No selection for change in polyandry under experimental evolution
Abstract
What drives mating system variation is a major question in evolutionary biology. Female multiple mating (polyandry) has diverse evolutionary consequences, and there are many potential benefits and costs of polyandry. However, our understanding of its evolution is biased towards studies enforcing monandry in polyandrous species. What drives and maintains variation in polyandry between individuals, genotypes, populations and species remains poorly understood. Genetic variation in polyandry may be actively maintained by selection, or arise by chance if polyandry is selectively neutral. In Drosophila pseudoobscura, there is genetic variation in polyandry between and within populations. We used isofemale lines to found replicate populations with high or low initial levels of polyandry and tracked polyandry under experimental evolution over seven generations. Polyandry remained relatively stable, reflecting the starting frequencies of the experimental populations. There were no clear fitness differences between high versus low polyandry genotypes, and there was no signature of balancing selection. We confirmed these patterns in direct comparisons between evolved and ancestral females and found no consequences of polyandry for female fecundity. The absence of differential selection even when initiating populations with major differences in polyandry casts some doubt on the importance of polyandry for female fitness.
1 INTRODUCTION
Female multiple mating (polyandry) has many important consequences for sexual selection (Birkhead & Moller, 1998; Parker, 1970; Simmons, 2001), population viability (Holman & Kokko, 2013; Lumley et al., 2015; Price, Hurst, & Wedell, 2010a), genetic variation (Balloux & Lehmann, 2003), genome evolution (Mank, Wedell, & Hosken, 2013) and may even drive speciation (Gavrilets, 2014). Polyandry is extremely widespread across the animal kingdom, with evidence for multiple paternity from 89% of all natural populations investigated across animal taxa (Taylor, Price, & Wedell, 2014). Much research has focused on the costs and benefits of polyandry (Arnqvist & Nilsson, 2000; Jennions & Petrie, 2000; Slatyer, Mautz, Backwell, & Jennions, 2012; Zeh & Zeh, 1996), finding substantial support for direct, and mixed support for indirect benefits of multiple mating for females. Nonetheless, given the many factors that potentially influence the dynamics of polyandry, polyandry remains a puzzling trait.
If polyandry is beneficial, how is variation between populations maintained? An intriguing observation shows that polyandry appears to correlate with latitude in many taxa (Taylor et al., 2014), but the reasons for this remain elusive (Price, Hoskyns, Rapley, Evans, & Wedell, 2012; Taylor, Price, Skeats, & Wedell, 2016). Nevertheless, this points towards a strong role of ecology for regulating a population's mating frequency, either directly by altering the costs/benefits of polyandry (Välimäki et al., 2008) or indirectly by altering the intensity of sexual conflict (Arbuthnott, Dutton, Agrawal, & Rundle, 2014). Sexual conflict over mating rate is very common, and realized mating rates will reflect the outcome of male persistence at making mating attempts and female resistance to such attempts (Parker, 2006). The costs and benefits of accepting or resisting multiple matings can take many forms given a set of ecological circumstances, and females are likely to adjust their mating strategy to optimize their fitness, balancing the costs and benefits of multiple mating (Arnqvist & Nilsson, 2000). Thus, directional selection should lead the frequency of polyandry towards an externally derived local optimum (Candolin & Heuschele, 2008; Emlen & Oring, 1977). Support for a role of ecological drivers of polyandry comes from observations of laboratory adaptation with evolution towards higher or lower frequencies of polyandry (Burton-Chellew, Beukeboom, West, & Shuker, 2007; Harano & Miyatake, 2005), presumably because the costs and benefits of (multiple) mating are altered in the laboratory relative to the wild (Markow, 2011).
The costs and benefits of polyandry are typically assumed to be uniform for all females, such that the same strategy maximizes fitness for all females (for reviews, see Jennions & Petrie, 2000; Slatyer et al., 2012). Most laboratory experiments on the benefits of polyandry involve drastic manipulations, where females are moved away from evolved optima. Because monandrous species typically cannot be forced to remate (but see, e.g., Arnqvist & Andrés, 2006; King & Bressac, 2010), experimenters commonly deny females from polyandrous species any opportunity for remating and then assess the fitness consequences (e.g. Evans & Magurran, 2000; Gowaty, Kim, Rawlings, & Anderson, 2010; Newcomer, Zeh, & Zeh, 1999). However, these studies can only explain why monandry does not evolve in polyandrous species but not vice versa. Other studies have used experimental evolution while manipulating the number of males a female mates with, and have revealed adaptations to mating systems both in males and females (e.g. Crudgington, Fellows, & Snook, 2010; Demont et al., 2014; Martin, Hosken, & Ward, 2004; Perry et al., 2016; Wigby & Chapman, 2004). In comparison, relatively few studies have experimentally manipulated aspects of the evolving populations to observe how the frequency of polyandry evolves in response (e.g. sex ratio distorter: Price, Hodgson, Lewis, Hurst, & Wedell, 2008; inbreeding: Michalczyk et al., 2011; male sterility: Kuriwada et al., 2014). Studies demonstrating experimental evolution of polyandry highlight that genetic variation within the starting population is an essential requirement for an adaptive response in polyandry to the local conditions. In natural populations, the costs and benefits of polyandry are likely to change dynamically, and females may adopt a flexible strategy that relies on phenotypic plasticity (Gowaty, 2013; Gowaty & Hubbell, 2009). However, evidence that genetic variation in polyandry is commonly present within populations is accumulating (Price et al., 2014; Sgrò, Chapman, & Partridge, 1998; Shuker, Phillimore, Burton-Chellew, Hodge, & West, 2007; Simmons, 2003; Solymar & Cade, 1990; Taylor et al., 2014; Torres-Vila, 2013; Torres-Vila, Gragera, Rodriguez-Molina, & Stockel, 2002; Torres-Vila, Rodríguez-Molina, Gragera, & Bielza-Lino, 2001; Travers, Simmons, & Garcia-Gonzalez, 2016; Wedell, 2001). This evidence of standing genetic variation for polyandry opens questions about what maintains it. If there is a single optimum for females, what maintains genetic variation once that optimum has been reached? To better understand polyandry evolution, we need to understand its fitness consequences in situations that better incorporate selective forces that act in natural populations, including social interactions (e.g. Takahashi & Kawata, 2013).
