New perspective on fecal microbiota transplantation in liver diseases
Declaration of conflict of interest: The authors have no potential conflicts of interest.
Abstract
Chronic liver disease including non-alcoholic fatty liver disease and alcohol-related liver disease is one of the most common diseases worldwide. The gut–liver axis plays an important role in the pathogenesis of liver disease. Small intestinal bacterial overgrowth, dysbiosis, leaky bowel, bacterial translocation, and imbalanced metabolites are related to the progression of chronic liver disease. Recently, novel therapeutic approaches for microbiota modulation such as personalized diet, probiotics, prebiotics, antibiotics, engineered microbiotas, phage therapy, stomach operation, and fecal microbiota transplantation (FMT) have been proposed with numerous promising results in the effectiveness and clinical application. Although the evidence is still lacking, FMT, a type of fecal bacteriotherapy, has been known as a candidate for the treatment of liver disease. This review article focuses on the most recent advances in our understanding of FMT in chronic liver disease such as non-alcoholic and alcohol-related liver disease.
Introduction
Chronic liver disease is one of the most common diseases worldwide and is a health-related disease with a high mortality rate of 2 million per year.1 Recent evidence suggests that the microbiota is associated with the progression and prognosis of chronic liver disease.2 The gut–liver axis is a widely used word containing a close interaction between the gut and liver through the portal vein. The gut microbiota metabolizes gut nutrients and its products are transported to the liver through the portal vein.3 Dysbiosis of the gut microbiome and therapeutic manipulation of the gut–liver axis have been investigated. Novel therapeutic approaches for microbiota modulation, such as multibiotics and fecal microbiota transplantation (FMT), have been proposed with numerous promising initial reports on the effectiveness and clinical applications of these approaches.4, 5
Trillions of microorganisms (bacteria, protozoa, archaea, fungi, and viruses) live in the human gastrointestinal tract, and the assemblage of living microorganisms in a defined environment is called microbiota.6 The human intestinal microbiota plays an important role in human health and diseases. When the balance between human and intestinal microbiota is disorganized, the composition and function of microbiota have been changed, which is referred to as dysbiosis.7 Strategies for the therapeutic modulation of intestinal microbiota are expected to contribute to the treatment of disorders associated with dysbiosis. Among these options, FMT achieves renown.8 The clinical response from FMT for different disorders provided evidence for human–microbiota interactions associated with human diseases, including inflammatory bowel disease, diabetes mellitus, cancer, gut–brain disease, and liver diseases.9 In particular, Clostridioides difficile infection demonstrates that intestinal microbiota can be modulated to procure therapeutic effects and FMT may become an important treatment option of a total therapeutic approach to effectively treat liver diseases.10 In this review, this report strongly recommends the application of FMT for the treatment of chronic liver diseases.
