Reproductive fitness consequences of progenesis: Sex-specific pay-offs in safe and risky environments
Abstract
Progenesis is considered to have an important role in evolution because it allows the retention of both a larval body size and shape in an adult morphology. However, the cost caused by the adoption of a progenetic process in both males and females remains to be explored to explain the success of progenesis and particularly its biased prevalence across the sexes and environments. Here, through an experimental approach, we used a facultative progenetic species, the palmate newt (Lissotriton helveticus) that can either mature at a small size and retain gills or mature after metamorphosis, to test three hypotheses for sex-specific pay-offs of progenesis in safe versus risky habitats. Goldfish were used because they caused a higher decline in progenetic than metamorphic newts. We determined that progenetic newts have a lower reproductive fitness than metamorphic newts. We also found that, when compared to metamorphs, progenetic males have lower reproductive activity than progenetic females and that predatory risk affects more progenetic than metamorphic newts. By identifying ultimate causes of the female-biased sex ratios found in nature, these results support the male escape hypothesis, that is the higher metamorphosis rate of progenetic males. They also highlight that although progenesis is advantageous in advancing the age at first reproduction, it also brings an immediate fitness cost and this, particularly, in hostile predatory environments. This means that whereas some environmental constraints could favour facultative progenesis, some others, such as predation, can ultimately counter-select progenesis. Altogether, these results improve our understanding of how developmental processes can affect the sexes differently and how species invasions can impair the success of alternative developmental phenotypes.
1 INTRODUCTION
Paedomorphosis is a key developmental mode that involves the retention of larval ancestral traits in the adults of descendants (Garstang, 1922, 1928). When this heterochrony bypasses metamorphosis—a spectacular body transformation—paedomorphosis produces reproductive organisms largely different from their ancestors (Gould, 1977; Laudet, 2011). As these changes involve the relative timing and rate of development of the somatic versus gonadal tissues, they do not require deep genetic changes (Gould, 1982; McKinney & McNamara, 1991). Consequently, this leads to the consideration that paedomorphosis plays a major role in both micro- and macro-evolution (Bonett & Blair, 2017; Garstang, 1954; Gould, 1977; Long, 1990; McNamara, 2012). However, the understanding of this role necessitates that we focus at the level of the developmental processes that produce paedomorphosis (Gould, 1977). Actually, there are two main distinct recognized processes determining paedomorphosis: neoteny and progenesis. Although neoteny results in paedomorphosis through a retardation of somatic development, progenesis makes it possible through a precocious sexual maturation, and therefore a truncation of ontogeny and a reproduction at a small size (Alberch, Gould, Oster, & Wake, 1979; Giard, 1887; Gould, 1977; McNamara, 1988). Progenesis is often considered more adaptive than neoteny in opening the exploitation of vacant spatial niches out of reach by larger individuals and in improving fitness through a precocious access to sexual partners (Gould, 1977). Typical examples of exploitation of new habitats refer to the meiofauna which is composed of miniaturized adults that can colonize and move easily through the sediment (Snyder & Bretsky, 1971; Struck et al., 2015; Westheide, 1987) and to parasites that specialize on their larger hosts (Lefebvre & Poulin, 2005; Stunkard, 1959). With regard to reproduction itself, progenesis is regarded as advantageous because an early breeding can provide a higher fitness, therefore reducing generation time and, ultimately, possibly favouring speciation (Gould, 1977; Hanken & Wake, 1993; Kon & Yoshino, 2002; Ryan & Semlitsch, 1998).
