Prognosis and clinicopathological features of patients with early-onset and late-onset colorectal cancer with second primary malignancies
Declaration of conflict of interest: The authors declare no conflicts of interest.
Author contribution: Methodology: Xinxiang Li and Qingguo Li. Writing—original draft: Fan Chen and Jiayu Chen. Writing—review and editing: Xinxiang Li and Qingguo Li. Project administration: Dakui Luo. Formal analysis: Ruijia Zhang. Data curation: Yufei Yang. Visualization: Fan Chen.
Ethical approval: No animal studies are presented in this manuscript. The studies involving humans were approved by the Ethical Committee and Institutional Review Board of the Fudan University Shanghai Cancer Center. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) and minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Financial support: This work was supported by the National Natural Science Foundation of China (81972260 and 82103259) and the Natural Science Foundation of Shanghai (21ZR1414400). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abstract
Background and Aim
The risk of developing a second primary malignancy differs among colorectal cancer patients in different age groups. This study aimed to investigate the differences in prognosis and clinicopathological features of patients with early-onset colorectal cancer and late-onset colorectal cancer who developed second primary malignancies.
Methods
The study included 15 489 patients who underwent surgery for colorectal cancer at Fudan University Shanghai Cancer Center between January 2008 and December 2018. Data pertaining to these patients were derived from the database.
Results
A total of 680 (4.5%) patients subsequently developed a second primary malignancy. Considering death as a competing event, the 10-year cumulative risk of second primary malignancy for early-onset colorectal cancer was 5.3%, compared with 7.3% for late-onset colorectal cancer. Cox analysis showed that late-onset colorectal cancer, colon cancer, smaller tumor size, and fewer tumor nodules without residual lymph node structure, chemotherapy, and radiotherapy were independent risk factors for second primary malignancy. In our patient cohort, early-onset colorectal cancer was associated with better prognosis compared to late-onset colorectal cancer, for both overall survival and second primary malignancy-free survival. In addition, there was insufficient evidence that early-onset colorectal cancer also affected prognosis after the occurrence of second primary malignancies.
Conclusions
The risk of early-onset colorectal cancer subsequently developing second primary malignancy was significantly lower than late-onset colorectal cancer, and the second primary malignancies of early-onset colorectal cancer were more likely to be colorectal cancer. Overall survival and second primary malignancy-free survival of early-onset colorectal cancer were consistently better than late-onset colorectal cancer.
Graphical Abstract
Introduction
Colorectal cancer (CRC) ranks as the third most common malignancy worldwide and the second most common cause of cancer-related deaths.1
The full implementation and popularization of CRC screening strategies in recent years have led to a significant decrease in morbidity and mortality rates in the USA and European countries.2, 3 However, the incidence of early-onset CRC (eoCRC; diagnosed < 50 years of age) has been on the rise globally.4-6 The reasons for this upward trend are not fully understood and may be related to risk factors such as diabetes, obesity, diet, sedentary time, drinking, and smoking.7 In China, the morbidity and mortality of CRC have increased in recent years, whether it is late-onset CRC (loCRC; diagnosed ≥ 50 years of age) or eoCRC.8-10 Between 1990 and 2019, the number of patients with CRC increased from 0.44 million to 3.42 million, whereas that with eoCRC increased from 0.12 million to 0.59 million. It is estimated that the annual number of eoCRC cases in China will reach a peak of about 101 000 by around 2034.8
Recent studies have shown that about 13% of eoCRC patients have hereditary cancer syndromes and about 30% of eoCRC patients have a family history of cancer, but most CRC cases are still sporadic.11-14 Compared with loCRC, eoCRC tends to be more heterogeneous, with more advanced staging, poor histological features, and variations in molecular features.15-17
Previous findings suggest that a history of prior CRC is an independent prognostic factor for second primary malignancies (SPMs).18, 19 Furthermore, different subgroups of patients with CRC show variable risk of developing SPMs, with individuals with eoCRC, not loCRC, at a significantly higher risk than the general population.20-24 However, studies supporting the association between this increased risk and specific clinicopathological features and prognostic profiles are limited. Notably, as survival rates for patients with CRC increase and the proportion of younger patients with CRC increases, these patients are more likely to develop an SPM over a longer survival period. Additionally, the prognosis of patients with CRC with SPMs is generally worse than that of patients diagnosed with CRC alone.25, 26
This study aimed to analyze the differences between eoCRC and loCRC in SPM development, to explore the risk factors affecting SPM development, and to assess the impact of SPM development on prognosis. The findings aimed to provide valuable insights for the monitoring and screening of SPMs and potentially contribute to the exploration of genetic and environmental factors associated with this phenomenon.