Most previous studies have simply addressed the question whether polyandry is subject to directional selection, manifested as a fitness difference between monandrous and polyandrous females. However, directional selection should lead to the depletion of genetic variation and does not explain the presence of genetic variation in polyandry within populations (Taylor et al., 2014). Balancing selection under negative frequency dependence (nFDS) is a pervasive force for maintaining genetic variation (Clarke, 1979; but see Brisson, 2018). Under nFDS, the fitness of a certain genotype or phenotype depends on its frequency in the population, increasing at low frequencies and decreasing when high frequencies are reached (Ayala & Campbell, 1974). In the context of polyandry, the fitness effects of multiple mating may depend on what other females in the population do. Traditionally, evidence for nFDS on reproductive strategies has come from males (e.g. Sinervo & Lively, 1996), but has more recently included female mating strategies (Neff & Svensson, 2013). A thoroughly demonstrated example is female colour-dependent harassment by male Ischnura damselflies (Svensson, Abbott, & Hardling, 2005; see also Takahashi & Kawata, 2013). More generally, Svensson and Råberg (2010) suggested that sexual conflict could generally lead to nFDS on female mating strategies, if females avoid the costs of male harassment by tolerance rather than by resistance. Sexual conflict over remating is common, with males trying to manipulate females away from reaching their optimum remating rate. However, females will in turn counteract these manipulations (Arnqvist & Rowe, 2005). If the majority of females mate with multiple males, males may respond to increased levels of sperm competition by increasing attempts to prevent females from remating, including seminal fluids that decrease female longevity (Chapman, Arnqvist, Bangham, & Rowe, 2003). This may give females that mate only once an advantage over polyandrous females through reduced cost of receiving male ejaculates, especially if the costs of mating increase more than linearly (Kuijper, Stewart, & Rice, 2006). As female mating frequency decreases, males may reduce costs to females (Hollis, Houle, & Kawecki, 2016; Hollis, Houle, Yan, Kawecki, & Keller, 2014), in turn favouring polyandrous females that gain potential benefits of polyandry with reduced exposure to mating costs. At equilibrium, different female mating strategies may have equal net fitness.
Alternatively, genetic variation in polyandry need not be actively maintained through selection. Instead, genetic variation could be maintained by random mutation, especially if polyandry is a highly polygenic trait (e.g. Torres-Vila et al., 2001). Polyandry may be selectively neutral, and the frequency of polyandry might change only through genetic drift. This could be true especially in benign conditions such as laboratory environments, where reduced exposure to predators, pathogens and competing species might limit the benefits and costs of multiple mating.
Studying the fitness consequences of polyandry and its evolution in a population context is notoriously difficult and is not possible in many experimental systems. Here, we use naturally occurring genetic variation in polyandry in the fruit fly Drosophila pseudoobscura to investigate selection on polyandry through experimental evolution over multiple generations in a laboratory population context. Using genetic variation in polyandry enabled us to test for fitness consequences of multiple mating in a population setting without manipulating the adult sex ratio or females’ access to mates. D. pseudoobscura shows remarkable genetic variation in polyandry, both between and within populations. There is genetic variation in average degree of polyandry between populations across a latitudinal cline across North America (Price et al., 2014). Moreover, genetic variation exists within populations, revealed by comparisons of wild-caught females with their descendants (Price, Lewis, Smith, Hurst, & Wedell, 2011) and through variation between isofemale lines (Herrera, Taylor, Skeats, Price, & Wedell, 2014; Taylor et al., 2016) that represent a snapshot of the genetic variation in a population (David et al., 2005; Nouhaud, Tobler, Nolte, & Schlötterer, 2016). Laboratory experiments show that genetic variation in polyandry is stable with respect to temperature variation (Taylor et al., 2016), and is largely under female control (Price et al., 2008; but see Crudgington, Fellows, Badcock, & Snook, 2009 and Price, Lewis, Smith, Hurst, & Wedell, 2010b). Except for in very long-lived females, males provide no direct fitness benefits to females (Turner & Anderson, 1983). Polyandry can however provide indirect benefits for offspring survival (Gowaty et al., 2010). In the presence of a naturally occurring sex ratio distorter, polyandry can have strong fitness benefits by allowing females to avoid fertilization by distorter-carrying males (Price et al., 2010a). In the presence of this sex ratio distorter, polyandry showed a clear increase within nine generations in experimental evolution (Price et al., 2008). In nature, the distorter correlates negatively with the latitudinal polyandry cline, likely due to polyandry regulating the frequency of the distorter by reduced transmission success (Price et al., 2014). However, what drives and maintains variation in polyandry between populations, and especially within populations, remains unknown (Price et al., 2014; Taylor et al., 2016).
Here, we investigated whether in the absence of the sex ratio distorter, balancing or directional selection acts on polyandry in evolving populations where we eliminated differences in the abiotic environment, but started with an initially high or low representation of polyandrous genotypes. If balancing selection is the main force maintaining variation in polyandry, we would expect all populations to evolve towards an intermediate frequency of polyandry. If polyandry is consistently beneficial or costly, all populations should evolve towards a high or low frequency of polyandry, irrespective of their initial starting frequency. Finally, if polyandry is selectively neutral, polyandry should remain the same as its initial high or low frequency. We first characterized isofemale lines for female mating behaviour and selected lines that represented differences in the genetic predisposition to mate multiply. Variation in polyandry was continuous, but to create contrasting backgrounds, we grouped isolines into two categories with more polyandrous versus relatively monandrous lines, respectively. Using the selected isolines, we then initiated replicate populations that differed in their initial average frequency of polyandry, and tracked the frequency of polyandry over seven consecutive generations during experimental evolution. Finally, after a generation of common garden breeding, we compared the evolved populations directly with the ancestral isolines with regard to female remating behaviour and fecundity, and male ability to inhibit female remating. Using tester flies that had not co-evolved, we tested female and male effects on polyandry independently. This allowed us to compare the observed patterns to those predicted under different scenarios regarding the evolution of polyandry.