The pay-offs of reproducing at a small juvenilized stage are expected to differ across the sexes and may have given rise to a contrasted evolution of progenesis between the sexes. In protandrous progenesis, the size reduction of males allows them to live on or within their female host (Giard, 1887; McKinney & McNamara, 1991) and to get an earlier access to them (Adamson, 1990). The sex-specific prevalence of progenesis suggests that progenesis can also result in some costs due to its associated miniaturization (Adamson, 1990). In most ectotherms, such as arthropods, fish, amphibians and reptiles, there is indeed a positive correlation between body size and clutch size in females (fecundity selection) and between body size and mate choice (sexual selection; Edward & Chapman, 2011; Pincheira-Donoso & Hunt, 2017). Accordingly, progenesis was shown to cause a lower fecundity in females in several groups such as millipedes and molluscs (Gould, 1977), but also in amphibians (Wake, 1986). However, because of their adaptation to unstable habitats, progenetic organisms have also been considered as r-strategists, with a high fecundity (McNamara, 1988). Finally, because reproduction may be riskier for one of the sexes as a consequence of visibility or vulnerability to predators (Magnhagen, 1991) and because prey–predator interactions are mediated by the size of organisms (Cohen, Pimm, Yodzis, & Saldaña, 1993), it can be expected that progenesis would have a different payoff in males and females across safe and risky environments.
Polymorphisms are excellent models in which to explore the fitness consequences of progenesis because they can produce alternative size-dimorphic morphologies that have a common evolutionary history and share similar or even identical habitats during a part of their life (Kalezić, Cvetković, Djorović, & Džukić, 1996; Lefebvre & Poulin, 2005; West-Eberhard, 2003; Whiteman, 1997). Gould (1977) used the term “facultative progenesis” to denote these polymorphisms. This has been shown since to be typical of many populations of newts and salamanders where both sexes are progenetic, leading to the coexistence of small and young gilled adults with larger and older metamorphosed adults in the same water bodies (Denoël, Ivanović, Džukić, & Kalezić, 2009; Denoël & Joly, 2000). Although both phenotypes can exhibit sexual traits, such as a developed cloaca (Denoël, 2017) and seasonal epigamous characters (Winandy & Denoël, 2015b), the progenetic males are rarer than progenetic females in newt populations, with sex ratios that can be as biased as five females per male (Gabrion, 1976). This is in part explained by the higher probability in males to metamorphose and escape water for land (Mathiron, Lena, Baouch, & Denoël, 2017; Denoël, Drapeau, Oromi, & Winandy, 2019) but this then suggests that this sex-specific response may be due to a reproductive cost borne only or mainly by males (see also Roff & Fairbairn, 1993; Whiteman, 1997). Moreover, by their aquatic mode of life, progenetic newts and salamanders are more vulnerable to predation than metamorphosed newts and may have their reproductive fitness differently affected. This is supported by the high declines found in natural populations of progenetic newts (Denoël & Winandy, 2015), but this needs to be experimentally tested.
By using a facultative progenetic species, the palmate newt, Lissotriton helveticus (Razoumowsky, 1789) as a model system (Denoël et al., 2009; Mathiron, Lena, Baouch, & Denoël, 2017), we aim to test the following hypotheses on the pay-offs of progenesis across the sexes and in risky versus safe environments: (a) under the “male escape” hypothesis, we predict that the pay-offs for progenetic males are lower than those of the progenetic females; (b) under the “reproductive cost” hypothesis of progenesis, we predict that progenesis induces a lower reproductive output (courtship in males, egg laying in females) because progenetic newts are smaller than metamorphic newts; and (c) under the “predator-risk” hypothesis of progenesis, we predict that, in predator-rich environments, progenetic newts are more affected than metamorphic newts.
2 MATERIALS AND METHODS
2.1 Study species and phenotypes
Palmate newts are pond-breeding salamandrid amphibians from Western Europe (Sparreboom, 2014). They are long-term aquatic breeders that start to breed after overwintering: males are not territorial and exhibit multiple courtship displays, after which females can lay eggs one by one, often on aquatic leaves (Griffiths, 1987; Miaud, 1993; Sparreboom, 2014). Sexual dimorphism and courtship are conspicuous. Males are distinguished from females by the shape of their cloaca as well as by secondary sexual traits, such as a tail filament (Halliday, 1975). The courtship includes mainly caudal movements, such as the fan, during which males vibrate their tails at females (Figure 1) but also the whip, during which males lash their tail more powerfully towards the female (Halliday, 1977; Wambreuse & Bels, 1984).
