No mate preference associated with the supergene controlling social organization in Alpine silver ants
Abstract
Disassortative mating is a powerful mechanism stabilizing polymorphisms at sex chromosomes and other supergenes. The Alpine silver ant, Formica selysi, has two forms of social organization—single-queen and multiple-queen colonies—determined by alternate haplotypes at a large supergene. Here, we explore whether mate preference contributes to the maintenance of the genetic polymorphism at the social supergene. With mate choice experiments, we found that females and males mated randomly with respect to social form. Moreover, queens were able to produce offspring irrespective of whether they had mated with a male from the same or the alternative social form. Yet, females originating from single-queen colonies were more fertile, suggesting that they may be more successful at independent colony founding. We conclude that the pattern of asymmetric assortative mating documented from mature F. selysi colonies in the field is not caused by mate preferences or major genetic incompatibilities between social forms. More generally, we found no evidence that disassortative mate preference contributes to the maintenance of polymorphism at this supergene controlling ant social organization.
1 INTRODUCTION
The genetic basis of behavioural variation and the maintenance of adaptive diversity within populations are central questions in evolutionary biology. Recently, supergenes controlling complex social phenotypes have been discovered in ants and birds (Küpper et al., 2016; Purcell, Brelsford, Wurm, Perrin, & Chapuisat, 2014; Tuttle et al., 2016; Wang et al., 2013). Supergenes are large genomic region of suppressed recombination. Because of tight linkage, alternate haplotypes of supergenes harbour clusters of coadapted alleles that are transmitted together and cause coordinated variation in multiple traits, including morphology, physiology and behaviour (Schwander, Libbrecht, & Keller, 2014; Thompson & Jiggins, 2014). This unusual genomic architecture raises immediate questions on the origin, evolution and maintenance of supergenes.
The long-term persistence of polymorphic supergenes indicates that they are subject to balancing selection, generally through some form of heterozygote advantage or frequency-dependent selection (Llaurens, Whibley, & Joron, 2017; Wellenreuther & Bernatchez, 2018). In several cases, the mutant haplotype is a recessive lethal, while heterozygotes have a fitness advantage (e.g. fire ant, ruff; Küpper et al., 2016; Wang et al., 2013). Spatial or temporal variation in selection can also contribute to stabilize polymorphisms (e.g. mimetic butterfly, land snail; Joron et al., 2011; Richards et al., 2013). Last, supergenes controlling alternative reproductive phenotypes can be balanced by disassortative mating.
Disassortative mating, a process whereby mates are less similar than expected by chance, is a powerful mechanism balancing polymorphism through frequency-dependent selection, because the rarer type gains a reproductive advantage over the more frequent type (Fisher, 1930). Obligate disassortative mating maintains polymorphism at sex chromosomes and mating type chromosomes (Beukeboom & Perrin, 2014; Branco et al., 2018; Charlesworth & Mank, 2010). Disassortative mating also stabilizes supergenes that do not determine sex in plants and animals. The common primrose, Primula vulgaris, has heteromorphic flowers that have either long style and low anthers, or short style and high anthers (heterostyly). Obligate out-crossing with the alternative flower morph balances the frequencies of alternate allelic variants at the supergene controlling heterostyly (Li et al., 2016). In the white-throated sparrow, Zonotrichia albicollis, near-perfect disassortative mating between alternative morphs leads to a balanced polymorphism at a supergene controlling plumage colour and social behaviour (Hedrick, Tuttle, & Gonser, 2018; Sun, Huh, Zinzow-Kramer, Maney, & Yi, 2018; Tuttle et al., 2016). Finally, mate preference for morphs with alternative wing pattern contributes to the maintenance of polymorphism at a supergene regulating Müllerian mimicry in Heliconius numata (Chouteau, Llaurens, Piron-Prunier, & Joron, 2017). In that mimetic butterfly, disassortative mate preference prevents the fixation of the morph that is most abundant and best protected from predators.
