Endoscopic diagnosis of early neoplasia of the esophagus with narrow band imaging: Correlations among background coloration and iodine staining findings
Abstract
Background and Aim
It was previously reported that high-grade intraepithelial neoplasia of the esophagus turns pink within a few minutes after iodine staining (pink-color sign; PCS); however, iodine staining is uncomfortable. By using narrow band imaging (NBI), color change in the area between the intraepithelial papillary capillary loop (background coloration; BGC) is often observed within the brownish area. The diagnostic usefulness of BGC findings for differentiating high-grade intraepithelial neoplasia from low-grade intraepithelial neoplasia was evaluated.
Methods
In a prospective observational study from September 2010 to August 2012, 285 patients who were in a high-risk group for esophageal squamous cell carcinoma underwent endoscopic examination. Lesions with both endoscopic findings of dilated intraepithelial papillary capillary loop on NBI and iodine-unstained areas were studied, in which endoscopic biopsy or endoscopic resection was subsequently performed. The esophageal background mucosa was also evaluated on the basis of the iodine staining pattern (uniform type: Group U, scattered type: Group S).
Results
One hundred three esophageal lesions in 87 patients were studied. When BGC was used as the differentiation index, sensitivity was 93.8%, specificity was 88.2%, and accuracy was 91.3%. When PCS was used, sensitivity was 97.9%, specificity was 88.2%, and accuracy was 93.2% (P = 0.79). In Group U (n = 54), BGC had an accuracy of 93.8%, and PCS had an accuracy of 92.3% (P = 1.0). On the other hand, in Group S (n = 33), BGC had an accuracy of 86.8%, while PCS had an accuracy of 94.7% (P = 0.27).
Conclusions
Diagnosis using BGC on NBI may substitute for diagnosis based on PCS in many patients.
Introduction
The development in recent years of techniques for endoscopic diagnosis has led to the detection of increasing numbers of cases of early-stage esophageal squamous cell neoplasia.1-4 Although local treatments such as endoscopic resection and local surgical excision are the standards for high-grade intraepithelial neoplasia (HGIN), follow-up is recommended for low-grade intraepithelial neoplasia (LGIN).5-8 Endoscopic diagnosis to differentiate HGIN from LGIN is very important. We previously reported that HGIN turns pink within a few minutes after iodine staining and named this phenomenon pink-color sign (PCS).9 PCS allows accurate endoscopic diagnosis of intraepithelial neoplasia. Ishihara et al. subsequently investigated the mechanism underlying PCS by applying quantitative analysis and confirmed the usefulness of PCS for endoscopic diagnosis of early esophageal lesions.10 However, diagnosis based on PCS is time-consuming, and iodine staining can cause chest pain, even severe discomfort, in some patients. Recently, the usefulness of narrow band imaging (NBI) with magnifying endoscopy (NBI-ME) for detecting early squamous neoplasia of the esophagus has been reported.3, 4 The capacity of NBI for detecting HGIN has been reported to be equal to that of iodine staining.3 Such lesions are usually imaged as a brownish area with NBI.3, 4 The brownish area is composed mainly of irregularly dilated intraepithelial papillary capillary loops (IPCLs). However, color change in the area between the IPCL (background coloration; BGC) is also often observed within the brownish area. Previously, Ishihara et al., who referred to BGC-positive lesions as brownish epithelium, conducted a retrospective analysis and concluded that brownish epithelium and dilated IPCL were confirmed to be significant findings in the diagnosis of HGIN.11 We assumed that HGIN would be identifiable as brownish areas with BGC because there is almost no remaining non-neoplastic epithelium and diagnosis using BGC would substitute for diagnosis based on PCS. In this study, we prospectively evaluated the diagnostic usefulness of BGC findings for differentiating HGIN from LGIN of the esophagus and investigated the histopathological mechanism underlying BGC findings.