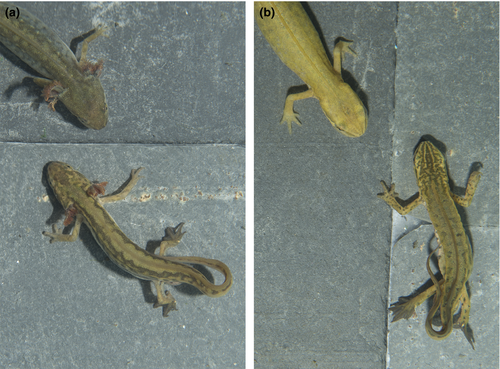
Facultative progenesis is frequent in palmate newts, with most known populations being in southern France where both phenotypes occur in syntopy in the same ponds (Figure 1; Denoël & Ficetola, 2014, 2015). The progenetic phenotype is smaller than the metamorphic phenotype as a result of an early maturation. Whereas progenetic newts become mature in their first year of life, metamorphosed newts spend a few years on land before their first reproduction (Denoël, 2017; Denoël & Joly, 2000; Denoël et al., 2009; Miaud, 1991).
2.2 Sampling and maintenance
Palmate newts were sampled by dip-netting in Mas d'Azirou pond in Larzac, France, at the start of the breeding season, that is, on 15 March 2015 (64 adults, 16 of each sex and phenotype). They were stored in tanks and carried to the laboratory in a large cooler. Their snout-vent length (from the tip of the snout to the end of the cloaca) and the length of their caudal filament (from the tip of the tail to the end of the filament) were measured.
Newts were then distributed into 16 identical glass aquariums (60 × 60 × 60 cm, 45 cm water depth) with two males and two females of either metamorphic or progenetic phenotype in each. Phenotypes were not mixed to be able to compare fecundity of progenetic versus metamorphic females and to consider reproductive pay-offs of both phenotypes independently. Drawings of each newt were done to recognize them individually inside each aquarium. All aquariums were independent, and opaque plates were placed between them. One half of the aquariums (n = 8) were devoid of goldfish, and the other half of the aquariums (n = 8) had two goldfish, Carassius auratus (Linnaeus, 1758) in them to simulate a predator-risk environment. The size of the goldfish (mean ± SE = 10.5 ± 0.2 cm) was specifically selected to scare the newts without hurting them (Winandy & Denoël, 2015a). Water temperature was maintained at 15°C, an optimal temperature for reproduction of the study species (Galloy & Denoël, 2010). The photoperiod followed the natural regime at the capture location. A shelter was provided in all aquariums, and the floor was covered by slates of natural rocks. Fish could not access the shelter of the newts. Sheets of coffee filters were provided to function as egg supports, simulating plants. Newts and fish were fed ad libitum everyday with unfrozen, washed bloodworms.
2.3 Behavioural observations and fecundity measures
Courtship observations were done 180 times per individuals (n = 32 males), that is three times a day (separated by at least 30 min), five mornings a week over a period of 12 weeks (i.e., 5,760 observations in total). At each observation session (1 min per aquarium), the presence versus absence of courtship activity from a male towards a female was noted. Courtship can be easily assessed because it involves stereotyped movements of the tail of the male (Figure 1; Wambreuse & Bels, 1984). The scores of the courtship activities were obtained by totalizing individual observations for each 2-week periods (i.e., 30 observations per individual per time interval of 2 weeks; six replicates).
The egg supports were checked every day for 12 weeks to collect eggs. They were replaced if eggs had been deposited and if they had started to degrade. The number of eggs was totalized for each 2-week periods (six time replicates), which resulted in a total of 96 scored observations.
2.4 Ethics
All applicable institutional and national guidelines for the care and use of animals were followed. The capture permit was issued by DREAL Languedoc-Roussillon (decree 2013274-0001). All experiments were approved by the University of Liège's animal ethical committee (authorization 1613).