Chronic liver disease
Chronic liver disease refers to a group of diseases that includes fatty liver, hepatitis, fibrosis, and cirrhosis.11 The etiology of chronic liver disease includes virus, alcohol abuse, obesity, autoimmune diseases, toxins, and environmental and genetic factors.12 Chronic liver disease is an important public health problem worldwide and a frequent and common clinical condition. Recently, viral liver diseases have been the leading cause of chronic disease. Preventive medication for the hepatitis B and treatment of hepatitis C improved general condition of chronic liver disease. Non-alcoholic fatty liver disease (NAFLD) and alcohol-related liver disease (ALD) have been increasing because of increasing obesity rates and increasing alcohol consumption.11
Non-alcoholic fatty liver disease is defined as fat accumulation in the liver without consuming excessive alcohol drinking. NAFLD has gradual stages with typical characteristics. The first stage is steatosis, which is the deposition of fat in the liver. Steatosis progresses to non-alcoholic steatohepatitis (NASH), which reveals inflammation. Globally, NAFLD shows a 24% of prevalence rate, and approximately 59% of NAFLD patients have steatohepatitis in the liver.13 Dysbiosis, leaky gut syndrome, bile acid dysregulation, metabolite dysregulation, and endogenous ethanol production are relating factors related to the development of NAFLD.14 In addition, blood endotoxin biomarkers have also been implicated in NAFLD and endotoxemia is related with NAFLD.15, 16
Alcohol is the cause of cirrhosis-associated deaths in 30–50% of global mortality.17 Activation of Kupffer cells has been demonstrated to be a major factor in the progression of ALD.18, 19 Alcohol-induced small intestinal bacterial overgrowth and translocation to the portal vein are the pathophysiologies of ALD.20, 21 Endotoxins, such as lipopolysaccharide (LPS), are key elements of the inflammation in the gut–liver axis.22, 23 ALD shows similar histopathological findings compared with NAFLD although they are different etiologies.24
Chronic liver disease typically shows a gradual decrease in liver function such as abnormal findings of liver function tests, decreased albumin production, or coagulation factors. A pathologic process including inflammation, fibrogenesis, and regeneration of liver tissue leads to cirrhosis and carcinogenesis in chronic liver disease.25, 26
Liver fibrosis varies in the rate of progression depending on etiology, environment, and genetic factors.27 Onset of the pathophysiology of liver fibrosis occurs as a result of chronic liver injury. Inflammatory cells are recruited to the hepatic parenchyma, and some hepatocytes undergo programmed cell death. After this process, Kupffer cells and stellate cells are activated and proliferate in myofibroblasts. Extracellular matrix proteins such as collagen, glycoprotein, and proteoglycans are remodeled during this process.28 Disruption of the homeostasis between deposition and disruption of extracellular matrix proteins causes structural changes in the liver by the formation of fibrosis and cirrhosis.29 In addition, advanced liver fibrosis, METAVIR stage 3–4, is considered a reversible condition and is a major risk factor for carcinogenesis.
Gut–liver axis
Recent evidence regarding the positive roles of the gut microbiota has changed many perspectives on human diseases.30 The gut microbiome is maintained like an ecosystem, which consists of many millions of species that total approximately 1–2 kg in weight.31, 32 The gut microbiota has critical functions in immune reactions, hormonal responses, inflammatory pathways, and metabolites.33 As many factors are involved in homeostasis of the microbiome, research based on big data is necessary.
A close interaction between the gut and liver may be a major factor in the pathogenesis of liver injury (Fig. 1). The liver, which receives most of its blood and nutritional supply from the gut through the portal vein, is the first organ to be exposed to gut-derived toxic factors including bacteria, metabolites, damage-associated molecular patterns, or pathogen-associated molecular patterns.34 Microbiotas produce ammonia, endogenous ethanol, and acetaldehyde, which are related with the inflammatory response in the liver.35 Additionally, both dysbiosis, which refers to quantitative and qualitative changes in the intestinal bacteria, and bacterial overgrowth in the small intestine lead to an increase in intestinal permeability and the translocation of endotoxins to the portal tract; the latter activates signaling pathways of a wide variety of inflammatory cytokines in the liver.36
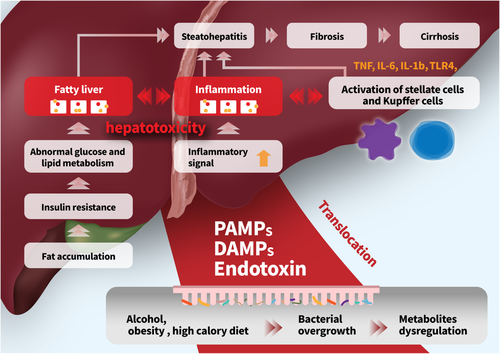
Under disease conditions, the composition and function of the microbiome are changed, which may cause small intestinal bacterial overgrowth, increasing pathogenic bacteria such as Escherichia and Streptococcus.37 The production of endotoxins, including LPS, lipoteichoic acid, peptidoglycan, porin, flagellin, or lipoprotein, is induced by pathogenic microbiotas. Dysbiosis may disrupt the gut barrier function, leaky bowel, and cause bacterial translocation. Pathogen-associated molecular patterns and LPS are recognized by Toll-like receptors (TLRs) in the liver and trigger the inflammatory pathway.