2 MATERIALS AND METHODS
2.1 Establishment of isofemale isogenic lines
2.1.1 Collection and maintenance
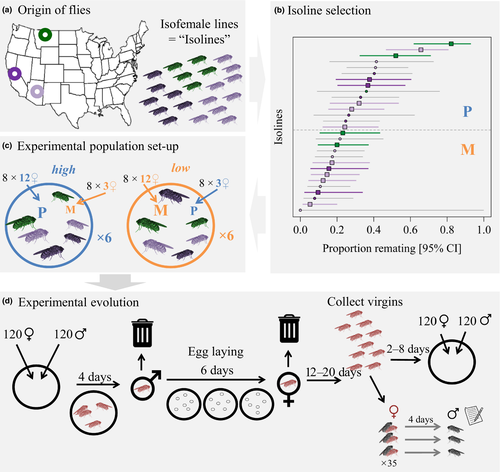
We established isofemale isogenic lines using wild female D. pseudoobscura from three populations across the Western USA (Lewistown Montana, Show Low Arizona, and Shaver Lake California). We reared full-sib inbred offspring of wild-caught females for 15 or more generations, maintaining flies under standardized laboratory conditions throughout. We give a schematic overview of our methods in Figure 1 and describe full details for our methods in the Supporting Information.
2.1.2 Preliminary assays
We first quantified variation in genetic predisposition for polyandry in 29 isolines using a remating assay routinely performed in our laboratory (Herrera et al., 2014; Price et al., 2011; Taylor et al., 2016). We aspirated sexually mature virgin females from each isoline individually into a vial containing a single male from the same isoline. Males had been separated into individual vials the day before the mating assay to reduce effects arising from prior male–male interactions. We observed matings by scan sampling, and after 2 hr, we discarded all males and females that had not mated. Scan sapling was performed by one or two observers (depending on the size of the assay) who checked vials for mating pairs, observing every vial for a few seconds approximately every 2 min. Females were left to oviposit for 4 days, after which we aspirated them into the vial of a second male from their isoline and observed them for 2 hr by scan sampling. Female D. pseudoobscura do not remate within 24 hr (Snook & So, 2000), such that females had a maximum of two matings across the two assay days. We confirmed first matings by the presence of larvae in the oviposition vial, but were not able to ascertain sperm transfer in second matings. The proportion of females that remated ranged from 0 to 0.83 for individual isolines (mean 0.28; 28 ± 10 females tested per isoline; Figure 1b and Table S1). A likelihood ratio test between binomial GLMMs including or excluding isoline identity as a random effect confirmed that this variation between isolines was substantial and statistically significant (χ2 = 42.1, df = 1, N = 821, p = 8.7 × 10−11).
2.1.3 Selecting focal isolines
To establish our experimental evolution replicates, we chose 16 isolines from the three populations fulfilling the following three criteria: (a) eight isolines had to have a relatively high (i.e. more polyandrous P lines) versus relatively low (i.e. relatively monandrous M lines) frequency of polyandry (see Figure 1), (b) P and M isolines had to be balanced with regard to population of origin, and (c) polyandry had to have been tested for a satisfactory number of females (N = 21–41). Although this meant that the exact threshold that separated P from M isolines was arbitrary, our method helped avoid biases with respect to representation of the three populations of origin. We repeated the polyandry assay for the 16 chosen isolines before starting experimental evolution, this time giving females two mating opportunities with outbred tester males (population from Chiricahua, Arizona) to minimize male effects on polyandry estimates. The remating proportion of isolines was significantly correlated between this and the prior assay (linear regression weighted by sample size: R2 = 0.43, F1,14 = 12.15, p = 0.004; see Table S1).
2.2 Experimental evolution
2.2.1 Population set-up and maintenance
We established six replicate experimental evolution populations for each of two treatments. We used all 16 isolines (eight P, eight M isolines) in all 12 replicates, but varied the relative representation of the isolines between the treatments. We initiated low polyandry replicate populations with twelve females and twelve males from each of the eight M isolines, and three females and three males from each of the eight P isolines. In contrast, we founded high polyandry replicate populations with three flies of both sexes from each M isoline and twelve flies of both sexes from each P isoline (Figure 1c). Thus, we founded all 12 replicate populations with 120 virgin females and 120 virgin males, maintained in large plastic tubs within a single incubator under standard conditions. From day one to five, flies mated freely for 4 days. On day five, we removed males and left females to oviposit for further 6 days across three sets of vials (Figure 1d). Adult offspring eclosing from these vials were collected as virgins across multiple days and used to create the next generation. Population identity was blinded for all procedures after the initial population set-up. See our supplementary methods for detailed procedures.
Every generation, we obtained an estimate of the frequency of polyandry for each of the twelve experimentally evolving populations as described in detail above and in the supplementary methods. We used tester males from the unrelated Chiricahua population and allowed a minimum of 90 min of observation in each assay.
2.2.2 Statistical analyses
We used r version 3.4.2 (R Core Team, 2018) for all statistical analyses and figures, running linear mixed effects models (LMM) and generalized linear mixed effects models (GLMM) implemented in the lme4 package version 1.1-14 (Bates, Maechler, Bolker, & Walker, 2015). We extracted effect sizes and p values from full models to avoid biasing effect sizes through the removal of nonsignificant terms (Forstmeier & Schielzeth, 2011). p Values from LMMs were obtained from F tests using the Kenward-Roger approximation for denominator degrees of freedom implemented in lmerTest (Kuznetsova, Brockhoff, & Christensen, 2016). We centred all covariates to a mean of zero to facilitate the interpretation of main effects in the presence of interactions and to aid model convergence. Age covariates were mean-centred, and order was centred and scaled to a standard deviation of one. We centred contrasts between two factors (high and low populations, P and M isolines) by coding factor levels as −0.5 and 0.5, respectively (Schielzeth, 2010). We calculated approximate 95% confidence intervals (CI) for effect sizes as twice the standard error either side of the mean (Crawley, 2007).