In the Alpine silver ant, Formica selysi, a large supergene with two haplotypes, Sm and Sp, is associated with colony social organization (Avril, Purcell, Brelsford, & Chapuisat, 2019; Purcell et al., 2014). The species is socially polymorphic. Within the same populations, “monogynous” colonies harbour a single queen that monopolizes reproduction, whereas “polygynous” colonies contain multiple queens sharing reproduction (Chapuisat, Bocherens, & Rosset, 2004; Purcell & Chapuisat, 2013; Purcell, Pellissier, & Chapuisat, 2015). Queens, workers and winged males from polygynous colonies carry at least one copy of the Sp haplotype. Specifically, all queens and workers from polygynous colonies have the supergene genotypes Sp/Sm or Sp/Sp, whereas males produced by polygynous colonies have the supergene haplotype Sp (male ants are haploid; Avril et al., 2019; Purcell et al., 2014). In contrast, all individuals from monogynous colonies lack the Sp haplotype and carry exclusively the Sm haplotype (all queens and workers have the supergene genotype Sm/Sm, and males the haplotype Sm; Avril et al., 2019; Purcell et al., 2014). An unusual feature of the F. selysi supergene is that both homozygotes are viable. The mechanisms contributing to maintain the polymorphism at this social supergene are not yet known.
In principle, disassortative mate preference might contribute to balance the polymorphism at the supergene controlling social organization in F. selysi. Yet, genetic evidence from mature colonies in the field suggests a pattern of asymmetric assortative mating (Avril et al., 2019). In monogynous colonies, all queens had the Sm/Sm genotype and were mated with males having the Sm haplotype. In contrast, queens heading polygynous colonies were mated with Sp males or Sm males, the latter accounting for 22.9% of the matings (Avril et al., 2019). Polygynous colonies do not produce Sm males and Sm/Sm females because the Sp haplotype is a maternal effect killer. Specifically, eggs from heterozygous queens that did not inherit Sp failed to hatch (Avril, Purcell, Béniguel, & Chapuisat, unpublished results). Hence, Sp males are exclusively produced by polygynous colonies and Sm males by monogynous colonies (Avril et al., 2019; Purcell et al., 2014). Overall, all queens heading mature monogynous colonies had mated assortatively, whereas a fraction of queens from polygynous colonies had mated disassortatively, with males originating from the alternative social form.
The causes for the mating pattern documented in the field remain elusive (Avril et al., 2019). Indeed, the degree of disassortative mating in mature colonies depends on multiple factors, including mate availability, mate preference and genetic compatibilities. Because sex ratio, productivity and probably dispersal vary greatly between monogynous and polygynous colonies, queens originating from monogynous colonies may encounter primarily Sm males when mating in nuptial swarms, whereas queens originating from polygynous colonies may encounter primarily Sp males when mating close to their natal nest (Rosset & Chapuisat, 2006, 2007). Disassortative mate preference by polygynous queens could thus favour locally rare Sm males over Sp males, and such rare male advantage could contribute to balance the polymorphism. Conversely, assortative mate preference tends to restrict gene flow between social forms and might even lead to speciation, a process that is not supported by the absence of genetic differentiation between social forms at loci outside of the supergene (Avril et al., 2019; Purcell & Chapuisat, 2013; Purcell et al., 2014). Overall, it is of interest to investigate whether mate preferences or genetic incompatibilities between social forms play a role in the dynamics of this unusual genetic system.
With mate choice experiments, we assessed whether mate preferences or genetic incompatibilities between social forms explain the pattern of asymmetric assortative mating observed in mature field colonies. This would be the case if (a) queens of polygynous origin readily mate with males of monogynous origin, whereas (b) queens of monogynous origin do not mate with males of polygynous origin or (c) queens of monogynous origin mated to males of polygynous origin fail to produce offspring. At a more fundamental level, we test whether disassortative mate preference by queens of polygynous origin contributes to balance the polymorphism at this supergene controlling ant social organization.