The microbiome is defined as the combination of microbiota and its genomic element with postbiotics and the host environment.38, 39 The composition and number of microbiotas vary according to their location in humans. In the stomach and duodenum, 10–103/gram of microbiotas are present, and 104–107 and 1011–1012/gram of microbiotas are found in small and large bowel, respectively.40 Most bacteria belong to two phyla, Firmicutes and Bacteroidetes, followed by others such as Proteobacteria, Actinobacteria, Fusobacteria, and Verrucomicrobia.41, 42 In the composition, obesity correlates with a change in the abundance of Bacteroidetes (50% reduction in obesity) and Firmicutes. In animal study, Firmicutes-to-Bacteroidetes ratio was increased in the Western diet group (47.1) compared with the control group (1).43 Each phylum consists of one or more classes that compose of orders that in turn encompass families, genera, and species.44 In addition, significant pathogens, such as Escherichia coli, Campylobacter jejuni, Salmonella enterica, Vibrio cholerae, and Bacteroides fragilis, exist in the human gut but normally at very low levels (less than 0.1%).45, 46 A high abundance of general genera, such as Bacteroides, Prevotella, and Ruminococcus, mean a healthy eubiosis state in the gut.47 Bajaj et al.48 suggested that cirrhosis dysbiosis ratio [(Lachnospiraceae + Ruminococcaceae + Clostridiales Incertae Sedis XIV + Veillonellaceae)/(Enterobacteriaceae + Bacteroidaceae)] is useful index to describe microbiome alteration. This ratio was highest in controls (2.05) followed by compensated (0.89) and decompensated (0.66) cirrhosis. In another study, salivary microbiota ratio [(Lachnospiraceae + Ruminococcaceae + Clostridiales Incertae Sedis XIV)/Streptococcaceae] was significantly lower (indicates dysbiosis) in patients with cirrhosis, compared with controls (2.0 ± 6.0 vs 0.4 ± 1.0).49
A regular composition of the common phylum including Bacteroidetes, Firmicutes, Actinobacteria, and Proteobacteria is crucial for the normal function of the gut–liver axis.50 Changes to a dysbiosis including loss of beneficial taxa, pathogenic overgrowth, loss of diversity, and changes in metabolites cause disease initiation or progression in human.51, 52 Many initiating factors such as oxidative stress, bacterial overgrowth, and the production of bacteria l endotoxins are associated with dysbiosis in the microbiome.
Intestinal tight junctions prevent the translocation of gut microbiota and its metabolites from the intestine to the liver. Intestinal mucin, tight junction proteins, antimicrobial proteins, and immunologic cells are major components of the gut barrier.53 The mucin layer is the first physical barrier preventing the humans from the toxic components. Antimicrobial proteins of the inner mucin layer kill harmful microbiota and hamper translocation of bacteria. The significance of sustaining a sterile inner mucus layer has recently been demonstrated. Regenerating islet-derived 3 gamma (REG3G), a c-type lectin produced by intestinal epithelial and Paneth cells, defends against pathogens.54 Increased intestinal permeability was reported in patients with chronic liver disease.55-57
Microbiotas secrete biological metabolites and postbiotics, which enter the human blood circulation and liver. Microbiota-induced metabolites induce various signaling pathways and activate disease progression. Microbiotas generate many metabolites, such as short-chain fatty acids (SCFAs), bile acids (BAs), amino acid-derived metabolites, and endogenous ethanol, which lead to metabolic processes and regulate the progression of chronic liver disease.2 Regarding role of nutrients, dietary fructose may affect the intestinal mucosal cells and in turn modify food absorption.58 In another study, 30% fructose diet increased hepatic steatosis in wild-type mice and decreased hepatic triglyceride accumulation in TLR4 mutant mice.59
Some gut microbiotas secrete endogenous ethanol.60 NASH patients and obese mice showed significantly elevated blood ethanol concentrations compared with the control groups because of ethanol-producing microbiotas.61 FMT into mice using feces with Klebsiella pneumoniae, an alcohol producing strain, isolated from patients with NASH, resulted in NAFLD development in recipient mice.62 Taken together, NAFLD and NASH might progress due to the alcohol producing microbiota in the gut.