We analysed the evolution of the frequency of polyandry using female remating as our binary response variable in a binomial GLMM. Our main interest was in how the frequency of polyandry changed over generations from the two respective starting frequencies, that is backgrounds (low vs. high). Thus, our fixed effects were background, generation and their interaction. Generation was centred at the experimental evolution midpoint of four generations. We included as further fixed effects the age of the female and both males (first and second mate), as well as the order in the assay to control for potential variation arising from age variation and time available for mating in a given assay. To control for sources of nonindependence between measurements and for stochastic day effects, we modelled random intercepts for female post-eclosion vial ID (4.7 ± 1.3 females from the same post-eclosion vial were used in an assay), population replicate as well as assay day, and random slopes over the seven generations for each population replicate (Schielzeth & Forstmeier, 2009). We removed females (N = 74) for which we could not confirm fertilization during their first mating through the presence of larvae in their oviposition vial.
2.3 Assays after experimental evolution
After seven generations of experimental evolution, we subjected all experimental populations to one generation of common garden breeding and used the offspring for our final assays described below. Because polyandry assays can be subject to substantial block effects, comparisons of absolute estimates of the frequency of polyandry cannot be made across assays conducted on different days. Thus, to make direct comparisons not only between experimentally evolved replicate populations, but also between the ancestral isolines and the experimentally evolved populations, we simultaneously assayed flies from the twelve replicate evolved populations and from the 16 original ancestral isolines (see Nouhaud et al., 2016).
2.3.1 Female remating latency
To refine our comparisons, here we used female latency to remating (Price et al., 2008) as a more precise measure of polyandry that correlates with the proportion of females remating given one opportunity (Price et al., 2008, 2011). All 12 populations and 16 isolines were simultaneously tested in each of two experimental blocks. Mating assays followed our general methods for remating assays described above, with the difference that here females were given a remating opportunity every day from two to five days after their first mating, or until they remated. Due to logistical limitations in obtaining several hundreds of virgin tester males for every mating day, we re-used some males for remating opportunities, such that our assays included some nonvirgin tester males that had been sexually rested for at least 2 days. We found that female remating was not affected by mating status of tester males (data not shown).
Because data for remating latency were right-censored (23% of females did not remate in any of their four opportunities), we analysed remating analogous to death in survival models, using mixed effects cox models implemented in the coxme package (Therneau, 2015). We used days to remating as a right-censored response variable. As fixed effects, we included focal female background (two levels: P/high and M/low), female age, age of the first male and order in the assay. Fixed effects were centred and scaled as described above. Female post-eclosion vial, nested within population replicate or isoline, and experimental block were included as random effects. We first ran separate models on ancestral isolines and evolved populations, respectively. To ask whether populations had evolved polyandry levels different from their initial set-up, we then simulated resampling of our set-up of the 12 population replicates from the 16 ancestral isolines before experimental evolution, using for loops in r. We ran coxme models on 1,000 simulated data sets to obtain a distribution of the inferred initial difference between low versus high polyandry population replicates, with the sample size reflecting our remating latency assay (see supplementary methods). We compared the observed difference between evolved low and high polyandry populations to that distribution under the null hypothesis that the difference in polyandry between the populations did not change during experimental evolution. Similarly, we compared the simulated populations (i.e. inferred remating latencies in the population replicates before experimental evolution) with the observed remating latencies of the experimentally evolved populations.
2.3.2 Remating inhibition by males
To investigate potential male effects on female remating, we assessed variation in the ability of males from the 12 populations and 16 isolines to induce a refractory period (i.e. male remating inhibition) in females from the tester (Chiricahua) population. We used variation in the proportion of tester females that remated with tester males four days after mating with focal males as our proxy for variation in remating inhibition by focal males. We conducted the experiment across two blocks and used the same methods as for our polyandry assays during experimental evolution. In the second block, we quantified reproductive output after the first mating to test for its association with remating inhibition (see Supporting Information).
In this assay, higher tester female remating would indicate lower remating inhibition by focal males. Our main questions were whether our experimental evolution protocol had generally changed male remating inhibition, whether experimental evolution under our low versus high polyandry regime had manifested in differences in males’ ability to inhibit remating (Price et al., 2010b), and, if so, whether the difference already existed in the isolines used to initiate the populations. We used GLMMs with female remating as a binary response and included focal male background, the ages of the female and both her (potential) mates as well as order in the assay as fixed effects. Random effects were female post-eclosion vial nested within experimental block and the genetic background (isoline/replicate population) of the focal first-to-mate male. Ancestral and evolved populations were compared in analogy to female remating latency, using resampling to simulate the experimental set-up of the population replicates (see Female remating latency).
To explore a possible pre-existing genetic correlation between female mating behaviour and male remating inhibition, we first obtained predictions for isolines for both female remating latency and male remating inhibition. We used a linear model for remating latency and a generalized linear model for remating inhibition with isoline ID as well as age and order (centred and scaled) and block (centred) as fixed effects. Thus, we ignored variation between female post-eclosion vials, which was found to be very small in the previous mixed models (see Tables 2 and 3). To test for a correlation between female remating latency and male remating inhibition, we used linear regression on the predictions for the 16 isolines, backtransformed from the latent scale for male remating inhibition and weighted by the combined sample sizes of the female and male assays. We excluded evolved populations from this analysis to avoid pseudo-replication arising from repeated representation of isoline genotypes in the evolved population replicates.