Methods
Study population
The study included 15 489 patients who underwent surgery for CRC at Fudan University Shanghai Cancer Center (FUSCC) between January 2008 and December 2018. Data pertaining to these patients were derived from the FUSCC database. Figure S1 delineates the inclusive and exclusive criteria for the cohorts. The FUSCC database provided the following patient information: sex, age at diagnosis, tumor location, neoadjuvant therapy, histological type, differentiation, TNM stage, perineural invasion, vascular invasion, tumor nodules without residual lymph node structure (ND), survival data, surgical procedures, adjuvant chemotherapy, adjuvant radiotherapy, and SPM-related information. The study was approved by the Ethics Committee of FUSCC, and informed consent was obtained from each patient.
Second primary malignancies
Multiple primaries were defined as more than one synchronous or metachronous cancer in a single individual. The study adhered to the guidelines set by the International Agency for Research on Cancer and the International Association of Cancer Registries, the International Code for Multiple Primary Cancers (ICD-0, version 3): (i) the recognition of an SPM does not depend on the time interval from the first primary malignancy; (ii) a primary malignancy is defined as originating from a primary site or tissue and is not a recurrence or metastasis; and (iii) a tumor is considered to originate from only one organ or tissue, except for systemic cancers and polymorphic tumors.
Postoperative follow-up
For postoperative follow-up, the survival data of patients were collected through outpatient follow-ups, telephonic follow-ups, and death registry data linkage. The last follow-up date was recorded as March 15, 2021.
All patients were advised to undergo colonoscopy 3–6 months after surgery and every 1–2 years thereafter; computed tomography scans of the chest and abdomen initially and annually thereafter; blood levels of tumor markers including CEA,carcinoembryonic antigen, cancer antigen 19-9, cancer antigen 125, and cancer antigen 72-4 were measured every 3–6 months after surgery; and normal screening was performed 5 years after surgery. Patients with abnormal test results will be diagnosed by a specialist in the appropriate department. Patients who meet the earlier requirements for SPM will be recorded in the follow-up information. Some patients are diagnosed accidentally outside the regular postoperative examination.
Statistical analysis
Statistical analyses were performed using spss version 26.0 (SPSS, Chicago, Illinois, USA). The t-test, chi-squared test, and Mann–Whitney U-test were used to analyze the clinicopathological differences between the eoCRC and loCRC groups.
Death as a competing event leads to an overestimation of the incidence of SPM when standard Cox regression is applied with SPM as an outcome.27 Therefore, the Fine and Gray subdistribution risk model was used to calculate the cumulative risk of SPM when death is considered a competing event.28
The Kaplan–Meier method was used to compare the survival curves of patients in the two groups. Cox regression was used for multivariate analysis to determine independent risk factors. The impact of diverse factors on prognosis was assessed through multiple temporal variables. Overall survival (OS) denotes the duration from the initial diagnosis of CRC to mortality from any cause. SPM latency represents the interval from the diagnosis of CRC to the identification of a subsequent primary malignancy. SPM-free survival encompasses the duration from the diagnosis of CRC to mortality or the occurrence of an SPM as competing events. SPM OS (SPMOS) signifies the duration from the diagnosis of an SPM to mortality from any cause. These temporal parameters were all quantified in months. A P value of < 0.05 indicates a statistically significant difference.
Results
Pathological characteristics of patients in the early-onset colorectal cancer and late-onset colorectal cancer groups
A total of 15 489 patients diagnosed with CRC were included in our investigation. Among them, 3230 (20.9%) individuals presented with eoCRC, while 12 259 patients (79.1%) had loCRC. Over an average follow-up period of 4.2 years, 30 (0.2%) refused follow-up, 1874 (12.1%) were lost to follow-up, and 3699 (23.9%) died. Ultimately, 680 patients (4.5%) subsequently developed SPMs.
In all cases, eoCRC was linked to female gender, neoadjuvant therapy, advanced disease stage, aggressive tumor characteristics, chemotherapy, radiotherapy, laparoscopic surgery, and a reduced propensity for subsequent SPM development. However, eoCRC was not associated with the location of CRC or the duration of follow-up. Among cases with SPMs, eoCRC was only correlated with female gender and poorer histological subtypes and not with other pathological factors, follow-up duration, or SPM latency (Table 1).