2 MATERIALS AND METHODS
2.1 Sampling
Virgin queens (=young winged females) and males of F. selysi were collected in central Valais, Switzerland, in summer 2015 from 12 colonies in Finges (7°36′30″ E, 4°18′30″ N, altitude: 565 m) and 30 colonies in Derborence (7°12′56″E, 46°16′50″ N, altitude: 1450 m). The social organization of each colony had been previously determined based on direct observations of queens that warm up under stones in early spring, microsatellite genotyping, RAD-seq genotyping and PCR-RFLP genotyping of SNPs diagnostic for social form (Avril et al., 2019; Purcell & Chapuisat, 2013). Most colonies of F. selysi specialize in the production of one sex (Rosset & Chapuisat, 2006). Virgin queens or males from each colony were kept separate in small plastic boxes, with workers from the same parent colony, at 24°C and under a relative humidity of 50% (Avril et al., 2019). The ants had access to water and ad libitum food.
2.2 Mate preferences
With mate choice experiments, we examined whether queens and males prefer to mate with partners of the same or the alternative social form. In each trial, a single virgin queen and four males, two from each social form, had the opportunity to mate. The queen and males originated from different colonies of the same population. Males of alternative social forms were colour-marked, with colours randomized across trials. Queens were unmarked. The queen and males were transferred to a mating arena consisting of a box covered by a net (35 × 22 × 15 cm). Each box had a masked label, so that during the mate choice experiment, the observers were kept blind with respect to the social origin of queens and males. For the mating trials, the boxes were placed outdoor, in the morning and in daylight, which elicits flying and mating behaviour (Reber, Meunier, & Chapuisat, 2010). We monitored the behaviour of queens and males until mating, if any, or up to 30 min otherwise. Queens and males that did not mate in the first trial were returned to their laboratory colonies and used in at most one other trial.
2.3 Genetic incompatibilities
To detect potential genetic incompatibilities between social forms, we assessed the success of each mated queen at founding incipient colonies and producing brood. Immediately after mating, the queen was isolated in a glass test tube labelled with a unique number, so that the subsequent observers were kept blind to the social origin of the queen and her mate. Each tube had water blocked by cotton wool at the bottom and was wrapped in aluminium foil for darkness, which mimics independent claustral colony founding by solitary queens (Brütsch, Avril, & Chapuisat, 2017). In each incipient colony, the number of eggs, larvae, cocoons and workers, as well as the status of the queen (dead or alive), was recorded every other day over 80 days after mating.
2.4 Statistical analyses
Mate preferences and queen mating propensity were analysed with generalized mixed effect models (GLMM), using a binomial error distribution. For mate preferences, we built a model in which the response variable was the social origin of the queen's mate (monogynous or polygynous, respectively). The social origin of the queen was included as fixed effect. Random effects comprised colony of origin of queen and males, colour marks, trial date, and whether the queen or males did mate in the first or second trial, if any. A Wald test on the intercept was used to detect significant departure from random mating. To estimate the power of this analysis, we simulated 1,000 data sets with 20% of disassortative mating, a degree of deviation comparable to the ones documented from field colonies (Avril et al., 2019). With our sample scheme, the power to detect deviations from random mating of this magnitude was 92.3% and 77.3% for queens of monogynous and polygynous origin, respectively. For the mating propensity of queens, the response variable was the mating status of the queen (mated or not) at the end of the trial. The queen social origin was included as fixed effect, while random effects comprised the trial date and whether the queen or males did mate in the first or second trial.