2.3.3 Fecundity after experimental evolution
Finally, we measured fecundity of females that evolved in populations with relatively high versus relatively low levels of polyandry. We used the same methods as for our standardized polyandry assays, except that females were paired with males from their own replicate population. Females were subjected to different remating regimes to test for phenotypic effects of polyandry on fecundity. We randomly chose four to five females per population that were not given a remating opportunity (i.e. forced monandry), aspirating the male out of his vial before the female was introduced. The remaining females (12–15 per population) had one opportunity to remate 4 days after their initial mating. After their denied or realized remating opportunity, females oviposited for 6 days across two vials. We incubated vials under standard conditions and counted the total number of offspring eclosed 9 days after the first eclosion in a given vial.
To explore variation in female fecundity, we pooled counts of eclosed offspring from the two vials in which females had oviposited for 3 days each after their second mating opportunity, thus matching the oviposition period used during experimental evolution. Our full LMM included female background (low vs. high), remating regime (forced monandry, elected monandry and polyandry), their interaction, and age of the female and her first mate (both centred) as fixed effects. We included post-eclosion vial nested within replicate population as random effects.
3 RESULTS
3.1 Experimental evolution of polyandry
The overall frequency of polyandry across all mating assays over seven generations was 34.1%, but there was substantial variation between generations and between replicate populations (Figure 2). Each generation, we aimed to test 35 females per population. However, failed first matings (8%) mortality between the two assays (3%) and the absence of larvae in the oviposition vial (2%) meant that we estimated the frequency of polyandry for each replicate population at every generation from an average of 30.5 females (N = 2,559 across seven generations).
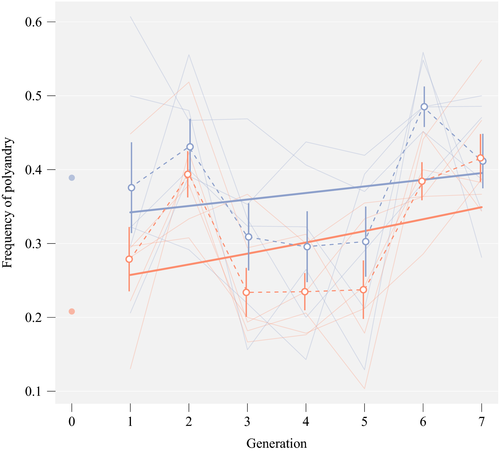
Inspection of our binomial GLMM on polyandry revealed that the interaction between generation and background was small and not significantly different from zero (effect size [approx. 95% CI] on the logit scale = 0.03 [–0.07;0.14]; p = 0.517; Table 1), meaning that there was neither evidence for convergence nor divergence of the frequency of polyandry between the populations with high and low polyandry backgrounds. There was a clear main effect of background indicating that polyandry was indeed lower in the low background (–0.30 [–0.52;–0.08]; p = 0.006), that is the populations that had been set up with predominantly low polyandry genotypes. There was also a slight positive trend of generation showing a general increase in polyandry over time (0.06 [–0.02;0.13]; p = 0.119). The first male's age had a clear negative effect on remating, meaning that females mated to older males were less likely to remate 4 days later. The age of the female and of the second male had no significant impact on polyandry. The order in the assay showed a minor negative trend, with flies entering the assay later having a slightly lower probability of remating (Table 1).
| Polyandry exp. evolution (N = 2,517) | glmer (logit scale) | |||
|---|---|---|---|---|
| Fixed effects | Coef | SE (coef) | z | p |
| Intercept | −0.690 | 0.072 | −9.64 | <0.001 |
| Female age (centred) | 0.048 | 0.038 | 1.27 | 0.204 |
| First male age (centred) | −0.199 | 0.053 | −3.78 | <0.001 |
| Second male age (centred) | 0.039 | 0.027 | 1.45 | 0.146 |
| Order (centred & scaled) | −0.075 | 0.046 | −1.63 | 0.103 |
| Generation (centred) | 0.055 | 0.036 | 1.56 | 0.119 |
| Background (centred; low vs. high) | −0.302 | 0.111 | −2.73 | 0.006 |
| Generation:background | 0.035 | 0.054 | 0.65 | 0.517 |
| Random effects | Var | SD | ||
|---|---|---|---|---|
| Post-eclosion vial (545 levels) | <0.001 | <0.001 | ||
| Replicate (12 levels) | 0.117 | 0.342 | ||
| Generation:replicate (12 random slopes) | 0.003 | 0.056 | ||
| Assay day (7 levels) | 0.014 | 0.120 |
Note
- Coefficients, standard errors, test statistics and variance components are taken from a GLMM on female remating (binary response) and are consequently on the logit scale. Continuous and factorial covariates were centred and scaled as described in the main text, such that the global intercept describes the prediction for the midpoint for all covariates. Effects associated with a p value smaller than 0.05 are highlighted in bold.