Methods
Patients
Patients who had previously undergone endoscopic resection for esophageal squamous cell carcinoma (SCC) and patients with current or previous SCC of the head and neck, known to be a high-risk group for metachronous esophageal SCC, were enrolled in this prospective observational study. Patients referred from other hospitals with newly diagnosed esophageal intraepithelial neoplasia because they required more detailed examination were also included (patients of whose biopsy specimen was pathologically reviewed in our institute prior to endoscopic examination were excluded). Patients with prior radiotherapy or chemoradiotherapy for esophageal SCC were excluded. The study subjects underwent endoscopic examination with NBI-ME followed by iodine staining between September 2010 and August 2012 at Hokkaido University Hospital. All lesions showing both endoscopic findings of dilated IPCL on NBI-ME and iodine-unstained areas were evaluated.
The protocol for this study was approved by the institutional review board of our hospital, and written informed consent was obtained from all patients.
Endoscopic examination
The endoscopic examinations in this study were performed using a zoom gastrointestinal endoscope, endoscopic system, and light source (GIF-Q240Z or GIF-H260Z, CV-260SL processor, and CLV-260SL light source; Olympus Medical Systems Co, Tokyo, Japan) in conjunction with an NBI system by two experienced endoscopists (MT and YS). When a well-demarcated brownish area was identified by NBI endoscopy, a magnifying examination was conducted to evaluate dilated IPCL and BGC. Brownish areas without dilated IPCL (such as melanosis) were not evaluated. If a brownish color change in the area between the IPCL was observed, the lesion was regarded as being BGC-positive. If there was no color change, the lesion was regarded as being BGC-negative (Fig. 1). After NBI-ME, the esophageal mucosa was stained with 1.5% iodine solution. After confirmation of the lesion as an iodine-unstained area, the lesion was observed for approximately 3 min (until the brown color of the iodine solution on the mucosal surface began to fade and dissipate), and its discoloration was then evaluated. If a light pink area appeared in the iodine-unstained area, the lesion was regarded as being PCS-positive. If no light pink area was observed in the lesion within 3 min, the lesion was regarded as being PCS-negative (Fig. 2).9 The esophageal background mucosa was also evaluated on the basis of the iodine staining pattern. Background mucosa showing a uniformly stained pattern was classified as uniform type, while that showing many minute iodine-unstained areas was classified as scattered type (Fig. 2).12, 13 Because most carcinomas in situ (HGIN) appearing as unstained lesions are reportedly at least 5 mm in longest diameter,14, 15 the target lesion in this study was defined as a flat lesion ≥ 5 mm in longest diameter. All patients with target lesions underwent endoscopic biopsy. Patients who were confirmed to have HGIN or SCC by endoscopic biopsy subsequently underwent endoscopic resection or argon plasma coagulation. Lesions showing apparent invasive carcinoma (with irregular surface, depression, or elevation) and reflux esophagitis (linear erosion located near the esophagogastric junction) were diagnosed accordingly and excluded from the study.
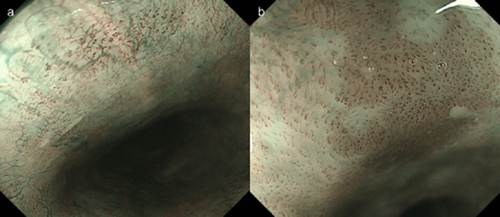
(a) Endoscopic image with narrow band imaging with magnifying endoscopy (NBI-ME) shows the lesion without color change in the area between the dilated intraepithelial papillary capillary loops. The lesion was diagnosed as background coloration-negative. (b) Endoscopic image with NBI-ME shows the lesion with brownish color change in the area between the dilated intraepithelial papillary capillary loops. The lesion was diagnosed as background coloration-positive.
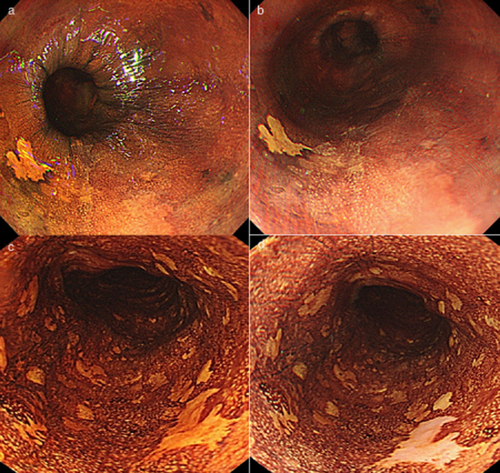
(a) Endoscopic image with iodine staining shows a demarcated flat-type lesion not stained by iodine detected in the uniformly iodine-stained background mucosa of the esophagus (uniform type). (b) Endoscopic image obtained approximately 3 min after iodine staining shows a yellowish-white lesion. The lesion was diagnosed as pink color sign-negative. (c) Endoscopic image with iodine staining shows multiple small iodine-unstained areas in the middle esophagus (scattered type). (d) Endoscopic image obtained approximately 3 min after iodine staining shows a demarcated reddish lesion and other minute unstained areas that are yellowish-white in color. The reddish lesion was diagnosed as pink color sign-positive.