| Variable | With SPMs (n = 680) | All patients (n = 15 489) | |||||
|---|---|---|---|---|---|---|---|
| eoCRC (n = 106) | loCRC (n = 574) | P value | eoCRC (n = 3230) | loCRC (n = 12 259) | P value | ||
| Sex | Male | 50 (47.2%) | 345 (60.1%) | 0.013 | 1799 (55.7%) | 7506 (61.2%) | < 0.001 |
| Female | 56 (52.8%) | 229 (39.9%) | 1431 (44.3%) | 4753 (38.8%) | |||
| Location | Rectum | 32 (31.1%) | 180 (31.4%) | 0.135 | 1793 (55.8%) | 6646 (54.3%) | 0.181 |
| Left-sided colon | 32 (21.1%) | 129 (22.5%) | 693 (21.6%) | 2652 (21.7%) | |||
| Right-sided colon | 39 (37.9%) | 264 (46.1%) | 726 (22.6%) | 2947 (24.1%) | |||
| Unknown | 3 | 1 | 18 | 14 | |||
| Neoadjuvant therapy | No | 96 (90.6%) | 518 (90.2%) | 0.918 | 2614 (80.9%) | 10 743 (87.6%) | < 0.001 |
| Yes | 10 (9.4%) | 56 (9.8%) | 616 (19.1%) | 1516 (12.4%) | |||
| Histological type | Adenocarcinoma | 86 (81.9%) | 507 (88.9%) | 0.041 | 2675 (83.5%) | 10 798 (88.6%) | < 0.001 |
| Mucinous | 17 (16.2%) | 59 (10.4%) | 406 (12.7%) | 1224 (10.0%) | |||
| Signet ring cell | 2 (1.9%) | 4 (0.7%) | 121 (3.8%) | 162 (1.3%) | |||
| Unknown | 1 | 4 | 28 | 75 | |||
| Differentiation | Poor | 28 (30.1%) | 118 (22.2%) | 0.074 | 870 (30.8%) | 2679 (23.8) | < 0.001 |
| Moderate | 63 (67.7%) | 393 (73.9) | 1916 (67.8%) | 8384 (74.1) | |||
| Well | 2 (2.2%) | 21 (3.9%) | 40 (1.4%) | 248 (2.2%) | |||
| Unknown | 13 | 42 | 404 | 948 | |||
| Vascular invasion | Negative | 83 (78.3%) | 459 (80.0%) | 0.696 | 2275 (70.4%) | 9225 (75.3%) | < 0.001 |
| Positive | 23 (21.7%) | 115 (20.0%) | 955 (29.6%) | 3034 (24.7%) | |||
| Perineural invasion | Negative | 88 (83.0%) | 475 (82.9%) | 0.976 | 2313 (71.6%) | 9436 (77.0%) | < 0.001 |
| Positive | 18 (17.0%) | 98 (17.1%) | 917 (28.4%) | 2821 (23.0%) | |||
| Unknown | 0 | 0 | 0 | 2 | |||
| T stage | 0–2 | 26 (26.3%) | 158 (29.0%) | 0.267 | 643 (21.4%) | 2846 (24.4%) | < 0.001 |
| 3 | 24 (24.2%) | 155 (28.5%) | 947 (31.6%) | 4110 (35.2%) | |||
| 4 | 49 (49.5%) | 231 (42.5%) | 1408 (47.0%) | 4727 (40.5%) | |||
| Unknown | 7 | 30 | 232 | 576 | |||
| N stage | 0 | 68 (64.2%) | 350 (61.0%) | 0.619 | 1544 (47.8%) | 6703 (54.7%) | < 0.001 |
| 1 | 26 (24.5%) | 161 (28.0%) | 971 (30.1%) | 3616 (29.5%) | |||
| 2 | 12 (11.3%) | 63 (11.0%) | 715 (22.1%) | 1940 (15.8%) | |||
| M stage | 0 | 97 (91.5%) | 512 (89.2%) | 0.475 | 2727 (84.4%) | 10 725 (87.5%) | < 0.001 |
| 1 | 9 (8.5%) | 62 (10.8%) | 503 (15.6%) | 1534 (12.5%) | |||
| TNM stage | 0–I | 23 (23.2%) | 125 (22.7%) | 0.635 | 461 (15.1%) | 2188 (18.6%) | < 0.001 |
| II | 34 (34.3%) | 178 (32.4%) | 819 (26.7%) | 3693 (31.3%) | |||
| III | 33 (33.3%) | 185 (33.6%) | 1279 (41.8%) | 4380 (37.1%) | |||
| IV | 9 (9.1%) | 62 (11.3%) | 503 (16.4%) | 1534 (13.0%) | |||
| Unknown | 7 | 24 | 168 | 464 | |||
| ND | Mean ± SD | 0.11 ± 0.558 | 0.18 ± 0.808 | 0.419 | 0.48 ± 1.824 | 0.35 ± 1.262 | < 0.001 |
| Chemotherapy | No | 36 (35.0%) | 228 (41.2%) | 0.238 | 1271 (41.3%) | 5884 (51.5%) | < 0.001 |