We explored whether genetic incompatibilities between social forms affected the success of solitary queens at founding incipient colonies, as well as brood production in successful colonies. For the success at founding incipient colonies, we used a GLMM with a binomial error distribution. Colony success, that is whether the queen had survived until the end of the experiment and succeeded in producing workers, was the binomial response variable. Queen social origin, male social origin and the interaction between the two factors were included as fixed effects. Random effects comprised the trial date and whether the queen or males did mate in the first or second trial. For brood production, we used a generalized additive mixed model (GAMM), which can model nonlinear time series data (Zuur, Ieno, Walker, Saveliev, & Smith, 2009). The response variable was the number of brood items (eggs, larvae, cocoons and workers) per queen, across fertile queens that survived until the end of the experiment. Queen social origin, male social origin and the interaction between the two factors were included as fixed effects. The number of days after mating was used as the smoothing covariate. Random effects comprised queen identity and whether the queen or males did mate in the first or second trial. All statistics were performed with the R statistical package v. 3.3.2 (R Development Core Team, 2015). GAMM and GLMM models were built using the “mgcv” package v1.8 (Wood, 2011) and the “lme4″ package v1.1 (Bates, Mächler, Bolker, & Walker, 2015), respectively.
3 RESULTS
3.1 Mate preferences
In mate choice experiments involving a virgin queen and two males from each social form, mating occurred randomly with respect to social form. No significant mate preference was detected for queens of monogynous origin (Figure 1; GLMM binomial, z1 = −0.25, p = 0.81), nor for queens of polygynous origin (Figure 1; GLMM binomial, z1 = 0.78, p = 0.43). However, queens of monogynous origin were more likely to mate than queens of polygynous origin (Figure 1; mating occurred for 74.2 % of 62 queens of monogynous origin and 40.7 % of 59 queens of polygynous origin, respectively; GLMM binomial,  = 14.1, p < 0.001).
= 14.1, p < 0.001).
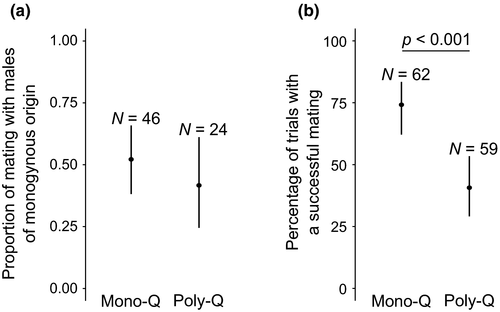
3.2 Genetic incompatibilities
The success of incipient colonies did not depend on whether the founding queen had mated with a male from the same or the alternative social form (Table 1; GLMM,  = 0.05, p = 0.81) and was not influenced by male social origin (Table 1; GLMM,
= 0.05, p = 0.81) and was not influenced by male social origin (Table 1; GLMM,  = 0.43, p = 0.51). Colony success rate tended to be higher for queens of monogynous origin than for queens of polygynous origin, but the difference was not statistically significant (Table 1; GLMM;
= 0.43, p = 0.51). Colony success rate tended to be higher for queens of monogynous origin than for queens of polygynous origin, but the difference was not statistically significant (Table 1; GLMM;  = 3.0, p = 0.08).
= 3.0, p = 0.08).
| Queen of monogynous origin | Queen of polygynous origin | |
|---|---|---|
| Mated to male of monogynous origin | 0.71 (24) | 0.60 (10) |
| Mated to male of polygynous origin | 0.68 (22) | 0.43 (14) |
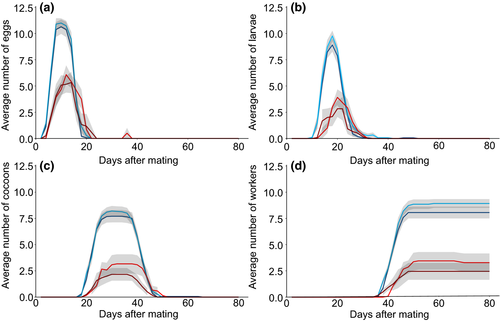
Brood production in successful incipient colonies was not influenced by whether the founding queen had mated with a male of the same or the alternative social origin (Figure 2; interaction between social origins of queens and their mates, for eggs: F1,1 = 0.72, p = 0.40; larvae: F1,1 = 1.03, p = 0.31; cocoons: F1,1 = 0.58, p = 0.45; workers: F1,1 = 0.92, p = 0.34). Male social origin did not influence brood production by queens (Figure 2; eggs: F1,1 = 0.18, p = 0.67; larvae: F1,1 = 1.63, p = 0.20; cocoons: F1,1 = 0.02, p = 0.88; workers: F1,1 = 0.70, p = 0.40). In contrast, queen social origin had a strong effect on brood production, with queens of monogynous origin producing significantly more brood than queens of polygynous origin (Figure 2; eggs: F1,1 = 26.7, p < 0.0001; larvae: F1,1 = 42.5, p < 0.0001; cocoons: F1,1 = 44.5, p < 0.0001; workers: F1,1 = 54.8, p < 0.0001).