In addition, after completion of the study, we conducted an examination on consistencies in the diagnosis of PCS and BGC among doctors. Endoscopic photographs, with a random arrangement of all lesions, were presented. For each lesion, two to four NBI images were presented. Three endoscopists (with endoscopic experience of 25 years, 18 years, and 8 years) conducted the evaluations by selecting BGC-positive or BGC-negative images. In the same manner, with random arrangement of all lesions, two to four endoscopic photographic images showing iodine staining were presented for each lesion. Again, the endoscopists were asked to determine whether the images were positive or negative for PCS.
Histological evaluation
All biopsy materials attached to a filter paper in an extended state were fixed in formalin and then embedded vertically. All resected specimens were cut into longitudinal slices measuring 2 mm in width. The slices were embedded in paraffin and stained with hematoxylin and eosin. All specimens were microscopically reviewed according to World Health Organization (WHO) classification5 by two pathologists blinded to the clinical characteristics of the patients. In the study on non-neoplastic epithelia remaining in the superficial layers of neoplastic lesions, we measured the three most representative sites in terms of thickness, and we used the average value thereof (Fig. 3). As for the endoscopic therapy specimens and biopsy specimens containing all mucosal epithelial layers, the thickness of the residual epithelium and that of all mucosal epithelial layers at the same three sites were measured, and average values were calculated in the same manner (Fig. 4).
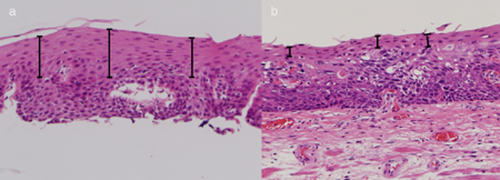
(a) Photomicrograph of an esophageal biopsy specimen shows that more than half of the non-neoplastic epithelium remains in the superficial layers of neoplastic lesions. (b) Photomicrograph of a resected esophageal specimen shows thinning of the non-neoplastic epithelium remaining in the superficial layers of neoplastic lesions.
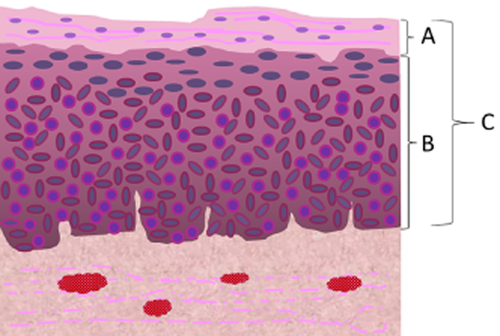
The schema of the histological evaluation. “A” is “the thickness of the non-neoplastic epithelium remaining in the superficial layers of neoplastic lesions.” “B/C” is “the proportion of neoplastic cell layers to total epithelial thickness.”
Statistical analysis
Differences in frequency distribution were tested using Fisher's exact test, and quantitative data were tested using the t-test. A P value less than 0.05 was considered to indicate a statistically significant difference. The interobserver agreement was calculated by using the multirater kappa value. The kappa values were interpreted according to Landis and Koch.16 All analyses were carried out with JMP pro 10 (SAS Institute, Inc., Cary, NC, USA).
