Early events in hepatitis B virus infection: From the cell surface to the nucleus
Abstract
While most adults are able to clear acute hepatitis B virus (HBV) infection, chronic HBV infection is recalcitrant to current therapy because of the persistence of covalently closed circular DNA in the nucleus. Complete clearance of the virus in these patients is rare, and long-term therapy with interferon and/or nucleoside analogues may be required in an attempt to suppress viral replication and prevent progressive liver damage. The difficulty of establishing HBV infection in cell culture and experimental organisms has hindered efforts to elucidate details of the HBV life cycle, but it has also revealed the importance of the cellular microenvironment required for HBV binding and entry. Recent studies have demonstrated an essential role of sodium–taurocholate cotransporting polypeptide as a functional receptor in HBV infection, which has facilitated the development of novel infection systems and opened the way for more detailed understanding of the early steps of HBV infection as well as a potential new therapeutic target. However, many gaps remain in understanding of how HBV recognizes and attaches to hepatocytes prior to binding to sodium–taurocholate cotransporting polypeptide, as well as events that are triggered after binding, including entry into the cell, intracellular transport, and passage through the nuclear pore complex. This review summarizes current knowledge of the initial stages of HBV infection leading to the establishment of covalently closed circular DNA in the nucleus.
Introduction
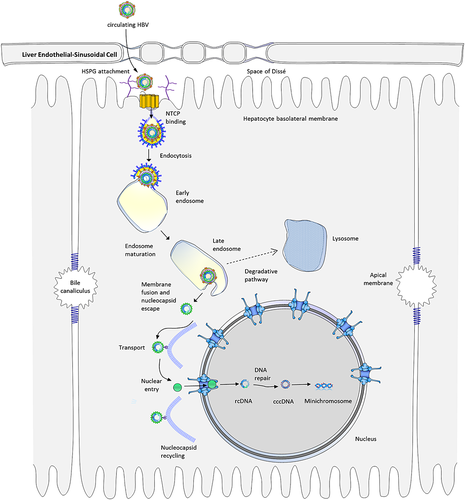
The liver is a multifunctional organ responsible for metabolizing carbohydrates, lipids, and proteins as well as clearing pathogens and toxins and modulating the immune response. Because of its central role in portal circulation and direct exposure to material absorbed from the intestine, the liver is relatively tolerant to self and foreign antigens.1 As a result, the liver is a favored target of many viral pathogens, including hepatitis A, B, C, D, and E viruses. Of these, chronic hepatitis B virus (HBV) infection is the most difficult to treat and poses the greatest long-term public health challenge. This review discusses recent highlights on the molecular mechanism of the journey of HBV from its initial attachment on the cell surface to entry into the nucleus (Fig. 1). As the role of host factors in HBV infection are elucidated (Table 1), novel therapeutic approaches await discovery, but many gaps remain in understanding the details of this process. Current therapies act late in the HBV life cycle, but drugs that target viral entry prior to the formation of covalently closed circular DNA (cccDNA) (Table 2) may be effective as post-exposure prophylaxis as well as to prevent reinfection following liver transplantation.2 As some fraction of newly synthesized capsids re-enter the nucleus during viral replication to replenish the nuclear cccDNA,3 it might also be possible to treat chronic infection and reduce drug resistance by preventing re-entry of capsids into the nucleus.