Fecal microbiota transplantation in liver diseases
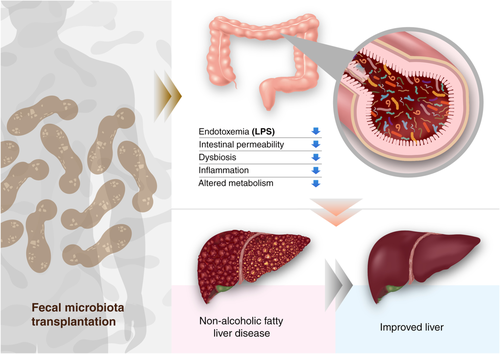
Fecal microbiota transplantation is a method to modulate the gut microbiome by fecal transplantation from healthy donors to patients with various diseases including gastrointestinal disease, infectious disease, or diabetes. Given the relationship between the microbiome and liver disease, FMT has been investigated to modify the dysbiosis of the intestine and treat chronic liver disease.
Most of the clinical studies conducted so far have shown that FMT is safe in patients with liver disease. However, because most of the studies enrolled a small number of patients, a study with a large number of patients is needed. In a previous study, donor screening is important in FMT to limit the transmission of pathogens.63 Drug-resistant E. coli was transmitted to two patients who underwent FMT and one patient died after FMT due to bacteremia.
Fecal microbiota transplantation in non-alcoholic fatty liver disease
Non-alcoholic fatty liver disease is a well-known disease of the liver in adults and children. Histopathologic manifestations associated with NAFLD include storage of lipids in the liver and inflammation in NASH. NAFLD is described as a multifactorial complication due to genetic susceptibility, metabolic malfunction, inflammation, environmental factors, and disturbed intestinal microbiota.64
The intestinal microbiota can influence energy production, homeostasis, and the development of obesity and metabolic syndromes.65 Clinical and experimental evidence supports the importance of the composition of intestinal microbiota for the development of obesity and demonstrates that the intestinal microbiota population can determine important characteristics of metabolic pathways that play an important role in obesity.66, 67 The microbiota can regulate the release of fasting-induced adipose factor from intestinal epithelial cells. FIAF can act as a lipoprotein lipase inhibitor and regulate the storage of peripheral fat.68 Metabolites such as SCFAs can bind to conjugate receptors of G protein on mucosal epithelial cells to regulate energy from intestinal peptide hormones and regulate inflammatory response.69 The gut microbiota of obese patients might present idiosyncrasy, which may induce chronic inflammation.70 Intestinal dysbiosis can cause leaky intestine, resulting in the initiation of inflammatory processes in the liver. Disruption of the gut barrier can enhance translocation of intestinal microbes into the intestinal wall, which can induce microbial metabolites (butyrate, SCFA, or endotoxins) through the bloodstream and exude into the liver.71 The microbiota and its metabolites can also contribute to the development of NAFLD. Increasing intestinal permeability can stimulate monosaccharide absorption from the gut lumen, leading to microbiota translocation. Endotoxins can penetrate the portal vein, promote de novo fatty acid synthesis and triglyceride production, and activate inflammatory TLRs in hepatocytes.72, 73 The production of nuclear factor-κB and tumor necrosis factor-α is activated by LPS derived from gram-negative bacteria in the intestinal microbiota. This supports that intestinal microbiota can increase live exposure to endotoxin as an important factor of the progression of NASH.74, 75
Recent clinical studies have shown a beneficial effect of FMT from healthy donors by extenuating metabolic syndrome and increasing insulin sensitivity, intestinal microbiota diversity, and production of SCFAs after FMT.76 In another study, FMT in mice with NASH can significantly decreased body weight, body fat content, and serum aminotransferases. FMT can recover the diversity of the gut, increase the amount of Bacteroidetes, decrease the number of Actinobacteria and Firmicutes, promote butyrate production, and improve tight junction function and endotoxemia.77 A recent randomized clinical trial showed that FMT did not improve insulin resistance as measured by HOMA-IR or hepatic PDFF. However, it did have the potential to reduce small intestinal permeability in NAFLD.78 Although FMT has logistical challenges, several infection risks, and unexpected microbiota-associated diseases, FMT allows us to study the pathophysiology of NALFD and gives us a therapeutic option to treat diseases (Fig. 2).