4 DISCUSSION
A large nonrecombining region with two haplotypes determines colony social organization in the Alpine silver ant, F. selysi (Purcell et al., 2014). Females and males originating from monogynous colonies carry exclusively the Sm haplotype, whereas females and males originating from polygynous colonies have one or two copies of the Sp haplotype (Avril et al., 2019). This polymorphism is shared across populations and appears stable (Avril et al., 2019; Chapuisat, Goudet, & Keller, 1997; Chapuisat et al., 2004; Purcell & Chapuisat, 2013; Purcell et al., 2015), but so far the mechanism of balancing selection remains unclear. Disassortative mating maintains polymorphism at sex chromosomes and other supergenes (Chouteau et al., 2017; Hedrick et al., 2018; Llaurens et al., 2017; Tuttle et al., 2016). This prompted us to investigate whether some degree of disassortative mate preference could contribute to the maintenance of the polymorphism at the social supergene of F. selysi.
In mate choice experiments, females and males of F. selysi mated at random with respect to their social origin. These behavioural data provide no support to the hypothesis that disassortative mate preference balances the polymorphism at the supergene controlling social organization. Disassortative mating is associated with lethal homozygosity of one haplotype in many sex chromosomes and in at least two supergenes controlling social phenotypes (Charlesworth & Mank, 2010; Küpper et al., 2016; Wang et al., 2013). The absence of disassortative mate preference in F. selysi is consistent with the fact that homozygotes for both haplotypes are viable and that we detected no major genetic incompatibility within social forms.
Mate preference has not been investigated in fire ants, because their mating takes place high in the air and cannot be observed or manipulated in controlled conditions (e.g. Mikheyev, 2003). Fire ant social organization is controlled by a supergene that evolved independently from the one of F. selysi (Purcell et al., 2014; Wang et al., 2013). Fire ant queens in mature monogynous and polygynous colonies had mated predominantly with males originating from the monogynous social form (Fritz, Vander Meer, & Preston, 2006; Lawson, Vander Meer, & Shoemaker, 2012; Shoemaker & Ross, 1996). This unusual mating pattern might be linked to the lower fertility of males carrying the supergene haplotype associated with polygyny (Lawson et al., 2012).
In our experiment, F. selysi queens from each social form were able to produce offspring, independently of whether they had mated with a male from the same or the alternative social form. Two lines of evidence indicate that there are no major genetic incompatibilities within or between social forms. First, the success of queens at founding incipient colonies did not depend on the social origin of their mates. Second, offspring production in successful colonies was independent of whether the queen had mated with a male of the same or the alternative social form. These results corroborate earlier findings based on a larger number of colonies (Reber et al., 2010). They also confirm that workers can develop into adults irrespective of their supergene genotype—at least some to the Sm/Sm, Sp/Sp and Sm/Sp offspring are viable (Avril et al., 2019; Purcell et al., 2014). Due to the small number of incipient colonies that produced brood, the power of this experiment was not sufficient to detect more subtle genotypic incompatibilities. There is strong selection for disassortative mate preference when genetically similar partners are incompatible and conversely for assortative mate preference when genetically dissimilar partners are incompatible (Mays & Hill, 2004; Tregenza & Wedell, 2001). Overall, we detected no major genetic incompatibilities within or between social forms of F. selysi, based on a small number of crosses monitored during the early stages of colony development in protected laboratory conditions. Thus, such incompatibilities are unlikely to promote assortative or disassortative mating with respect to the social origin of queens and males.