| Phase | Host factors | Function | Virus-interacting domain |
|---|---|---|---|
| First contact | HSPG | Binds to protein ligands and regulates biological activity. Serves as primary receptor for a variety of viruses. | HBsAg, particularly pre-S1 epitope |
| Receptor binding | NTCP | Responsible for Na+-dependent transport of conjugated bile salts. Functionally mediates HBV and HDV entry into hepatocytes. | Pre-S1 epitope |
| Endocytosis | Clathrin | Plays a major role in cellular vesicles formation. Forms complexes together with the clathrin adaptor protein AP-2 and LHBsAg. | Large HBsAg |
| Endosome trafficking | Rab5, Rab7 | Direct vesicles from the plasma membrane to early endosomes, then to late endosomes and lysosomes. Assumed to be necessary for HBV particle trafficking. | HBsAg |
| Intracellular transport | Tubulin | Maintains the structure of the cell and provides a platform for intracellular transport. Allows efficient shuttling of HBV cccDNA to the nucleus. | Capsid |
| Nuclear import | Importin-β | A type of karyopherin. Transports the HBV genome into the nucleus by binding to a NLS in the C terminus of the core protein. | Capsid |
| Inhibitor | Target | Function |
|---|---|---|
| Heparin | HSPG | Similar structure to HSPG. Competitively binds to the large HBV S antigen. Blocks initial HBV attachment to HSPG. |
| Myrcludex-B | NTCP | A synthetic lipopeptide derived from the HBV preS1 domain, which specifically targets NTCP. Blocks HBV entry both in vitro and in vivo. |
| Cyclosporin A | NTCP | A cyclic peptide consisting of eleven amino acids. Binds to NTCP directly, overlapping HBV pre-S1 domain binding site. |
| Chlorpromazine | Clathrin | A cationic amphipathic drug. Inhibits clathrin-mediated endocytosis while HBV entry. |
| Nocodazole | Tubulin | Depolymerizes microtubules. Prevents HBV nucleocapsids from reaching the nucleus. |
| Importazole | Importin-β | A 2,4-diaminoquinazoline. Interferes with the binding of RanGTP and importin-β. Might block importin-β-mediated nuclear import of the HBV genome. |
First contact: heparan sulfate proteoglycans
HBV is highly species-specific, infecting only humans and some non-human primates.4 The virus is also highly hepatotropic and replicates mainly or exclusively in hepatocytes. One reason for this high specificity is that HBV recognizes distinctive features characteristic of the hepatocyte cell surface, including highly sulfated heparan sulfate proteoglycans (HSPGs).5, 6 HSPGs, which help to confer tissue specificity, are formed from several heparan sulfate glycosaminoglycan chains and are abundantly expressed on the surface of cells and in the extracellular matrix.7 Several viruses, including Herpes simplex virus-1, HIV, cytomegalovirus, and Dengue virus, interact with heparan sulfate during viral entry,7, 8 and HBV attachment to HSPGs has been implicated in HBV entry into hepatocytes.5, 6 HSPGs on the hepatocyte surface are more highly sulfated than those on endothelial and dermal cells,9 and electrostatic interactions with two highly conserved positively charged residues, R122 and K141, in the antigenic loop region of the HBsAg have been shown to precede pre-S1 binding to hepatocytes.10 HSPG interaction can be disrupted using a highly sulfated competitive inhibitor such as heparin, but not with the less sulfated chondroitin sulfate, and HBV attachment can be prevented by pretreatment with heparinase.6 After the virus binds to the membrane, however, the disruptive effect of heparin is lost, suggesting that the virus forms irreversible, higher affinity attachment to one or more surface receptors. Although HSPG interacts only transiently with the antigenic loops of individual proteins, clustering of envelope proteins in the viral membrane is thought to stabilize the interaction and facilitate binding of the virus to its primary receptor.10
Post-attachment conformational changes
In other enveloped viruses such as HIV and HSV-1, interaction with surface receptors initiates a fusion reaction that may activate or enhance expression of both cellular and viral genes.7, 8 Attachment of HBV to HSPGs might trigger an immune-related or stress-related response in the host cell that may be exploited for viral entry. Hepatitis C virus has been reported to hijack the PI3K pathway for entry and persistence in hepatocytes,11 although no report has conclusively demonstrated this mechanism in HBV. Another possibility is that transient interaction via HSPGs facilitates irreversible conformational changes via interaction with accessory molecules.
High-affinity binding: the NTCP receptor
HBV encodes three envelope proteins that are essential for the attachment and entry of the virus into host cells, the small, middle, and large surface proteins. Of these, only the large hepatitis B surface antigen harbors the pre-S1 domain, which contains a critical region between amino acids 2 and 47 that has long been implicated in specific hepatocyte binding.12 While the importance of this region in HBV binding was recognized early on, the corresponding receptor molecule on the hepatocyte membrane has long been a mystery, and many candidate molecules have been proposed, including asiaglycoprotein receptor, transferrin receptor, and IL-6 receptor (reviewed in Glebe and Urban13 and Rehman et al.14). A recent study also proposed ferritin light chain and squamous cell carcinoma antigen 1 as co-receptors for HBV entry.15 However, conclusive evidence in support of any of these molecules as the primary HBV receptor has remained lacking.