3.2 Polyandry in isolines and after experimental evolution
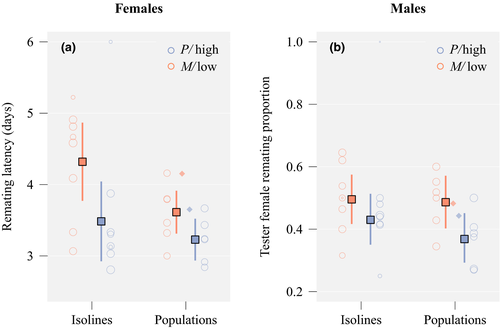
We assessed latency to remating in females from each of the 12 populations and 16 isolines. Figure 3a illustrates differences between isolines and experimentally evolved populations, and between high polyandry and low polyandry isolines and populations, assigning females that did not remate a maximum remating latency of 6 days. In total, 156 pairs of virgin flies did not mate (total N = 894). Failed matings were heavily biased towards three of the four isolines that originated from the Shaver Lake population (76–83% mating failure), resulting in small sample sizes for these isolines (N = 6–9 vs. N = 18–36 for other lines). After removal of females that died before their first remating opportunity, our final sample size for remating latency was 734 females, of which 169 (isolines: 86 M, 33 P; populations: 30 low, 20 high) were right-censored, that is had not remated by day six. Not surprisingly, M isolines had a longer remating latency than P isolines (odds ratio for remating [approx. 95% CI]: 0.49 [0.27;0.92]; N = 419; p = 0.023; Table 2, Figure 3a and Figure S1). In our evolved population replicates, we found correspondingly that low populations had a longer latency to remating than high populations (odds ratio 0.72 [0.53;0.99]; N = 315; p = 0.037). Females initially mated to older males were slower to remate, female age did not matter, and females with a later order in the assay (i.e. less time allowed for remating) showed delayed remating, which was statistically significant in the population subset but not in the isoline subset (Table 2). The comparison of the observed evolved populations to the populations simulated based on resampling of isoline females revealed the observed difference between low and high population replicates (odds ratio) to be remarkably similar to that in the simulated data sets (odds ratio observed 0.72; simulated 0.71 [0.53;0.93]; p = 0.866). However, females from evolved population replicates generally remated faster than expected based on the simulated ancestral composition of population replicates (odds ratio 1.70 [1.47;1.95]; p < 0.001; Figure 3a).
| Latency to remating | Isoline females (N = 419) | Evolved females (N = 315) | ||||||
|---|---|---|---|---|---|---|---|---|
| Fixed effects (coxme) | Coef | SE (coef) | z | p | Coef | SE (coef) | z | p |
| Female age (centred) | 0.004 | 0.047 | 0.08 | 0.930 | 0.015 | 0.046 | 0.32 | 0.750 |
| First male age (centred) | −0.164 | 0.053 | −3.10 | 0.002 | −0.144 | 0.056 | −2.58 | 0.010 |
| Order (centred & scaled) | −0.075 | 0.928 | −1.10 | 0.270 | −0.166 | 0.065 | −2.54 | 0.011 |
| Background (centred; low vs. high) | −0.704 | 0.495 | −2.28 | 0.023 | −0.323 | 0.155 | −2.09 | 0.037 |
| Random effects | Var | SD | Var | SD | ||||
|---|---|---|---|---|---|---|---|---|
| Housing vial | 0.058 | 0.242 | 0.045 | 0.211 | ||||
| Isoline/Population | 0.296 | 0.544 | 0.139 | 0.373 | ||||
| Block (2 levels) | 0.004 | 0.060 | <0.001 | 0.019 |
Note
- Remating latency was analysed analogous to survival using the coxme function, with females that did not remate entered as right-censored data points. Continuous and factorial covariates were centred as described in the main text. Effects associated with a p value smaller than 0.05 are highlighted in bold.
3.3 Male influence on female remating?
Analogous to the assay on female latency to remating, failed mating trials between focal males and tester females were heavily biased towards three of the isolines originating from the Shaver Lake population (76%–98% mating failure). Sample sizes for these isolines were consequently very small (N = 1–8 vs. N = 19–33 for other isolines/populations; total N = 710).
There was no difference in the likelihood of tester female remating after mating with males from M versus P isolines (effect on logit scale 0.23 [−0.21;0.67]; N = 363; p = 0.301). Males from low polyandry population replicates showed a tendency to be less effective at reducing tester female remating relative to males from high polyandry populations, although this was marginally nonsignificant (effect on logit scale 0.43 [−0.02;0.89]; N = 347; p = 0.059; Figure 3b). Male effects on female remating were not simply mediated through male effects on female reproductive output (see Supporting Information). Additionally, there were effects of the age of females and both males on the probability of remating, with consistent effect signs but varying effect sizes between tests on isolines and evolved populations (Table 3). Generally, older females were more likely to remate, older first males reduced remating later on, and females were more likely to remate when presented with younger tester males. These results were robust to omitting pseudo-polyandrous females (i.e. females with no larvae in their oviposition vial), thus only focussing on fertilized females (N = 694).
| Tester female remating | Isoline males (N = 363) | Evolved males (N = 347) | ||||||
|---|---|---|---|---|---|---|---|---|
| Fixed effects (binomial GLMM) | Coef | SE (coef) | z | p | Coef | SE (coef) | z | p |
| Intercept | −0.117 | 0.115 | −1.01 | 0.312 | −0.301 | 0.119 | −2.54 | 0.011 |
| Female age (centred) | 0.272 | 0.111 | 2.44 | 0.015 | 0.185 | 0.101 | 1.82 | 0.069 |
| First male age (centred) | −0.182 | 0.088 | −2.08 | 0.038 | −0.104 | 0.085 | −1.23 | 0.218 |
| Second male age (centred) | −0.270 | 0.139 | −1.94 | 0.052 | −0.260 | 0.157 | −1.66 | 0.097 |
| Order (centred & scaled) | 0.129 | 0.127 | 1.02 | 0.307 | −0.155 | 0.147 | −1.05 | 0.293 |
| Background (centred; low vs. high) | 0.228 | 0.220 | 1.04 | 0.301 | 0.434 | 0.229 | 1.89 | 0.059 |
| Random effects | Var | SD | Var | SD | ||||
|---|---|---|---|---|---|---|---|---|
| Tester female housing vial | 0.093 | 0.305 | 0.062 | 0.120 | ||||
| Male isoline/population | <0.001 | <0.001 | 0.002 | 0.041 | ||||
| Block (2 levels) | <0.001 | <0.001 | <0.001 | <0.001 |
Note
- Coefficients, standard errors, test statistics and variance components are taken from GLMMs on tester female remating (binary response) and are consequently on the logit scale. Continuous and factorial covariates were centred and scaled as described in the main text. Effects associated with a p value smaller than 0.05 are highlighted in bold.