| Yes | 67 (65.0%) | 326 (58.8%) | 1806 (58.7%) | 5550 (48.5%) | |||
| Unknown | 3 | 20 | 47 | 251 | |||
| Radiotherapy | No | 82 (79.6%) | 423 (76.4%) | 0.472 | 2355 (76.5%) | 9750 (85.3%) | < 0.001 |
| Yes | 21 (20.4%) | 131 (23.6%) | 722 (23.5%) | 1684 (14.7%) | |||
| Unknown | 3 | 20 | 47 | 251 | |||
| Surgical procedures | Open | 78 (83.0%) | 437 (84.7%) | 0.674 | 2208 (80.8%) | 8248 (82.5%) | 0.043 |
| Laparoscopic | 16 (17.0%) | 79 (15.3%) | 523 (19.2%) | 1747 (17.5%) | |||
| Unknown | 12 | 58 | 393 | 1690 | |||
| SPM | No | NA | NA | NA | 3124 (96.7%) | 11 685 (95.3%) | < 0.001 |
| Yes | NA | NA | 106 (3.3%) | 574 (4.7%) | |||
| Follow-up time | Mean ± SD | 68.74 ± 47.062 | 60.33 ± 36.613 | 0.083 | 50.74 ± 33.772 | 49.65 ± 32.374 | 0.098 |
| SPM latency | Mean ± SD | 27.75 ± 31.07 | 27.12 ± 31.21 | 0.848 | NA | NA | NA |
- eoCRC, early-onset colorectal cancer; loCRC, late-onset colorectal cancer; ND, tumor nodules without residual lymph node structure; SPM, second primary malignancy; NA, not applicable.
Second primary malignancies
CRC (22.1%), lung cancer (17.2%), breast cancer (8.2%), prostate cancer (7.5%), gastric cancer (6.9%), and thyroid cancer (6.5%) accounted for a high proportion of SPMs.
CRC (30.2%), thyroid cancer (9.4%), breast cancer (8.5%), lung cancer (7.5%), and endometrial cancer (7.5%) were the major SPMs in the eoCRC group. CRC (20.6%), lung cancer (19.0%), breast cancer (8.2%), prostate cancer (7.7%), and gastric cancer (7.1%) were the major SPMs in the loCRC group (Table 2).
| Location | eoCRC (n = 106) | loCRC (n = 574) | CRC (n = 680) |
|---|---|---|---|
| Colon and rectum | 32 (30.2%) | 118 (20.6%) | 150 (22.1%) |
| Lung, bronchus, and trachea | 8 (7.5%) | 109 (19.0%) | 117 (17.2%) |
| Breast | 9 (8.5%) | 47 (8.2%) | 56 (8.2%) |
| Stomach | 6 (5.7%) | 41 (7.1%) | 47 (6.9%) |
| Prostate | 0 (0.0%) | 44 (7.7%) | 44 (6.5%) |
| Thyroid gland | 10 (9.4%) | 27 (4.7%) | 37 (5.4%) |
| Kidney | 4 (3.8%) | 32 (5.6%) | 36 (5.3%) |
| Uterus | 8 (7.5%) | 19 (3.3%) | 27 (4.0%) |
| Esophagus | 0 (0.0%) | 23 (4.0%) | 23 (3.4%) |
| Liver | 3 (2.8%) | 20 (3.5%) | 23 (3.4%) |
| Bladder | 2 (1.9%) | 16 (2.8%) | 18 (2.6%) |
| Lymphoma | 3 (2.8%) | 11 (1.9%) | 14 (2.1%) |
| Cervix uteri | 5 (4.7%) | 8 (1.4%) | 13 (1.9%) |
| Oral cavity and pharynx | 4 (3.8%) | 9 (1.6%) | 13 (1.9%) |
| Pancreas | 2 (1.9%) | 11 (1.9%) | 13 (1.9%) |
| Ovary | 5 (4.7%) | 5 (0.9%) | 10 (1.5%) |
| Soft tissues | 4 (3.8%) | 5 (0.9%) | 9 (1.3%) |
| Head and neck | 0 (0.0%) | 7 (1.2%) | 7 (1.0%) |
| Malignant melanoma of skin | 0 (0.0%) | 5 (0.9%) | 5 (0.7%) |
| Small intestine | 0 (0.0%) | 3 (0.5%) | 3 (0.4%) |
| Biliary tract | 0 (0.0%) | 3 (0.5%) | 3 (0.4%) |
| Nervous system | 0 (0.0%) | 2 (0.3%) | 2 (0.3%) |
| Other malignant neoplasms | 1 (0.9%) | 9 (1.6%) | 10 (1.5%) |
- CRC, colorectal cancer; eoCRC, early-onset colorectal cancer; loCRC, late-onset colorectal cancer.