Finally in 2012, Yan et al.16 identified a promising receptor candidate for both HBV as well as HDV entry into primary hepatocytes of human and tree shrew (Tupaia belangeri). They used near-zero-distance photo-cross-linking and tandem affinity purification to isolate a single protein, which was later identified by mass spectrometry as the sodium–taurocholate cotransporting polypeptide (NTCP or SLC10A1).16 This finding was independently confirmed in a study by Ni et al., who examined differences in gene expression between differentiated and undifferentiated HepaRG cells and tested HBV binding against hairpin RNA-silenced candidate targets.17 They demonstrated that knockdown of NTCP prevented infection by HBV and HDV and showed that HepG2 and Huh7 cells gained the ability to bind HBV when human but not mouse NTCP was expressed. Interestingly, they identified two essential NTCP sequence motifs, aa 157-163,16 which is required for binding, and aa 84-87,18 which is required for HBV infection. The mouse NTCP, which is resistant to HBV infection, lacks the aa 84-87 motif.17, 19 Mouse NTCP gained the ability not only to bind to the preS1 region of HBV but also to support infection after key amino acids in the mouse NTCP were altered to match the human sequence.20 Similarly, short peptides with comparable properties are able to interact with NTCP, and a synthetic N-terminal myristoylated peptide sharing the same sequence is capable of blocking HBV and HDV infection.2 Because of their ability to act as inhibitors for HBV entry into hepatocytes by targeting NTCP, several molecules, including Myrcludex-B and cyclosporin A, may offer alternative therapeutic options for HBV infection.2, 17, 20, 21 Genetic differences affecting the amino acid sequence or expression levels of NTCP may also influence the risk of HBV infection. A recent report by Su et al.22 outlined the importance of two single nucleotide polymorphisms in the NTCP locus associated with the outcome of HBV infection in the Chinese Han population. One of the single nucleotide polymorphisms was associated with HBV clearance (rs7154439 AA), while the other was associated with risk of HBV-related hepatocellular carcinoma (rs4646287 AA).
NTCP secondary or tertiary structure may also play a role in susceptibility to HBV infection.23 A study using NTCP-expressing U20S cells suggested that NTCP may exist as a dimeric or heteromeric complex with other SLC10A family proteins (particularly SLC10A4 and SLC10A6) at the plasma membrane,24 although such interactions have not been confirmed to play a role in HBV infection in humans.
NTCP is an integral membrane glycoprotein responsible for most of the sodium-dependent import of bile salts reclaimed by the small intestine.25, 26 Because NTCP is expressed only on the basolateral membrane, NTCP localization depends on the unusual cell polarity observed in mature hepatocytes.27 Hepatocyte differentiation induced by treatment with dimethylsulfide (DMSO) has long been known to be facilitate HBV infection in cell lines,28 and treatment with DMSO has been shown to improve binding affinity of NTCP with pre-S1.29 The mechanism by which NTCP facilitates HBV entry is unclear, but binding of either HBV or Myrcludex-B to NTCP inhibits the normal activity of NTCP in importing bile salts.17 NTCP expression is highly regulated in order to maintain intracellular bile salt levels,26 and Oehler et al. found that HBV infection and Myrcludex-B were both able to promote compensatory changes in bile acid synthesis and cholesterol metabolism in chimeric mice.30 HBV infection resulted in 12-fold up-regulation of cholesterol 7α-hydroxylase (CYP7A1), a key enzyme required in the conversion of cholesterol to bile acids. NTCP expression is also strongly down-regulated by the pro-inflammatory cytokine IL-6.31 Interestingly, release of IL-6 by Kupffer cells has previously been shown to play an important role in suppression of HBV replication in the early stages of infection.32
The historical difficulties in establishing HBV cell culture infection systems are likely to be related to lack of sufficient NTCP expression or accessibility in candidate cell lines. Only freshly isolated primary human hepatocytes are able to support HBV infection, which may be because of a sharp decline in NTCP expression after culturing.33 Similarly, disruption of the epithelial barrier of HepaRG cells increased the rate of HBV infection, possibly by allowing greater access to the basolateral membrane.27 The study by Yan et al.16 has stimulated interest among many researchers in establishing a robust in vitro infection system for HBV by assessing the susceptibility of NTCP-expressing cells to allow effective attachment and entry of HBV (reviewed in Watashi et al.20). For example, several cell lines were transfected with the human NTCP gene (e.g. hNTCP-HepaRG, hNTCP-Huh, hNTCP-HepG2, and hNTCP-HEK293) and have become valuable tools for in vitro studies of HBV entry into the cell.16, 29
However, in vivo studies involving the role of NTCP in HBV infection suggest that this receptor may be necessary but not sufficient for robust HBV infection. Ectopic expression of NTCP in Huh7 and HepG2 cells alone induces HBV infection only at a low level, whereas administration of DMSO drastically improved the infection rate.17 This suggests that NTCP is not solely responsible for HBV entry.