A clinical study for patients with metabolic syndrome revealed that FMT from lean donors improved insulin sensitivity.79 Improved insulin sensitivity is related to butyrate-producing strains such as Roseburia intestinalis and Eubacterium hallii. In another study, improved peripheral insulin sensitivity and hemoglobin markers were transient and reverted to the initial state at 18 weeks. In accordance with markers, the composition of microbiota at 18 weeks after FMT followed the composition at baseline.80 Microbiota composition is correlated with metabolites including γ-aminobutyric acid, and improved insulin sensitivity after FMT depends on the diversity at baseline. FMT from donors who underwent gastric bypass operation to subjects with metabolic syndrome improved insulin sensitivity, energy expenditure, and colon transit time compared with controls.81 FMT with capsules for obesity with obesity over 6 weeks recovered the composition of the microbiome. However, comparison of metabolic effects did not show significant improvement (Table 1).85
| Conditions | Treatment | Main results | Ref |
|---|---|---|---|
| NAFLD patients | 15 allogenic or 6 autologous FMTs | Allogenic FMT patients with elevated small intestinal permeability at baseline had a significant reduction. | 71 |
| Metabolic syndrome | 9 allogenic FMTs from a thin donor and 9 autologous FMTs | Increase in insulin sensitivity in the allogenic transplant group. | 69 |
| Obese patients with steatohepatitis | Lean vegan donor, 10 allogenic FMTs or 11 autologous FMTs | Allogenic FMT using lean vegan donor: improved liver histology, expression of hepatic genes, microbial community, and plasma metabolites. | 82 |
| NAFLD versus healthy control | FMT from NAFLD patient and healthy individual to two groups of mice | Mice received FMT from NAFLD, gained more weight, and had a higher liver triglycerides level and higher plasma cholesterol than mice that received the human healthy microbiota. | 83 |
| Metabolic syndrome | Single allogenic FMT via nasojejunal tube from lean donor versus autologous FMT | No metabolic changes at 18 weeks after FMT, insulin sensitivity at 6 weeks after allogenic FMT was significantly improved. | 73 |
| Metabolic syndrome | Single allogenic FMT via nasojejunal tube from lean donor versus autologous FMT | Significantly improved peripheral insulin sensitivity 6 weeks after FMT. | 72 |
| Obese subjects without diabetes, NASH, or metabolic syndrome | Randomized, double-blind study, FMT by capsules from a lean donor or placebo capsules. | No significant changes in BMI at 12-week follow-up. | 84 |
| Obese subjects with mild to moderate insulin resistance | Randomized, double-blind study, weekly FMT by capsules versus placebo capsules for 6 weeks | No significant differences between groups for insulin sensitivity, body mass index, and fat mass. | 75 |
| Obese patients with metabolic syndrome who received FMT from post-Roux-en-Y gastric bypass donors versus obese patients with metabolic syndrome who received FMT from metabolic syndrome donors | FMT via nasoduodenal tube after completion of a clean out with polyethylene glycol solution | FMT from post-Roux-en-Y gastric bypass donors resulted in better insulin sensitivity, energy expenditure, and intestinal transit time in patients with metabolic syndrome 2 weeks after FMT compared with recipients of FMT from metabolic syndrome donors without gastric bypass. | 74 |
- BMI, body mass index; FMT, fecal microbiota transplantation; NAFLD, non-alcoholic fatty liver disease.