Compared with loCRC, a significant increase in the proportion of CRC, thyroid cancer, and uterine cancer, as well as a decrease in the proportion of lung cancer, gastric cancer, and prostate cancer, can be observed in the SPMs of patients with eoCRC.
Development of second primary malignancies
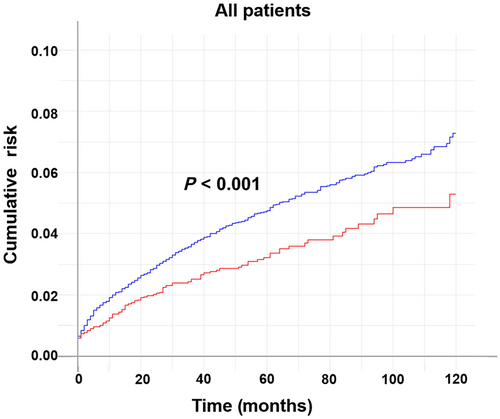
Considering death as a competing event, the cumulative risk of developing an SPM over a 10-year span was 7.3% for eoCRC and 5.3% for loCRC (P < 0.001, Fig. 1). The cumulative risk of developing an SPM over a decade was evenly increased in both eoCRC and loCRC patients.
 , loCRC;
, loCRC;  , eoCRC.
, eoCRC.The result of the multivariate Fine and Gray subdistribution risk model revealed that the risk of a subsequent SPM in eoCRC is only 72.5% of that in loCRC (eoCRC vs loCRC, hazard ratio [HR]: 0.725, 95% confidence interval [CI]: 0.583–0.901, P = 0.004). In addition, colon cancer (left-sided colon vs rectum, HR: 1.478, 95% CI: 1.211–1.803, P < 0.001; right-sided colon vs rectum, HR: 1.697, 95% CI: 1.407–2.047, P < 0.001), smaller tumor size (T4 vs T0–2, HR: 0.780, 95% CI: 0.639–0.952, P = 0.015), fewer lymph node metastases (N2 vs N0, HR: 0.662, 95% CI: 0.507–0.865, P = 0.003), fewer ND (HR: 0.858, 95% CI: 0.754–0.977, P = 0.021), chemotherapy (yes vs no, HR: 1.914, 95% CI: 1.659–2.444, P < 0.001), and radiotherapy (yes vs no, HR: 1.535, 95% CI: 1.208–1.949, P < 0.001) were independent risk factors for SPM development (Table 3).
| Variable | Univariate analysis | P value | Multivariate analysis | P value | |
|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | ||||
| Early-onset | No | Reference | Reference | ||
| Yes | 0.697 (0.567–0.858) | < 0.001 | 0.725 (0.583–0.901) | 0.004 | |
| Sex | Female | Reference | NI | ||
| Male | 0.918 (0.789–1.070) | 0.270 | |||
| Location | Rectum | Reference | Reference | ||
| Left-sided colon | 1.370 (1.132–1.654) | 0.001 | 1.478 (1.211–1.803) | < 0.001 | |
| Right-sided colon | 1.590 (1.340–1.901) | < 0.001 | 1.697 (1.407–2.047) | < 0.001 | |
| Neoadjuvant therapy | No | Reference | NI | ||
| Yes | 0.710 (0.551–0.915) | 0.008 | |||
| Histological type | Adenocarcinoma | Reference | NI | ||
| Mucinous | 1.030 (0.810–1.300) | 0.820 | |||
| Signet ring cell | 0.490 (0.219–1.091) | 0.082 | |||
| Differentiation | Poor | Reference | NI | ||
| Moderate | 1.041 (0.868–1.260) | 0.640 | |||
| Well | 1.887 (1217–2.941) | 0.005 | |||
| Vascular invasion | Negative | Reference | NI | ||
| Positive | 0.754 (0.626–0.909) | 0.003 | |||
| Perineural invasion | Negative | Reference | NI | ||
| Positive | 0.686 (0.562–0.838) | < 0.001 | |||
| T stage | 0–2 | Reference | Reference | ||
| 3 | 0.792 (0.645–0.973) | 0.026 | 0.810 (0.652–1.007) | 0.057 | |
| 4 | 0.765 (0.635–0.921) | 0.005 | 0.780 (0.639–0.952) | 0.015 | |
| N stage | 0 | Reference | Reference | ||
| 1 | 0.787 (0.663–0.935) | 0.006 | 0.903 (0.750–1.086) | 0.280 | |
| 2 | 0.545 (0.426–0.697) | < 0.001 | 0.662 (0.507–0.865) | 0.003 | |
| M stage | 0 | Reference | NI | ||
| 1 | 0.776 (0.607–0.992) | 0.043 | |||
| ND | 0.794 (0.691–0.912) | 0.001 | 0.858 (0.754–0.977) | 0.021 | |
| Chemotherapy | No | Reference | < 0.001 | Reference | < 0.001 |
| Yes | 1.830 (1.562–2.144) | 1.914 (1.659–2.444) | |||
| Radiotherapy | No | Reference | < 0.001 | Reference | < 0.001 |
| Yes | 1.589 (1.284–1.846) | 1.535 (1.208–1.949) | |||
| Surgical procedures | Open | Reference | 0.799 | NI | |
| Laparoscopic | 1.029 (0.825–1.283) | ||||
- CI, confidence interval; CRC, colorectal cancer; HR, hazard ratio; ND, tumor nodules without residual lymph node structure; SPM, second primary malignancy; NI, not included.