Into the cell
Bound to the hepatocyte membrane, the virus must then gain entry into the cell by inducing endocytosis, although the fine details of the intracellular trafficking events remain unclear.
Caveolae-mediated endocytosis
Cellular factors involved in endocytosis such as caveolin-1, clathrin heavy chain (CHC), and clathrin adaptor protein AP-2 play a role in HBV trafficking.34, 35 In the HepaRG cell line, infection with HBV was shown to depend on caveolae-mediated endocytosis. However, the cell line is permissive for HBV infection only after a differentiation process induced by treatment with DMSO and hydrocortisone.28 Furthermore, treatment of the original HepaRG cell line with inhibitors of caveolae did not significantly impair infection, consistent with an earlier report using primary tupaia hepatocytes.35
Clathrin-mediated endocytosis
Two studies reported clathrin-mediated endocytosis (CME) as a primary route of entry for HBV.34, 36 Using immortalized human primary hepatocytes, Huang et al. demonstrated that the pre-S1 domain of the large HBsAg specifically interacts with CHC and the CME adapter AP-2. Using chlorpromazine, an inhibitor of CME, and by silencing of CHC or AP-2, the susceptibility of cells to HBV was significantly reduced.34 Because the cell line used is phenotypically similar to hepatocytes, HBV may preferentially enlist CME to access the cell interior.
Endosome trafficking
After CME, subsequent translocation of the cargo along the endocytic pathway is regulated by Rab proteins.37 Rab proteins are small guanosine triphosphatases of the Ras superfamily that occupy specific endocytic compartments38 and direct endocytic vesicles to different cellular compartments.39 Rab5 is responsible for movement from plasma membrane to early endosomes,40 Rab7 to late endosomes and lysosomes,41 and Rab9 to the trans-Golgi network.42 Rab11 seems to be involved in vesicle recycling.43 Silencing of either Rab5 or Rab7 expression resulted in significant inhibition of the early stages of HBV infection, while Rab9 and Rab11 had no effect.44 These observations imply that translocation of the virus to late endosomes is critical for a successful infection and probably a prerequisite for dissociation of the enclosed nucleocapsid.36
Membrane potential-dependent escape from endosomes
Most enveloped viruses that use endocytosis for entry into their host cells rely on pH-dependent processing of endocytic vesicles.45 After the early endosome stage, translocation is associated with a gradually decreasing pH from about 6.2 in early endosomes to closer to 5.5 in late endosomes that aids fusion of the viral envelope with the endosomal membrane.46 However, in the case of duck hepatitis B virus (DHBV), pharmacological agents that raise the pH in the endocytic pathway did not affect infection.47, 48 While the vacuolar proton-ATPase acidifies the endosomal compartment,48 vacuolar proton-ATPase not only lowers the vesicular pH but also generates an internal positive electrical potential across the membrane,49 suggesting that membrane potential-dependent, but pH-independent, sorting and transportation of the virus within and beyond the early endosome is necessary for hepadnavirus infection to be established.48
Endo-lysosomal pathway
In principle, endocytosis is a high-risk strategy for viruses because late endosomes ultimately fuse with lysosomes, representing a dead-end for most viruses.45 In the case of HIV-1, infection was significantly augmented by raising the pH of endosomes and lysosomes, indicating that the virus is able to escape from lysosomes when rescued from degradation.50 In the case of HBV, however, while a substantial amount of viral particles were traced to late endosomes and endo-lysosomes after infection, inhibition of lysosomal activity by increasing pH or by treatment with protease inhibitors had no effect on infection.44 An earlier report also showed that internalized HBV core particles are transported to lysosomes and further disassembled as a result of endo-proteolytic cleavage within the arginine-rich domain of the core polypeptide by cysteine proteases.36 Therefore, HBV transport into the degradative branch of the endocytic pathway does not seem to be required for infection, and no evidence for a role of this compartment in promoting HBV infection has been demonstrated.