Fecal microbiota transplantation in alcohol-related liver disease
Alcohol-related liver disease is caused by chronic alcohol drinking, and the prevalence of ALD is 4.5% worldwide. In the case of severe alcoholic hepatitis, the 1-month mortality rate is approximately 35%. ALD is preventable and reversible by timely treatment. However, ALD is often asymptomatic in the early stages and can only be identified by laboratory findings. Abstinence is the most important therapeutic intervention for patients with ALD. Gut-derived microbial LPS, a component of the outer wall of gram-negative bacteria, has been known to play a central role in the pathogenesis of ALD.29, 86 Therefore, probiotics, prebiotics, antibiotics, or FMT have been suggested as therapies for ALD by modulating the gut–liver axis or repopulating the gut.
Sensitivity to ALD can be changed by gut microbiota in an alcohol-fed mouse model.87 Germ-free mice transplanted with fecal microbiota from humans with alcoholic hepatitis showed inflammatory changes in liver pathology after ethanol feeding. Susceptibility to ALD can be driven by intestinal microbiota, and ALD can be controlled by modulating the microbiome.
In clinical studies (Table 2), daily FMT from a control donor to patients with steroid-resistant severe alcoholic hepatitis resulted in significant survival improvement (87.5% vs 33.3%).89 These changes were associated with reduced proportions of some pathogenic microbiotas, including K. pneumoniae, and increased proportions of known good species, including Enterococcus villorum (9% vs 23%), Bifidobacterium longum (6% vs 50%), and Megasphaera elsdenii (10% vs 60%). Metabolic changes were typical in methane metabolism, bacterial invasion of epithelial cells, bile secretion, carotenoid biosynthesis, and pantothenate biosynthesis pathways. In another study, FMT from alcoholic patients with pectin to an ALD mouse model was performed. Tryptophan metabolites were associated with an improvement in liver injury, and an aryl hydrocarbon receptor (AhR) agonist reduced liver lesions. Inactivation of the AhR gene in alcohol-fed AhR-knockout mice abrogated the beneficial effects of the prebiotic.82 A study for alcohol use disorder and liver cirrhosis demonstrated that craving is reduced in 90% of FMT versus 30% in placebo at day 15 with lower urinary ethyl glucuronide/creatinine and improved cognition and psychosocial quality of life (Table 2).83
| Conditions | Treatment | Main results | Ref |
|---|---|---|---|
| Alcohol use disorder and liver cirrhosis | FMT (n = 20), placebo (n = 20) enema from a donor enriched in Lachnospiraceae and Ruminococcaceae | Craving is reduced in 90% of FMT versus 30% in placebo at day 15 with lower urinary ethyl glucuronide/creatinine and improved cognition and psychosocial quality of life. | 80 |
| ALD | FMT from alcoholic patients with pectin to ALD mice model |
Tryptophan metabolite was associated with an improvement of liver injury. AhR agonist reduced liver lesions. Inactivation of the AhR gene in alcohol-fed AhR-knockout mice abrogated the beneficial effects of the prebiotic. |
79 |
| 51 patients with severe alcoholic hepatitis | Corticosteroids (n = 8), nutritional support (n = 17), pentoxifylline (n = 10), and FMT (n = 16) for 7 days | Proportions of patients surviving at the end of 3 months in the steroids, nutrition, pentoxifylline, and FMT group were 38%, 29%, 30%, and 75%, respectively. | 88 |
| 8 severe AH patients, 18 historical controls with severe AH, both groups steroid ineligible | Daily FMT via nasojejunal tube for 7 days | 87.5% of patients who received FMT survived for 1 year, compared with 33.5% of matched controls. | 78 |
- AH, alcoholic hepatitis; AhR, aryl hydrocarbon receptor; ALD, alcoholic liver disease; FMT, fecal microbiota transplantation.