Results
During the study period, the 285 enrolled patients underwent endoscopic examination with NBI followed by iodine staining. Among them, 87 patients were found to have both endoscopic findings of brownish areas with dilated IPCL on NBI-ME and iodine-unstained areas (103 lesions in total) (Fig. 5). Of the 103 lesions, resected specimens obtained from 42 and biopsy specimens from 61 were examined. The male-to-female ratio was 75:12, and the mean age was 68.7 years (range 49–92 years). The histological diagnosis was SCC/HGIN for 48 lesions and LGIN/non-atypia for 55 lesions (Table 1). When BGC positivity was used as an index of SCC/HGIN, this finding allowed differentiation between SCC/HGIN and LGIN/non-atypia with a sensitivity of 93.8% (95% confidence interval [CI] 90.4–97.1%), specificity of 88.2% (95% CI 85.1–93.1%), and accuracy of 91.3% (95% CI 88.6–93.9%). When PCS was used as the differentiation index, sensitivity was 97.9% (95% CI 95.9–100%), specificity was 88.2% (95% CI 85.1–93.1%), and accuracy was 93.2% (95% CI 90.8–95.6%). The differences between these indices were not significant (P = 0.79) (Table 2). Next, differences in diagnostic accuracy for the uniform type (Group U) and the scattered type (Group S) were examined. A diagnosis was made in 54 patients with 65 lesions in Group U (63.1%) and in 33 patients with 38 lesions in Group S (36.9%). The sensitivity, specificity, and accuracy of BGC and PCS were compared between these two groups. In Group U, BGC had a sensitivity of 96.3%, specificity of 92.1%, and accuracy of 93.8%, and PCS had a sensitivity of 96.3%, specificity of 89.5%, and accuracy of 92.3%. Again, there were no significant differences (P = 1.0). On the other hand, in Group S, BGC had a sensitivity of 90.5%, specificity of 82.4%, and accuracy of 86.8%, while PCS had a sensitivity of 100%, specificity of 88.2%, and accuracy of 94.7%. BGC had lower values, although the differences did not reach statistical significance (P = 0.27) (Table 3).
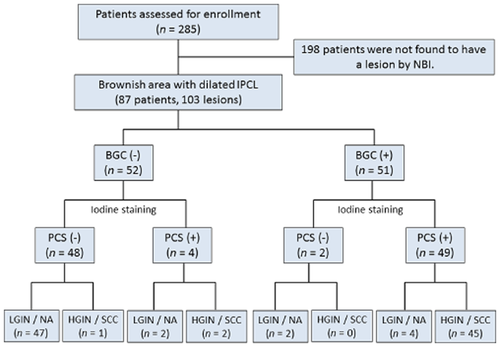
The flow-chart diagram of included lesions. BGC, background coloration; HGIN, high-grade intraepithelial neoplasia; IPCL, intraepithelial papillary capillary loops; LGIN, low-grade intraepithelial neoplasia; NA, non-atypia; NBI, narrow band imaging; PCS, pink-color sign; SCC, squamous cell carcinoma.
| Gender (n) | Male/female | 75/12 |
| Age (years old) | Mean | 68.7 (49–92) |
| Lesion location | Upper/middle/lower | 10/56/37 |
| Lesion size (mm) | Mean | 13.0 (5–40) |
| Histological diagnosis | Non-atypia/LGIN | 29/26 |
| HGIN/LPM | 18/25 | |
| MM, SM1 | 5 | |
| Specimen | Biopsy/EMR, ESD | 61/42 |
| Background mucosa | Uniform/scattered | 65/38 |
- EMR, endoscopic mucosal resection; ESD, endoscopic submucosal dissection; HGIN, high-grade intraepithelial neoplasia; LGIN, low-grade intraepithelial neoplasia; LPM, lamina propria mucosae; MM, muscularis mucosae; SM, submucosa.
| SCC or HGIN | LGIN or non-atypia | ||
|---|---|---|---|
| BGC | |||
| (+) | 45 (93.7%) | 6 (10.9%) | 51 |
| (−) | 3 (6.3%) | 49 (89.1%) | 52 |
| 48 | 55 | 103 | |
| Accuracy rate = 91.3% | |||
| PCS | |||
| (+) | 47 (97.9%) | 6 (10.9%) | 53 |
| (−) | 1 (2.1%) | 49 (89.1%) | 50 |
| 48 | 55 | 103 | |
| Accuracy rate = 91.3% | N.S. (P = 0.79) | ||
- BGC, background coloration; HGIN, high-grade intraepithelial neoplasia; LGIN, low-grade intraepithelial neoplasia; N.S., not significant; PCS, pink-color sign; SCC, squamous cell carcinoma.