Survival analyses
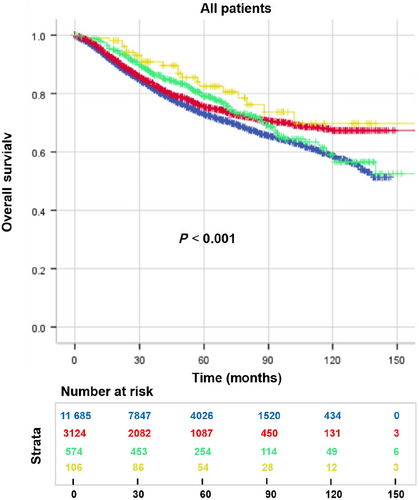
The OS exhibited disparities based on the occurrence of subsequent SPMs and the age at diagnosis (P < 0.001). Notably, patients with eoCRC demonstrated a more favorable prognosis compared with those with loCRC, irrespective of the presence of subsequent SPMs. Among eoCRC and loCRC patients, 5-year OS improved by 7.0% (82.5% vs 75.5%) and 6.2% (79.1 vs 72.9%), respectively, in patients with an SPM. As time progressed, this discrepancy diminished, with the OS rates remained consistent beyond the eighth year (Fig. 2). When comparing SPM-free survival between the two groups, the advantage in prognosis for eoCRC over loCRC only became discernible after 5 years (P < 0.001, Fig. 3a). Additionally, while the 5-year SPMOS rates for eoCRC and loCRC stood at 76.0% and 66.2%, respectively, no statistically significant difference was observed to support the notion that eoCRC exerted an influence on prognosis after the occurrence of SPMs (P = 0.161, Fig. 3b).
 , late-onset colorectal cancer (loCRC) + no SPM;
, late-onset colorectal cancer (loCRC) + no SPM;  , early-onset colorectal cancer (eoCRC) + no SPM;
, early-onset colorectal cancer (eoCRC) + no SPM;  , loCRC + SPM;
, loCRC + SPM;  , eoCRC + SPM;
, eoCRC + SPM;  , loCRC + no SPM;
, loCRC + no SPM;  , eoCRC + no SPM;
, eoCRC + no SPM;  , loCRC + SPM;
, loCRC + SPM;  , eoCRC + SPM.
, eoCRC + SPM.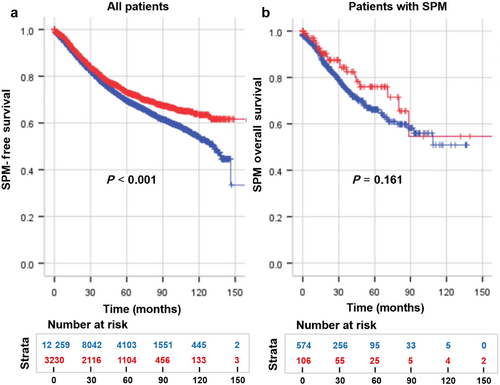
 , loCRC;
, loCRC;  , eoCRC;
, eoCRC;  , loCRC;
, loCRC;  , eoCRC.
, eoCRC.Multivariate Cox analysis unveiled that the risk of mortality for eoCRC patients was diminished by 38.0% in comparison with loCRC patients (eoCRC vs loCRC, HR: 0.636, 95% CI: 0.580–0.696, P < 0.001), and the SPM-free survival was reduced by 30.2% for eoCRC patients (eoCRC vs loCRC, HR: 0.698, 95% CI: 0.641–0761, P < 0.001). Moreover, patients who experienced subsequent SPMs exhibited a lower risk of death (SPM vs no SPM, HR: 0.793, 95% CI: 0.666–0.943, P = 0.009). Univariate and multivariate Cox analyses demonstrated the independent associations of tumor location, neoadjuvant therapy, histological type, differentiation, TNM stage, perineural invasion, vascular invasion, ND, chemotherapy, radiotherapy with OS, and SPM-free survival (Tables 4 and S1).