Fecal microbiota transplantation in cirrhosis
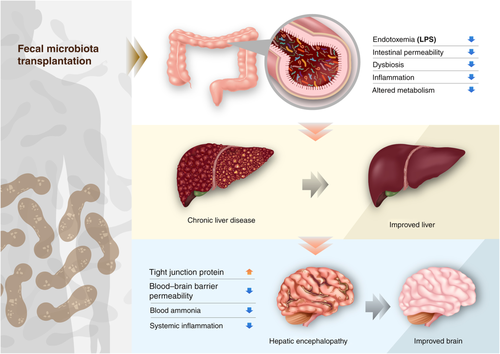
Recently, the effect of FMT in cirrhosis has been mainly focused on studies of encephalopathy (Table 3). In a randomized clinical trial, frozen enema FMT was performed on cirrhosis patients with recurrent hepatic encephalopathy. FMT with enema reduced the hospitalization rate and improved cognitive function at 15 days after FMT. FMT from a rational stool donor improves hepatic encephalopathy.84 However, FMT did not improve the mean model of end-stage liver disease (MELD) score, aspartate aminotransferase, alanine aminotransferase, or albumin at 30 days after transplantation. In a previous study, 10 cirrhosis patients with recurrent hepatic encephalopathy received FMT by colonoscopy from screened donors, and 6 patients revealed sustained responses after FMT.88 Antibiotics were used before the FMT, and FMT decreased the level of ammonia, Child–Pugh score, and MELD score at 20 weeks after FMT. In another FMT study with oral capsules, capsule FMT improved duodenal mucosal diversity, dysbiosis, antimicrobial peptide expression, LPS-binding protein, and cognition.90 In a next clinical trial with cirrhosis patients with encephalopathy.91 FMT significantly reduced inflammatory markers including serum interleukin-6 and improved cognitive function. The abundances of Ruminococcaceae, Verrucomicrobiaceae, and Lachnospiraceae were closely related to the improvement.
| Conditions | Treatment | Main results | Ref |
|---|---|---|---|
| Alcohol use disorder and liver cirrhosis | FMT (n = 20), placebo (n = 20) enema from a donor enriched in Lachnospiraceae and Ruminococcaceae | Craving is reduced in 90% of FMT versus 30% in placebo at day 15 with lower urinary ethyl glucuronide/creatinine and improved cognition and psychosocial quality of life. | 80 |
| Cirrhotic patients with MELD < 17 and recurrent encephalopathy | Single FMT enema following antibiotic treatment (n = 20) | FMT well tolerated, significantly less patients receiving FMT developed encephalopathy. FMT increased microbial diversity and increased beneficial bacterial taxa. | 86 |
| 10 male patients with liver cirrhosis and recurrent HE received FMT versus 10 matched patients who received standard of care | An enema of 90-mL FMT from a healthy volunteer from stool bank was performed after 5 days of broad-spectrum antibiotics (metronidazole, ciprofloxacin, and amoxicillin). Placebo group was not pretreated with antibiotics | FMT reduced hospitalizations secondary to encephalopathy over the observation period of 150 days and improved cognition in these patients 15 days after FMT. It did not change the MELD score, liver enzymes, or albumin levels 35 days after transplantation. | 81 |
| Retrospective study with liver cirrhosis and recurrent encephalopathy | FMT via colonoscopy 48 h after completion of 5 days of broad-spectrum antibiotics. At time of procedure, the patients were also started on 5 days of rifaximin and lactulose. Patients also received 1 dose of loperamide pre-procedure and 1 dose post-procedure to retain stool | 6 patients had sustained clinical response at 20 weeks post-treatment. This was associated significantly lower ammonia concentration, Child–Turcotte–Pugh score (9.5 to 8), and MELD score (18 to 15) at 20 weeks after FMT; one patient died within 2 months after the procedure. | 85 |
| Liver cirrhosis and recurrent encephalopathy with MELD < 17 received FMT versus 10 matched patients who received standard of care | 15 oral FMT capsules (4.125 g) versus placebo from a single donor enriched in Lachnospiraceae and Ruminococcaceae | FMT resulted in reduced serum IL-6 and LPS-binding protein and improved cognition at 4 weeks after FMT. FMT-assigned participants demonstrated higher deconjugation and secondary bile acid formation in feces and serum compared with baseline. | 87 |
- FMT, fecal microbiota transplantation; IL, interleukin; MELD, model for end-stage liver disease.