| SCC or HGIN | LGIN or non-atypia | ||
|---|---|---|---|
| Uniform type | |||
| BGC | |||
| (+) | 26 (96.3%) | 3 (7.9%) | 29 |
| (−) | 1 (3.7%) | 35 (92.1%) | 36 |
| 27 | 38 | 65 | |
| Accuracy rate = 93.8% | |||
| PCS | |||
| (+) | 26 (96.3%) | 4 (10.5%) | 30 |
| (−) | 1 (3.7%) | 34 (89.5%) | 35 |
| 27 | 38 | 65 | |
| Accuracy rate = 92.3% | N.S. (P = 1.0) | ||
| Scattered type | |||
| BGC | |||
| (+) | 19 (90.5%) | 3 (17.6%) | 22 |
| (−) | 2 (9.5%) | 14 (82.4%) | 16 |
| 21 | 17 | 38 | |
| Accuracy rate = 86.8% | |||
| PCS | |||
| (+) | 21 (100%) | 2 (11.8%) | 23 |
| (−) | 0 (0%) | 15 (88.2%) | 15 |
| 21 | 17 | 38 | |
| Accuracy rate = 94.7% | N.S. (P = 0.27) | ||
- BGC, background coloration; HGIN, high-grade intraepithelial neoplasia; LGIN, low-grade intraepithelial neoplasia; N.S., not significant; PCS, pink-color sign; SCC, squamous cell carcinoma.
Interobserver agreement among the three endoscopists for BGC was good, with an estimated kappa value of 0.644 (± 0.042). The agreement for PCS diagnosis was excellent, with an estimated kappa value of 0.827 (± 0.031).
The histological association between BGC and residual non-neoplastic epithelium thickness was examined. We examined all 42 resected specimens and all 61 biopsy specimens for evaluation of residual non-neoplastic epithelium thicknesses. Subsequently, we examined all 42 resected specimens and 19 of 61 biopsy specimens (confirmed to contain sufficient entire epithelium) for calculation of proportions of the entire thickness of the mucosal epithelium showing neoplasia. Residual non-neoplastic epithelium thicknesses were 118.2 ± 60.9 μm in the BGC-negative lesions and 14.3 ± 18.0 μm in the BGC-positive lesions, showing a significant difference (P < 0.01) (Fig. 6). Three lesions determined to be BGC-negative were histopathologically diagnosed as SCC/HGIN. In all three lesions, the residual epithelium thickness was 30 μm or less. Two of the three lesions were found in scattered type mucosa and were determined to be PCS-positive by iodine staining. The other lesion showed parakeratosis in the superficial layer of the neoplastic lesion and was also determined to be PCS-negative by iodine staining. Proportions of the entire thickness of the mucosal epithelium showing neoplasia were calculated in 18 BGC-negative lesions and 43 BGC-positive lesions in which the entire mucosal epithelium thickness was measured. The proportions were 0.493 ± 0.190 in the BGC-negative lesions and 0.900 ± 0.118 in the BGC-positive lesions, showing a significant difference (P < 0.01) (Fig. 6).
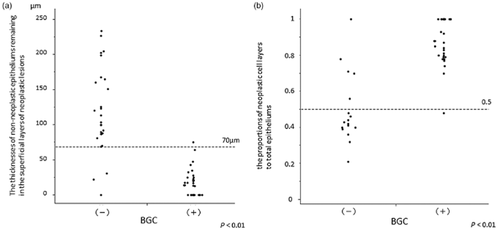
(a) The relationship between the thickness of the non-neoplastic epithelium remaining in the superficial layers of neoplastic lesions and background coloration (BGC): Thickness was lower in the BGC-positive than in the BGC-negative group (P < 0.01). (b) The relationship between the proportion of neoplastic cell layers to total epithelial thickness and BGC. The proportion was higher in the BGC-positive than in BGC-negative group (P < 0.01).
Discussion
Findings of dilated IPCL by NBI endoscopy are extremely useful for detecting early-stage esophageal squamous cell neoplasia.3, 4 Yoshida et al. classified IPCL dilation patterns and reported their usefulness for determining the depth of invasion.17 However, there are very few reports on color changes in areas surrounding IPCL.