2.5 Statistics
Newts were all measured for their body size (i.e., snout-vent length) and their secondary sexual traits (i.e., tail filament in males) before the beginning of the experiment. Using linear models, we assessed (a) the effect of phenotype and sex on newt snout-vent length and (b) the effect of phenotype on filament length in males. Since filament length is correlated with snout-vent length (R2 = 0.419, F1,30 = 23.39, p < 0.001), we used the residuals of the linear regression of filament length by snout-vent length. Compliance with requirements of the fitted linear models was checked using the Shapiro–Wilk normality test (for snout-vent length: W = 0.973, p = 0.18, n = 64; for filament length: W = 0.959, p = 0.25, n = 32).
We used generalized mixed models with a negative binomial error distribution to test the effect of fish (presence vs. absence), phenotype (progenetic paedomorphs vs. metamorphs) and their interaction on (a) the number of eggs laid by females and (b) the number of courtship displays by males. Because we repeated observations on the same individuals across time, we also included time as a (continuous) fixed factor (six replicated measures at identical time intervals, that is 2 weeks, over a period of 12 weeks), and we considered aquarium and identity as random factors (individuals nested in each aquarium). We used an information-theoretic approach based on the Akaike information criterion (AICc corrected for a small sample size) to select the best models, that is those with a delta AIC < 2, suggesting substantial evidence for the models (Burnham & Anderson, 2002). We calculated the significance of parameters using Type II Wald chi-square tests.
We chose an a priori level of significance of 0.05. Analyses were performed in R 3.4.2 using the lme4 and MuMIn packages.
3 RESULTS
3.1 Morphology: Size and secondary sexual trait
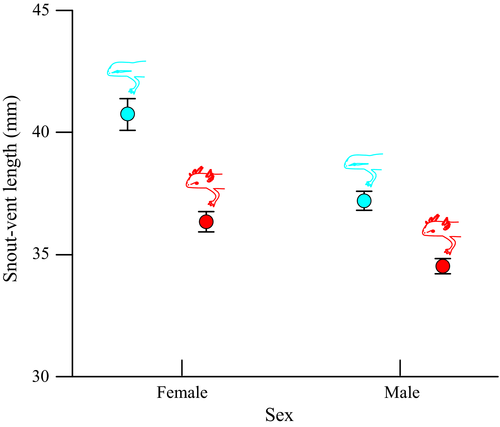
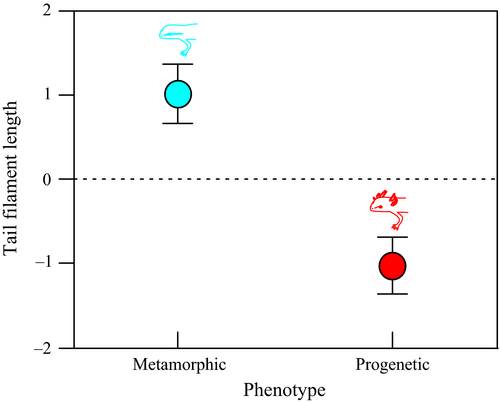
There was a significant effect of phenotype (F1,60 = 59.678, p < 0.001) and sex (F1,60 = 34.508, p < 0.001) on the size of newts showing that paedomorphs are smaller than metamorphs (mean ± SE = 3.54 ± 0.03 and 3.89 ± 0.05 cm, respectively) and males are smaller than females (mean ± SE = 3.59 ± 0.03 and 3.85 ± 0.05 cm, respectively; Figure 2 and Data S1). The interaction between phenotype and sex was not significant (F1,60 = 0.166, p = 0.06; Figure 2). The length of the tail filament (relative to body length) was significantly smaller in paedomorphic than in metamorphic males (F1,30 = 17.311, p < 0.001; Figure 3 and Data S1).
