1 activity (below vs. over the median value of 21.7 KU/L) are reported in Figure S2. Compared to subjects with low BACE1 activity, no differences in the incidence of dementia were observed for subjects with high BACE1 activity (HR: 1.23; 95% CI: 0.77–1.96) after adjustment for age, sex, baseline MMSE, education, and hypertension. No significant difference was found when the same Cox model was calculated for amnestic and non-amnestic MCI, separately (data not shown).
The significant results obtained among aMCIs with better cognitive performance led us to run the same Cox model (adjusted for age, sex, baseline MMSE, education, and hypertension) within this subgroup, dichotomized by median serum BACE1 activity levels (21.3 KU/L). As shown in Figure 3, a non-significant trend toward an increased risk of evolution to dementia in aMCIs with higher serum BACE1 activity was detected (HR: 1.65; 95% CI: 0.67–4.01).
4 DISCUSSION
We investigated serum BACE1 activity in a large sample of MCI patients followed for a median period of more than 30 months. The cross-sectional analysis showed that serum BACE1 activity was significantly higher in MCIs compared with Controls, regardless of potential confounders such as age, gender, hypertension, education, and MMSE score. Moreover, for the first time, the enzyme activity was found to be associated with radiological signs of AD-related neurodegeneration (i.e., atrophy) in aMCI and, as previously observed (Cervellati et al., 2020; Shen et al., 2018), with the degree of cognitive decline as assessed by MMSE.
These results are in good agreement with the findings from Shen et al. (Shen et al., 2018); these authors found that serum BACE1 activity was increased (about +50%) in subjects with aMCI. Collectively, these data support the concept of an early up-regulation of BACE1 serum activity before the onset of overt dementia. A few studies have previously investigated BACE1 activity in cerebrospinal fluid (CSF) and brain of subjects with MCI. Zhong et al. examined whether BACE1 could be identified in the CSF of patients with aMCI. They found that aMCIs, as well as patients with AD, showed increased BACE1 activity compared with controls (Zhong et al., 2007); moreover, BACE1 activity was significantly correlated with Aβ42 level. Successively, Cheng et al. found a significant increase in BACE1 activity in the brain of aMCI and AD patients (Cheng et al., 2014); interestingly, increased BACE1 activity was correlated with plaque numbers and cognitive status. Further compelling findings came from Shen's study (Shen et al., 2018) which showed that plasma BACE1 activity was positively and negatively correlated with CSF Tau and Aβ42, respectively. All these data suggest the presence of an early activation of BACE1 in AD, both in the brain and in periphery, although our recent data (Zuliani, Trentini, et al., 2020) suggest that serum BACE1 activity might increase also in VD and MIXED dementia.
The second part of our study focused on the possible relationship between serum BACE1 activity and the clinical evolution of MCI. In the whole sample, no difference in the enzyme activity emerged between converters and non-converters. Successively, we selected a subsample including MCI patients with a better cognitive performance (MMSE ≥24/30) in order to: (1) make our sample comparable to those of other authors (e.g., Shen et al.) who enrolled MCI with a much better cognitive performance (mean MMSE = 27/30); (2) reduce the effect of baseline cognitive status on MCI evolution. Indeed, we recently reported that a MMSE score below 25/30 is a major predictor of MCI progression to dementia (Zuliani, Polastri, et al., 2020). Actually, we found that serum BACE1 activity was significantly higher in aMCI progressing to dementia compared with stable aMCI. This finding is in good agreement with previous results from Shen et al. which found that aMCIs converting to probable AD exhibited significantly higher BACE1 activity compared with stable MCIs (Shen et al., 2018).
However, higher BACE1 activity did not predict the conversion from aMCI to dementia in multivariate Cox proportional hazard model, after a median follow-up of 32 months, although an interesting trend was observed. In this regard, it must be underlined that our final sample was definitely underpowered for this kind of analysis.