Regarding efficacy of FMT in patients with cirrhosis and ascites, previous case series reported that 20% (2/10) patients were re-admitted due to bacterial peritonitis after FMT.88 In another study, ascites was present in 17 patients (85%) and FMT did not cause more infection compared with control group.84 Therefore, comparative study with many patients is needed for the evaluation of FMT effects in patients with ascites.
Future perspective of fecal microbiota transplantation
Fecal microbiota transplantation consists of the infusion of feces from a healthy donor to a patient to treat a specific disease associated with alteration of intestinal microbiota. Many clinical studies have confirmed that FMT is an effective treatment for C. difficile infection. It has been accepted as a therapeutic option in many human diseases including liver diseases. Interest in FMT is increasing. Currently, a total of 34 FMT studies related to liver disease have been registered on ClinicalTrials.gov, and 7 trials are recruiting patients with liver disease. Further studies are expected in the future.
Clinicians are aware of its therapeutic importance. However, there is no clear protocol for the preparation, pretreatment, administration, or long-term stability of FMT. The exact mechanism involved in its effect has also not been elucidated. Thus, further studies on FMT are needed in the future. Because the definition of healthy feces is unclear, it remains controversial whether a stool without specific microorganisms is considered healthy and whether a higher proportion of some microorganisms is better. Additionally, determining the importance based on the number of microorganisms alone should be avoided, as small amounts of microbes in feces can also play a key role. Substances such as SCFAs produced by microorganisms in feces are also important. Each stool bank has different standards. Currently, stool donation is possible if there are no abnormalities in blood or stool tests. However, in the future, detailed screening tests are needed in this area. Studies such as customized stool transplantation that can select the most suitable healthy stool for a patient are needed rather than simply measuring the abundance of microorganisms. Recently, a method in which a suspension for transplantation is prepared from feces from healthy donors and then immediately frozen and administered to patients while preserving the intestinal microbial environment has been described. When defining a healthy stool, minimal side effects and maximal therapeutic effects should be considered by separating and administering only necessary microorganisms or active substances. Efforts are now being made to isolate specific microorganisms from feces and administer them to patients under the name of human microbiota transplant rather than FMT. However, many studies are needed as it is still controversial which part of the stool is effective.
In the future, for inflammatory bowel disease and hepatic encephalopathy in addition to C. difficile infection, if people occasionally store their feces once a while for a certain period, analyze them in reverse when a disease occurs, and perform autologous fecal transplantation, it will have the effect of returning to the pre-disease state. Although many studies are needed in the future on extraction methods, storage, administration, and later stability, the results of studies performed thus far indicate that FMT could be defined as a new treatment method in the future. Diseases that have not been properly treated with antibiotics or various drugs thus far can be overcome by FMT. Thus, the future of FMT is expected to be positive.
Conclusion
The relationship between the human intestinal microbiota and liver provides a new potential and promising therapeutic methods in chronic liver diseases. In particular, FMT appears to be the most promising and efficacious treatment for many liver diseases, including NAFLD, NASH, ALD, and hepatic encephalopathy (Fig. 3). However, the number of randomized clinical studies using FMT in chronic liver disease remains limited. Further investigations into the safety and efficacy of FMT in chronic diseases are needed.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2018M3A9F3020956, NRF-2019R1I1A3A01060447, and NRF-2020R1A6A1A03043026).