In addition to electrostatic changes resulting from establishment of the proton gradient, the composition of host-independent cholesterol in the viral envelope has been shown to play a role in facilitating escape of DHBV capsids from endosomes,51 but how DHBV nucleocapsids trigger membrane fusion and what happens after endosomal escape has not been well studied. A cell-permeable peptide translocation motif (TLM) identified within the surface protein of DHBV appears to be a general feature of hepadnaviruses,52 and processing of the TLM by endosomal proteases may facilitate viral particle translocation through the endosomal membrane. DHBV TLM mutant viruses were shown to bind to cells and enter the endosomes but were not able to escape,52 suggesting that release of nucleocapsids from endosomes was blocked in these mutants. On the other hand, the TLM model has been disputed on the grounds that HDV entry does not depend on TLM functionality,53, 54 and mutation or deletion of the TLM does not appear to affect HBV infectivity.55
Intracellular transport
The precise location and timing of nucleocapsid release remains unknown, but for naked core particles, active transport along microtubules towards the nucleus has been described.56 HBV genomes accumulate in host cell nuclei within 15 min after entry of capsids into the cell membrane,56 suggesting that the virus employs rapid, directed transport to the nucleus. The viscosity of the cytoplasmic environment is 10 to 100 times that of water, with a protein density of 170–350 g/mL, making diffusion impractical for particles larger than 50 nm, and even smaller particles may be trafficked using active transport.57 Unlike microfilaments, which localize near the cell membrane, microtubules connect the cell periphery to the interior of the cell. The less dynamic minus ends converge at the microtubule organizing center near the nucleus. These highly polarized structures function in transport of organelles and macromolecules as well as play a role in endocytosis. Most viruses examined so far exploit the microtubule network to enable efficient transport throughout the cell, both indirectly through transport within endocytic vesicles as well as directly by interacting with motor proteins. While some transport is mediated by polymerization and depolymerization of microtubules, movement of macromolecules is mainly accomplished via motor proteins, including kinesin, which transports material toward the periphery (anterograde), and dynein, which transports material towards the nucleus (retrograde).
Many viruses, including retroviruses, herpesviruses, parvoviruses, and adenoviruses, require nuclear localization and exploit the polarization of the microtubule network for efficient transport towards the nucleus.58 In the case of adenovirus, following endosomal escape, an adenovirus capsid protein called Hexon is responsible for interacting with the motor protein dynein.59 Although intracellular transport is less well studied in HBV, recent efforts have shown that HBV also takes advantage of the microtubule network and dynein motor proteins for intracellular transport. Depolymerization of microtubules with nocodazole prevents HBV nucleocapsids from reaching the nucleus, and microtubules have been shown to be essential for cccDNA formation.56, 60 Microtubule-dependent transport of the HBV capsid has been reported,56 but so far no reports have shown a direct interaction between the HBV capsid and dynein.
Nuclear import
Unlike hepatitis C virus, which remains in the cytoplasm throughout the viral life cycle, HBV must enter the nucleus in order to generate cccDNA and begin transcription and replication. However, entry into the nucleus is tightly regulated by nuclear pore complexes (NPCs) embedded in the nuclear membrane. NPCs are large, approximately 125 MDa structures consisting of multiple copies of more than 30 different types of nucleoporins to form an aqueous channel comprised of 500 to 1000 proteins in eigthfold symmetry.61 A docking site surrounds the pore on the cytoplasmic side, whereas the structure constricts to form a narrow basket on the nuclear side, establishing a physical limit for cargo of about 45 nm. While particles less than 9 nm can pass through NPC by diffusion, larger particles are transported through the NPC via importins. Transport of material through the nuclear pore ultimately requires interaction with the karyopherin importin β, either directly, in proteins that contain a classical nuclear localization signal (NLS) (e.g. PKKKRKV), or indirectly by binding instead to the adaptor protein importin-α, which in turn attaches to importin-β.61 This complex diffuses through the nuclear pore, whereupon RanGTP displaces importin-β and CAS displaces importin-α, releasing the cargo protein into the nucleus. Although the actual translocation through the nucleus is passive, the process is powered by the hydrolysis of ran-GTP, which establishes a gradient that drives unidirectional transport through the NPC.