| Variable | OS | SPM-free survival | SPMOS | ||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||
| Early-onset | No | Reference | < 0.001 | Reference | < 0.001 | NI | |
| Yes | 0.636 (0.580–0.696) | 0.698 (0.641–0.761) | |||||
| SPM | No | Reference | NI | NI | |||
| Yes | 0.793 (0.666–0.943) | 0.009 | |||||
| Sex | Female | NI | NI | Reference | |||
| Male | 1.473 (1.036–2.094) | 0.031 | |||||
| Location | Rectum | Reference | Reference | NI | |||
| Left colon | 0.857 (0.782–0.938) | < 0.001 | 0.876 (0.805–0.955) | 0.002 | |||
| Right colon | 1.035 (0.950–1.126) | 0.432 | 1.019 (0.941–1.105) | 0.642 | |||
| Neoadjuvant therapy | No | Reference | Reference | NI | |||
| Yes | 1.585 (1.412–1.779) | < 0.001 | 1.466 (1.310–1.640) | < 0.001 | |||
| Histological type | Adenocarcinoma | Reference | Reference | NI | |||
| Mucinous | 1.073 (0.960–1.199) | 0.214 | 1.057 (0.951–1.175) | 0.303 | |||
| Signet ring cell | 1.748 (1.439–2.125) | < 0.001 | 1.690 (1.395–2.046) | < 0.001 | |||
| Differentiation | Poor | Reference | Reference | NI | |||
| Moderate | 0.742 (0.684–0.805) | < 0.001 | 0.740 (0.685–0.799) | < 0.001 | |||
| Well | 0.829 (0.592–1.159) | 0.272 | 0.822 (0.623–1.083) | 0.164 | |||
| Vascular invasion | Negative | Reference | Reference | NI | |||
| Positive | 1.271 (1.173–1.378) | < 0.001 | 1.233 (1.141–1.332) | < 0.001 | |||
| Perineural invasion | Negative | Reference | Reference | NI | |||
| Positive | 1.372 (1.271–1.481) | < 0.001 | 1.341 (1.247–1.443) | < 0.001 | |||
| T stage | 0–2 | Reference | Reference | NI | |||
| 3 | 1.339 (1.176–1.525) | < 0.001 | 1.258 (1.119–1.413) | 0.001 | |||
| 4 | 1.680 (1.490–1.893) | < 0.001 | 1.393 (1.252–1.550) | < 0.001 | |||
| N stage | 0 | Reference | Reference | Reference | |||
| 1 | 1.350 (1.183–1.540) | < 0.001 | 1.484 (1.365–1.615) | < 0.001 | 1.286 (0.871–1.898) | 0.206 | |
| 2 | 1.718 (1.521–1.941) | < 0.001 | 2.166 (1.963–2.390) | < 0.001 | 2.416 (1.536–3.800) | < 0.001 | |
| M stage | 0 | Reference | Reference | NI | |||
| 1 | 4.347 (4.015–4.706) | < 0.001 | 3.776 (3.501–4.074) | < 0.001 | |||
| ND | 1.076 (1.061–1.093) | < 0.001 | 1.070 (1.053–1.085) | < 0.001 | 1.129 (0.999–1.276) | 0.053 | |
| Chemotherapy | No | Reference | Reference | NI | |||
| Yes | 0.757 (0.684–0.836) | < 0.001 | 0.798 (0.727–0.874) | ||||
| Radiotherapy | No | Reference | Reference | NI | |||
| Yes | 0.802 (0.701–0.915) | < 0.001 | 0.734 (0.646–0.831) | ||||
| Surgical procedures | Open | NI | NI | NI | |||
| Laparoscopic | |||||||
- CI, confidence interval; HR, hazard ratio; ND, tumor nodules without residual lymph node structure; OS, overall survival; SPM, second primary malignancy; SPMOS, second primary malignancy overall survival; NI, not included.
There was insufficient evidence to suggest that eoCRC was associated with better SPMOS. Notably, male gender (male vs female, HR: 1.473, 95% CI: 1.036–2.094, P = 0.031) and advanced N stage (N2 vs N0, HR: 2.416, 95% CI: 1.536–3.800, P < 0.001) were independently linked to a heightened risk of mortality following SPMs.