Mate preference or genetic incompatibilities did not explain the fact that all queens in mature monogynous colonies had mated with males of monogynous origin, while 22.9% of the queens in mature polygynous colonies had mated with males of monogynous origin (asymmetric assortative mating; Avril et al., 2019). In the mate choice experiments, queens showed no preference for males of monogynous origin and males of monogynous origin did not outperform males of polygynous origin. In particular, Sm/Sm queens did mate with Sp males and this cross produced viable offspring. Yet, Sm/Sp workers or Sm/Sm queens that had mated with Sp males have never been detected in mature monogynous field colonies, which are several years old (Avril et al., 2019; Purcell et al., 2014). It is possible that Sm/Sm queens do not encounter Sp males in the field, due to differences between social forms in the number, timing or dispersal behaviour of queens and males (Rosset & Chapuisat, 2006, 2007). Alternatively, when they age, incipient colonies founded by Sm/Sm queens that had mated with Sp males might be quickly converted into polygynous colonies headed by multiple Sp/Sm daughter queens.
Queens of alternative social forms showed some differences in their mating and reproductive strategies. Queens of monogynous origin were more likely to mate in our experimental settings that mimicked a mating flight, which suggests that they might be more prone to mate outside of their nests. More importantly, queens of monogynous origin produced three times as many brood than queens of polygynous origin, irrespective of the social origin of their mates. F. selysi queens of monogynous origin are slightly bigger than queens of polygynous origin (Meunier & Chapuisat, 2009; Rosset & Chapuisat, 2007), which may explain their higher productivity in experimental conditions mimicking independent claustral colony founding, without food and workers. In contrast, queens of polygynous origin may preferentially mate close to or within their natal nest and establish new nests with the help of workers. This shift in body size, dispersal and mode of colony founding is commonly associated with the transition to polygyny in ants (Hölldobler & Wilson, 1977; Keller & Passera, 1989). Consistent with more restricted dispersal and dependent colony founding by queens of polygynous origin, nestmate queens, as well as queens and their mates, are significantly related in polygynous colonies (Avril et al., 2019).
In summary, we found no evidence that disassortative mating contributes to stabilize the polymorphism at the social supergene of F. selysi. Moreover, mate preferences or strong genetic incompatibilities between social forms do not explain the pattern of asymmetric assortative mating observed in the field, which probably reflects differences in mate availability and colony development. Yet, queens of monogynous origin were more fertile than queens of polygynous origin, which is consistent with the hypothesis that queens of monogynous origin are more successful at independent colony founding. Differences between social forms in dispersal and mode of colony founding might play a key role in the maintenance of the polymorphism. A plausible scenario is that the monogynous form has higher success at colonizing novel habitat patches, whereas the polygynous form outperforms the monogynous form in old, saturated habitat patches (spatial heterogeneity in selection; Pedersen & Boomsma, 1999; Purcell et al., 2015). Heterozygote advantage might also contribute to stabilizing this genetic polymorphism controlling ant social organization.
ACKNOWLEDGEMENTS
We thank Jessica Purcell, Ornela De Gasperin and Nicolas Perrin for comments on the manuscript. Funding was provided by the Swiss National Science Foundation grant 31003A-173189/1 and the Fondation Herbette UNIL.
DATA ACCESSIBILITY
Data from this manuscript have been deposited in the Dryad database (https://datadryad.org/resource/doi:10.5061/dryad.9vb79kt).