The HBV core protein has been shown to contain a NLS in the C-terminal domain between amino acids 141 and 185,62 but cryoelectron microscopy previously revealed that the NLS is not exposed in capsids.63 However, while the C-terminal domain is oriented towards the interior in immature capsids,64 reverse transcription of pregenomic RNA into relaxed circular DNA during capsid maturation causes a conformational change resulting in exposure of the C-terminal domain in about 50% of the chains.65 Exposure of the NLS also requires extensive phosphorylation of the C-terminal domain.62 However, capsids bearing recombinant core proteins lacking the C-terminal NLS and phosphorylation sites were also shown to bind to the NPC.66 While the capsids are small enough to pass through the NPC, immature capsids are arrested at the nuclear basket by Nup153 and prevented from entering the nucleus.67 Surprisingly, maturation of the capsid does not lead to nuclear entry but instead to disassembly of the nucleocapsid, whereupon HBV DNA and a number of core proteins enter the nucleus.56, 67 Schmitz et al. showed that the core protein interacts at high affinity with Nup153 using a binding site shared with importin-β and demonstrated that siRNA knockdown of Nup153 increased viral replication efficiency in terms of increased HBsAg expression.67 They proposed that dissociation of the capsid results in saturation of Nup153 binding and clears the way for entry of HBV DNA and the covalently bound HBV polymerase.67 Following disassembly of the nucleocapsid at the NPC, entry of HBV DNA may be further aided by a putative bipartite NLS in the terminal protein domain of the HBV polymerase.68 Inhibition of a casein kinase II phosphorylation site in the NLS region of the polymerase was shown to impair nuclear import,68 suggesting that phosphorylation of the HBV DNA polymerase is necessary to expose an NLS required for final entry of the HBV genome into the nucleus.
Formation of cccDNA
Once inside the nucleus, partially double-stranded, relaxed circular DNA is converted into cccDNA, which then functions as a template for viral transcription and pregenomic RNA synthesis by host RNA polymerase II.69 Conversion to cccDNA in hepadnaviruses is poorly understood but involves removal of the covalently bound polymerase and the RNA primer, followed by DNA synthesis and ligation to form closed DNA.70 Interaction with histones (H1, H2A, H3, and H4) and non-histone proteins, including the HBV core protein, is also involved in minichromosome formation.69 Once established, cccDNA is highly stable, with a half-life of at least 33 to 57 days, based on estimates from DHBV.71 However, a small number of newly synthesized capsids re-enter the nucleus and establish additional copies of cccDNA during replication.3 As a result, the nucleus may contain 5–50 copies of cccDNA,72 and cccDNA may stably persist in patients over the course of decades. However, key differences in the efficiency of cccDNA generation in DHBV versus HBV have been observed,73 suggesting that conclusions drawn from cccDNA studies involving DHBV should be applied with caution. While reverse transcription inhibitors are able to suppress viral replication, the continued presence of cccDNA may result in viral rebound when the inhibitor is removed. Consequently, strategies to target cccDNA formation or maintenance are urgently needed for effective treatment of chronic HBV.74
Conclusions
The identification of NTCP as a primary HBV receptor represents the culmination of years of effort by numerous researchers and opens the door to new experimental approaches, affords development of more effective infection systems, and unveils a promising new drug target. On the other hand, binding to the hepatocyte surface is only one of many steps in the establishment of infection, and the number of false or contradictory leads in the search for the primary HBV receptor has revealed that the virus may interact with numerous other host proteins during infection. Some of these proteins should be re-examined in the light of current knowledge to identify potential co-receptors as well as other host factors that, while non-essential, may be recruited by HBV to maximize infection efficiency. Although several key details of HBV entry have been elucidated, many questions remain regarding the signaling pathways that orchestrate these changes. HBV achieves this navigational feat without the ability to initiate new transcription, using only the surface proteins (which are subsequently lost during fusion), the core proteins of the nucleocapsid, and the covalently bound viral polymerase and HBV DNA. HBV also accomplishes this task without its potent regulatory modifier, HBx, which plays an essential role in later stages of the life cycle.75 These limitations, as well as viral dependence on numerous evolutionarily conserved host factors, suggest that better understanding of the journey from the cell surface to the nucleus may reveal new opportunities to block HBV infection.
Acknowledgments
This work is partially supported by Grants-in-Aid for scientific research and development from the Ministry of Health, Labor and Welfare and the Ministry of Education, Culture, Sports, Science and Technology, Japan. The authors have no conflicts of interest to declare.