Discussion
Multiple retrospective studies have consistently shown a higher prevalence of advanced stages (stages III and IV) and more aggressive tumor characteristics in patients with eoCRC, which were the same characteristics as our patients. However, the analysis of its prognosis remains controversial.16, 29 It was important to note that these clinicopathological features were not fully manifested in patients with a subsequent SPM. Among them, eoCRC patients were more female and had more aggressive histological types. These patients may have specific genetic mutations due to genetic or environmental exposures that induce the development of more aggressive CRC and subsequent SPMs. Differences in treatment options may also play a role. A study demonstrated that surgeons in the USA chose more aggressive treatment regimens when treating patients with eoCRC. However, the patients did not benefit from these interventions.30 The choice of treatment regimen, such as chemotherapy and radiotherapy, can also affect the development of SPMs.31-34 Previous studies have indicated that individuals with eoCRC exhibited a heightened susceptibility to subsequent SPMs compared with the general population. Nevertheless, upon accounting for potential confounding factors, we observed that the risk of SPMs in eoCRC patients was merely 68.5% of that in loCRC patients, signifying a substantial disparity.
The laterality of CRC has been investigated in relation to both eoCRC and SPMs. Previous studies found that rectal cancer and left-sided colon cancer were more common in patients with eoCRC than in those with loCRC. However, this difference was not observed in our 15 489 patients. Studies conducted by Broman et al. and Liu et al. reported that the incidence of SPM following right-sided colon cancer was significantly higher than that following left-sided colon and rectal cancer.35, 36 Our findings were consistent with this, with right-sided colon cancer having the highest SPM risk and rectal cancer having the lowest SPM risk. Furthermore, right-sided CRC tended to have a worse prognosis.37, 38 In our study, after adjusting for confounding factors, left colon cancer had a 14.3% lower risk of death than rectal cancer, and no statistical difference in prognosis was observed between right colon cancer and rectal cancer.
Fewer ND and smaller tumor size are risk factors for SPM, possibly because patients with a better prognosis have a longer survival and a greater chance of developing subsequent SPM.
CRC accounts for the highest percentage of SPMs and occurs with the highest frequency, consistent with our findings.19, 20, 26, 36 A large study conducted in Taiwan showed that CRC was associated with an increased risk of uterine, ovarian, thyroid, kidney, bladder, breast, prostate, and lung cancer, while liver and biliary tract cancer were associated with a decreased risk.21 And this trend was evident in our results. Patients with eoCRC had significantly higher rates of subsequent colorectal, thyroid, and pelvic cancer and lower rates of lung and prostate cancer. There are several reasons for this phenomenon. First, eoCRC has a higher proportion of genetic syndromes,39-42 which increase the risk of second primary CRC and extraintestinal cancer (such as endometrial and thyroid cancer).43 Sporadic eoCRC also has specific genetic characteristics that may influence the development of SPMs.17 Second, from a therapeutic perspective, patients with eoCRC tend to be diagnosed as more advanced and receive more chemoradiotherapy as a choice of treatment. It has been suggested that radiotherapy reduces the overall risk of second primary cancer but induces an increased risk of second cancer in the irradiated area and adjacent organs.34 This also explains in part the increased proportion of second primary CRC and pelvic cancer. Finally, this difference is also correlated with demographic characteristics of eoCRC, with an increased proportion of cancers occurring in younger adults and in women.
OS was better in patients who subsequently developed SPM, especially in the first 5 years. This was mainly because patients with a better prognosis and long enough survival had a greater chance of developing SPM. After 5 years, the OS advantage gradually disappeared under the threat of SPMs.
The prognosis of patients with eoCRC versus those with loCRC remains controversial.29 However, a recent large cohort study conducted in the same region of China showed no significant differences in OS and disease-free survival between patients with eoCRC and loCRC.44 Our study found that the OS of eoCRC was better than that of loCRC, but this advantage could not be obviously reflected until 5 years later, so the prognostic significance was poor. In addition, the SPM-free survival of eoCRC was also better than that of loCRC. The 5-year post-SPMOS of eoCRC and loCRC was 76.0% and 66.2%, respectively, but there was no statistical difference. This difference may be due to younger eoCRCs having better physical status after surgery and chemotherapy.45 Only male and advanced N stages represented a worse SPMOS prognosis.
Our study had certain limitations. First, the patients included in the study were solely selected from the FUSCC database, leading to a potential selection bias when extrapolating the findings to the wider population in China. Second, the occurrence of SPMs was related to the duration of follow-up. As a result, some patients who developed SPM after the follow-up period were not included in the study, which might have affected the results to a certain extent. Finally, our database lacked some detailed information about family history and hereditary syndromes that influence the occurrence of SPMs.
Overall, the risk of eoCRC subsequently developing SPM was significantly lower than loCRC, and the SPMs of eoCRC were more likely to be CRC. OS and SPM-free survival of eoCRC were consistently better than loCRC. No difference in prognosis after SPM was observed between the two groups. Clarifying the variability in prognosis and clinical features might help in the screening and timely treatment of this high-risk group of patients.
Open Research
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.