The comparison of the observed evolved populations to the simulated populations based on resampling of remating inhibition by isoline males showed a minor trend for a greater difference between high and low population replicates after experimental evolution than expected based on the simulated initial population set-up (observed 0.43; simulated 0.09 [−0.33;0.53]; p = 0.139). This was probably mainly driven by evolved high polyandry replicates (Figure 3), with males from evolved population replicates overall inhibiting female remating more efficiently than expected based on the simulated ancestral composition of population replicates (effect size for tester female remating on logit scale −0.20 [−0.41;0.02]; p < 0.033).
Finally, we found no evidence for a genetic correlation between female remating latency and male remating inhibition in our 16 original isolines. The correlation coefficient was positive but not significantly different from zero (0.05 [−0.02;0.12], F1,14 = 2.17, p = 0.163).
3.4 Fitness effects of polyandry?
We pooled counts of offspring eclosing from the two vials in which individual females (N = 226) from evolved population replicates had oviposited over a combined period of 6 days. There was no significant influence of any of the variables included in the full model, except for significant variation between population replicates (p = 0.024; Table S2 and Figure S5). Thus, there was no significant difference in fecundity between females from a low versus high polyandry background, nor was there an effect of mating phenotype, that is of whether the opportunity to remate was experimentally prevented, or refused or accepted by the female. Finally, there was no support for the interaction between genetic background and mating phenotype.
4 DISCUSSION
What drives and maintains variation in polyandry between and within populations is poorly understood. Here, we used naturally occurring genetic variation in polyandry and investigated whether experimental populations that started with a high versus low initial frequency of polyandry would show evidence for balancing or directional selection, or evolve neutrally. We found that the frequency of polyandry remained remarkably stable over time, remaining relatively low in populations with an initially lower frequency, and relatively high in populations with an initially higher frequency of polyandry. Thus, we found no clear evidence for directional or balancing selection on polyandry. Despite starting with a substantial difference in polyandry in the high versus low polyandry populations, remarkably we found no difference in fecundity between females from these populations, and no significant change in the difference between these populations over time which would have indicated fitness consequences of polyandry. Data on male inhibition of female remating showed a trend consistent with previous findings that males evolve enhanced remating inhibition in response to elevated female remating (Price et al., 2010b). This indicates ongoing evolution in males in our experimental populations, but the absence of a correlation between polyandry and male remating inhibition in ancestral isolines suggests selection can operate independently on male and female traits. Overall, our findings are consistent with genetic control over female remating behaviour, but indicate that polyandry does not have strong fitness consequences under these conditions.
4.1 Neutral experimental evolution of polyandry?
Populations initiated with many polyandrous females maintained a higher frequency of polyandry than did populations initiated with relatively fewer polyandrous females (Figure 2). Our assay on female remating latency after one generation of common garden breeding allowed us to directly compare experimentally evolved populations with ancestral isolines and confirmed genetic differences between the high and low polyandry populations. Importantly, using tester males that had not co-evolved with females allowed us to assess selection on polyandry independent of selection acting on males. There was only a very minor tendency for populations to be more similar after experimental evolution than when they were initially founded; we found no clear evidence for convergence towards a common polyandry frequency. We experimentally evolved populations for only seven generations, admittedly limiting our power to detect convergence. Indeed, the best model estimates based on assays during experimental evolution (Table 1) suggested that high and low populations might indeed have converged after a few more generations. However, in our remating latency assays where we tested experimentally evolved and ancestral isolines simultaneously—arguably a more accurate comparison—the observed difference between high and low populations after seven generations of experimental was only very marginally smaller than expected based on our resampling simulation of the initial isoline composition (odds ratios 0.72 and 0.71, respectively), suggesting populations would only fully converge after more than 100 generations. This was in contrast to the trend observed for male remating inhibition (Figure 3b), which suggested that a rapid response was possible despite the limited timeframe. Rather than convergence in polyandry levels, the patterns from the female remating assays both during (Figure 2) and after experimental evolution (Figure 3a) suggested a parallel increase in polyandry in the evolved populations relative to the ancestral isolines. This increase was visible as a trend across seven assays during experimental evolution and reached statistical significance only in the direct comparison between ancestral and evolved females. The small number of matings between individuals from the Shaver Lake isolines and tester individuals from the Chiricahua population weakened our direct comparison between isolines and evolved populations. Generally, Shaver Lake flies appeared to have reduced compatibility with flies from the other populations (see Supporting Information for more details). However, Shaver Lake isolines represented average polyandry genotypes both within the P and M isoline groups (cf. Figure 1b) and our balanced design would have prevented a systematic bias in polyandry arising from selective disappearance of Shaver Lake genotypes. The observed increase in polyandry could indicate a selective advantage of polyandry alleles in all populations due to a superior fitness of highly polyandrous genotypes. Under this scenario however, selection should favour the high polyandry alleles both in high and low polyandry populations, and the populations to consequently converge towards a high frequency of polyandry. Alternatively, the increase in polyandry could be a manifestation of condition-dependent polyandry. Experimentally evolved females have high heterozygosity and might therefore have higher fecundity and remate more than highly inbred isoline females, for example due to reduced costs of mating (Perry, Sharpe, & Rowe, 2009) or higher demands for sperm numbers. Whether the observed increase in polyandry reflects a change in the frequency of high polyandry alleles or represents a phenotypically plastic response that is independent of allele frequency changes is currently unknown. Although we acknowledge that the duration of our experiment meant limited power to detect convergence, we believe that the phenotypic plasticity explanation is more consistent with our observation that the increase in polyandry was parallel in both the low and high polyandry populations.