Although we confirmed the results of Shen et al., some differences should be noted. First, they did not calculate a hazard ratio (HR) for the risk of progression from MCI to dementia, and this is an important analysis since it takes into account the time to conversion. Second, important differences in the characteristics of the sample are present. Indeed, the MCIs included into Shen's study were 8 years younger than ours. This is a crucial point, considering that age is a very strong risk factor for progression to dementia (Zuliani, Polastri, et al., 2020), and it is also associated with an increase in serum BACE1 activity (Cervellati et al., 2020).
Finally, we found no differences in serum BACE1 activity between MCIs converting to LOAD, VD, or mixed dementia. These results are in good agreement with our recent study (Zuliani, Trentini, et al., 2020) showing that BACE1 serum activity might be increased not only in LOAD, but also in VD and mixed dementia, and consequently might not be a very specific marker for LOAD (Cervellati et al., 2021). The finding of increased serum BACE1 activity in mixed dementia is somehow expected since, by definition, in this frequent type of dementia both the neurodegeneration (typical of AD) and the vascular lesions (typical of VD) (Cervellati et al., 2021; de la Torre, 2002) coexist. More intriguing and still not explained is the possible increase in serum BACE1 activity in VD (Zuliani, Trentini, et al., 2020).
As previously suggested (Cheng et al., 2014; Fukumoto et al., 2002; Zhong et al., 2007; Zuliani, Trentini, et al., 2020), the increase in BACE1 activity seems to be an early event in the long way to MCI and eventually to dementia. As a consequence, several steps downstream of the increase in BACE1 activity may modulate the progression of the pathology including amyloid-beta clearance, synaptic dysfunction, tau mediated neural injury, brain damage and, finally, cognitive impairment (Sperling et al., 2011). Nevertheless, it appears that increased BACE1 serum activity may be associated with the progression from aMCI to dementia; it could be viewed like a kind of “necessary” but, perhaps, not sufficient condition for the development of LOAD and possibly of VD.
Some limitations and strengths of the present study should be noted.
First, we cannot exclude that some biases or unmeasured confounders might limit the reliability of our findings. Second, a single timepoint of BACE1 activity measurement precluded our ability to assess its trend in subjects converting or not to dementia. Third, LOAD diagnosis was based on the NIA-AA workgroups criteria (McKhann et al., 2011) with evidence of neuronal injury (structural MRI/FDG-PET) without confirmation from CSF biomarkers or Aβ PET (probable AD dementia with intermediate probability). However, it is worth noting that many studies dealing with BACE1 mainly enrolled a younger population than ours (around 70 years vs. around 76 years in our study). As such, we believe that our population composed of elderly subjects better resemble the general patients affected by dementia. We also have to acknowledge that the lack of CSF Aβ measurement prevented us from confirming the blood-CSF correspondence observed in Shen's work. Finally, the results obtained by comparing MCI subsamples should be replicated in a much larger cohort of patients, since it was limited by the low effect size.
Some strengths of our study would be the longitudinal design, and the highly standardized clinical follow-up. Moreover, to the best of our knowledge, this is the first longitudinal attempt to evaluate the predictive value of serum activity of BACE1 by using a Cox multivariate regression analysis.
5 CONCLUSIONS
We found that serum BACE1 activity significantly increased in MCI patients (both amnestic and non-amnestic) compared with Controls. Moreover, higher serum BACE1 activity was observed only among aMCI with a better cognitive performance who progressed to dementia, suggesting that a dysregulation of this enzyme might be an early event primarily associated with neurodegeneration. Further larger studies employing validated CSF biomarkers are required to confirm these findings.
ACKNOWLEDGMENTS
No specific funding was received. We thank Dr. Federica Gentili for the correction of the English language.
All experiments were conducted in compliance with the ARRIVE guidelines.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
AUTHOR CONTRIBUTION
G.Z. and C.C. were involved in conceptualization, writing original draft, data analysis, and review and editing; A.T. was involved in writing original draft, data analysis, and review and editing; G.B, V.R., and T.R. were involved in data analysis, data curation, validation, and review and editing; R.G. and S.P. were involved in data analysis, data curation, review and editing, and formal analysis; P.G., M.P., and L.M. were involved in data analysis, investigation, data curation, and review and editing; D.P. and C.P. were involved in investigation, and review and editing; D.S. was involved in data curation, investigation, and review and editing.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.