According to the WHO classification, morphological features of intraepithelial squamous neoplasia of the esophagus include both architectural and cytological abnormalities, and intraepithelial neoplasia is graded as high-grade when greater abnormalities are detected in the upper half of the epithelium.5 As for the mechanism underlying PCS, HGIN and SCC react only minimally with iodine because of the small number of glycogen-containing cells and are therefore seen as completely unstained areas with a reddish color change after the brown iodine solution fades. On the other hand, LGIN reacts slightly with iodine because of surviving glycogen-containing cells and is therefore seen as an unstained area with a yellowish-white color.9 In this study, examination of BGC also demonstrated residual epithelium thickness to be associated with BGC. Moreover, the thicknesses of the entire mucosal epithelium showing neoplasia essentially corresponded to the HGIN and LGIN criteria defined by WHO. Regarding BGC, Kanzaki et al., who referred to BGC-positive lesions as brownish epithelium, conducted a retrospective study involving mainly patients undergoing HGIN/SCC resection and reported that thinning of the keratinous layer is an important pathogenic factor.18 Our results are consistent with the results of their study. However, the actual mechanism responsible for the color changes remains unknown. The central wavelengths of the NBI filters are 415 and 540 nm, and each has a bandwidth of 30 nm. NBI-ME can clearly visualize the microvascular structure of the organ surface because 415 nm light is well absorbed by hemoglobin.19 A dense distribution of hemoglobin in the areas between vessels associated with extravascular red blood cells (RBCs) may therefore be one of the causes of BGC. However, in this study, extravascular RBCs were rarely observed microscopically in most cases. Further basic studies may be needed to assess the actual mechanism.
In this study, the diagnostic utility of BGC used as an index of SCC/HGIN was equivalent to that of PCS as an index in many of the patients. However, in patients with scattered-type background mucosa, the accuracy of BGC was lower than that of PCS (although the difference was not significant). The main factor explaining this was the lower sensitivity of BGC. This might be attributable to the color changes often being difficult to assess in scattered-type lesions because nondemarcated or minute areas with dilated IPCL were scattered within areas that appeared to correspond to minute iodine-unstained areas. Moreover, this might also have contributed to the values for BGC being lower than those for PCS, in terms of consistency of diagnosis, among the endoscopists. One possible reason for a significant difference not being obtained is the small number of misdiagnosed cases. Significant differences might have been obtained if the number of cases was large. We previously reported that metachronous esophageal SCC often arose after initial EMR in patients with scattered-type background mucosa.12 Muto et al., who refer to the scattered-type lesions as multiple Lugol-voiding lesions, reported that patients with such lesions are a high-risk group for development of esophageal SCC.20 We advocate that endoscopic screening with iodine staining and diagnostic tests based on PCS be routinely performed in patients with a diagnosis of scattered type. However, patients with scattered-type background mucosa accounted for only 36.9% of our study subjects. In the other approximately 60% of patients, diagnosis using BGC on NBI may substitute for diagnosis based on PCS, which is time-consuming and can cause severe discomfort for patients. Although, iodine staining should be necessary for first endoscopic examination, it would become unnecessary for patients who were diagnosed to be with uniform type background mucosa in following periodic examination.
Although findings of irregularly dilated IPCL by NBI endoscopy are useful for diagnosis of early neoplasia of the esophagus and also useful for determining the depth of invasion for esophageal SCC, diagnosis to differentiate HGIN from LGIN by IPCL pattern alone would not be easy. However, diagnosis of BGC is evaluation of simple color change and would be easy even for non-expert endoscopists. In this study, favorable results regarding interobserver agreement for diagnosis of BGC (as well as diagnosis of PCS) were obtained. Although there may be some bias caused by selection of best images taken from the best areas, the results suggest that diagnosis of BGC could be standard diagnostic index. In conclusion, although this study was a single center study and the sample size was rather small, our results suggest that diagnosis using BGC on NBI is useful for diagnosis to differentiate HGIN from LGIN and may substitute for diagnosis based on PCS in many patients.