Experimentally investigating the evolution of polyandry without manipulating access to mates is challenging, because monandrous females can typically not be forced to mate multiply (but see Arnqvist & Andrés, 2006; King & Bressac, 2010). As a consequence, the majority of evidence for the benefits of polyandry has come from experiments where naturally polyandrous females were denied the possibility for multiple mating. Although experimentally manipulating sex ratio may offer much insight into how selection from sperm competition acts on males, enforcing a particular mating frequency on females may reveal little about why there is so much variation in female mating strategies (Taylor et al., 2014). Our design allowed us to initiate replicate populations with substantial differences in the average frequency of polyandry without altering the sex ratio or manipulating female access to mates, allowing for a more realistic competition between different female strategies. To our knowledge, only one previous study has employed genetic variation in female mating behaviour to manipulate sexual selection. Using a sex peptide receptor knockout to render females hyper-promiscuous, the study highlighted that purely manipulating the mating frequency may have consequences for sexual selection that are different from those of sex ratio manipulations (Perry et al., 2016). Genetic variation in polyandry is potentially very widespread (Taylor et al., 2014), so utilizing it offers an invaluable experimental tool for improving our understanding of the evolution of polyandry in semi-natural conditions.
4.2 Consequences of polyandry for males
Consistent with previous findings in D. pseudoobscura, we found that males had some effect on female remating behaviour. Across all experiments, age of the first male had a consistently negative effect on female remating (Tables 1-3). This effect could have been driven by age-dependent variation in male accessory gland size (Ruhmann, Wensing, Neuhalfen, Specker, & Fricke, 2016) and/or by older males allocating larger ejaculates during mating (Avent, Price, & Wedell, 2008). We cannot tell whether reduced remating after mating with older males represents male suppression of female remating decisions or adaptive female mate choice, given that females can benefit directly from mating with older males (Avent et al., 2008; Verspoor, Cuss, & Price, 2015). However, we found no evidence for a preference for older males during rematings (in fact, there was a trend for the opposite effect), thus favouring the idea that reduced remating propensity reflects a male effect. Indeed, our results on experimentally evolved males were in agreement with previous results showing that more frequent remating by females selects for improved remating inhibition in males (Crudgington, Beckerman, Brüstle, Green, & Snook, 2005; Price et al., 2010b; Figure 3b). Our direct comparison between isolines and evolved populations indicated that the tendency for higher remating inhibition by males that had experimentally evolved with high polyandry was not driven by a pre-existing genetic correlation between polyandry and male remating inhibition. In support of this interpretation, there was no difference in remating inhibition in M versus P isolines, and no correlation between female remating latency and male remating inhibition across the 16 isolines (Figure S2).
4.3 Polyandry does not affect fecundity
After seven generations of experimental evolution and one generation of common garden breeding, we found no evidence that genetic polyandry was associated with higher fecundity. Although we found variation between evolved populations (Figure S5), this variation did not co-vary with polyandry levels, suggesting polyandry does not evolve simply through a genetic correlation between polyandry and fecundity. Indeed, early-life fecundity was neither linked to genetic variation in polyandry nor to phenotypic variation in polyandry (Table S2). Moreover, we found no evidence that females evolving with higher polyandry levels became dependent on polyandry, which would have manifested in increased costs of forced monandry. In combination, this means that the overall increase in polyandry after experimental evolution (see above) is unlikely to have been caused by a direct or correlated response to selection on fecundity. Unlike our fecundity assay after experimental evolution which focused on the effect of polyandry on a single fitness measure in isolated females, tracking polyandry during experimental evolution was an integrated measure of the costs and benefits of polyandry. Thus, potential costs of polyandry manifesting through injury, sexually transmitted diseases or foregone foraging opportunities would have operated simultaneously with potential direct benefits of fertility assurance, and indirect genetic effects of good genes or sexy sperm (Arnqvist & Nilsson, 2000; Jennions & Petrie, 2000). The absence of clear changes in polyandry levels in our populations indicates that these costs and benefits are of small effect or that the costs and benefits are balanced, at least under our laboratory conditions.
4.4 What maintains genetic variation in polyandry?
Despite a considerable body of work on the costs and benefits of polyandry, and many empirical demonstrations of fitness effects, genetic variation in and experimental evolution of polyandry, what drives and maintains variation in polyandry between and within wild populations remains elusive. Given there are many factors that can influence multiple mating, including stochastic variation between females, phenotypic variation in polyandry rather than monandry may well be the null model (Gowaty, 2013; Kokko & Mappes, 2013). However, if polyandry is adaptively flexible, why should genetic variation in polyandry persist (Gowaty, 2013)? One potential answer is fluctuating selection imposed by fluctuating environmental conditions, which can favour the maintenance of alternative polyandry genotypes in butterflies (Välimäki et al., 2008; Wedell, Wiklund, & Cook, 2002). Or perhaps genetic variation is simply the product of mutation-selection balance? Indeed, if polyandry is a highly polygenic trait that is largely selectively neutral in many females, then we might expect substantial genetic variation arising through random mutation that is not counteracted by strong selection. If so, then we might expect to find genetic variation predominantly in species and populations where polyandry has little effect on reproductive fitness. To understand the evolution of polyandry, we need to better understand the genetic basis of polyandry and the evolutionary processes that increase and decrease genetic variation in polyandry.
4.5 Summary
In this study, we confirmed strong genetic control over remating decisions in female D. pseudoobscura. Populations initiated with a high versus low frequency of alleles conferring a predisposition for polyandry maintained their genetic differences in polyandry over time. We found no evidence for balancing selection, and little evidence for positive selection on polyandry.
ACKNOWLEDGMENTS
We thank Ali Skeats and Michelle Taylor for establishing isofemale isogenic lines. This work was supported by Swiss National Science Foundation fellowships awarded to AS (Grants P2ZHP3_164990 & P300PA_177906) and a Marie Skłodowska-Curie Fellowship to KO (Grant 746169 – IMMUNFUNC). We are grateful to editors and reviewers for constructive feedback on previous versions of this manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
NW and TARP conceived the study; AS, TARP and NW designed the study; AS collected the data with help from LMT, MW and KO; AS performed the analyses; AS wrote the manuscript, with input from NW, TARP and LMT; all authors approved the manuscript.
DATA ACCESSIBILITY
Data deposited at Dryad: https://datadryad.org/resource/doi:10.5061/dryad.h0m01sf/1