3.2 Courtship patterns
The AICc analyses showed that all factors and their interactions were included in the best model (i.e., only one model with a ΔAIC < 2; Table 1). Fish caused a significant decrease in the number of courtship events that are displayed by males to females (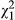 = 4.629, p = 0.031; mean ± SE = 2.94 ± 0.35 and 4.04 ± 0.49 courtship events in the fish and control treatments, respectively), and metamorphs displayed significantly more courtship events than paedomorphs (
= 4.629, p = 0.031; mean ± SE = 2.94 ± 0.35 and 4.04 ± 0.49 courtship events in the fish and control treatments, respectively), and metamorphs displayed significantly more courtship events than paedomorphs (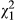 = 83.307, p < 0.001; mean ± SE = 5.93 ± 0.41 and 0.68 ± 0.15 courtships in metamorphs and paedomorphs, respectively). There was also a significant interaction between fish treatment and phenotype (
= 83.307, p < 0.001; mean ± SE = 5.93 ± 0.41 and 0.68 ± 0.15 courtships in metamorphs and paedomorphs, respectively). There was also a significant interaction between fish treatment and phenotype (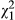 = 5.625, p = 0.018, Figure 4a and Data S2) showing that, in the absence of fish, metamorphs displayed 1.4 times more courtship events and paedomorphs 5 times more courtship events than in the presence of fish. There was also a significant effect of time on the number of courtship events that were displayed by males to females (
= 5.625, p = 0.018, Figure 4a and Data S2) showing that, in the absence of fish, metamorphs displayed 1.4 times more courtship events and paedomorphs 5 times more courtship events than in the presence of fish. There was also a significant effect of time on the number of courtship events that were displayed by males to females ( = 25.014, p < 0.001) showing that the number of courtship events decreased over time.
= 25.014, p < 0.001) showing that the number of courtship events decreased over time.
| Fish | Phenotype | Time | Fish × Phenotype | AICc | ΔAICc | Weight | |
|---|---|---|---|---|---|---|---|
| Courtship | + | + | −0.247 | + | 665.1 | 0 | 0.789 |
| + | + | −0.245 | 668.3 | 3.19 | 0.16 | ||
| + | −0.251 | 670.6 | 5.5 | 0.05 | |||
| Fecundity | + | + | + | 732.2 | 0 | 0.602 | |
| + | + | 0.046 | + | 733.8 | 1.61 | 0.27 | |
| + | + | 736.3 | 4.12 | 0.077 | |||
| + | + | 0.061 | 737.3 | 5.08 | 0.048 |
Note
- Coefficients of included parameters with the sign of the relationship (+ or −) are reported. The tested parameters were as follows: fish (control treatment vs. goldfish treatment), phenotype (progenetic paedomorph vs. metamorph) and time (six periods).

3.3 Fecundity
The AICc analyses showed that all factors and their interactions were included in the two best models (i.e., with a ΔAIC < 2; Table 1). Fish induced a significant decrease in the number of eggs laid (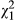 = 12.167, p < 0.001; mean ± SE = 17.67 ± 4.59 and 44.17 ± 4.88 eggs per 2-week period in the fish and control treatments, respectively), and metamorphs laid significantly more eggs than paedomorphs (
= 12.167, p < 0.001; mean ± SE = 17.67 ± 4.59 and 44.17 ± 4.88 eggs per 2-week period in the fish and control treatments, respectively), and metamorphs laid significantly more eggs than paedomorphs (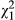 = 17.235, p < 0.001; mean ± SE = 46.17 ± 4.98 and 15.44 ± 4.10 eggs per 2-week period in metamorphs and paedomorphs, respectively). There was also an interaction between fish treatment and phenotype (
= 17.235, p < 0.001; mean ± SE = 46.17 ± 4.98 and 15.44 ± 4.10 eggs per 2-week period in metamorphs and paedomorphs, respectively). There was also an interaction between fish treatment and phenotype (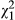 = 5.839, p = 0.016, Figure 4b and Data S3) showing that, in the absence of fish, metamorphs laid twice as many eggs and paedomorphs almost 25 times as many eggs than in the presence of fish. However, time had no significant effect on the number of eggs laid (
= 5.839, p = 0.016, Figure 4b and Data S3) showing that, in the absence of fish, metamorphs laid twice as many eggs and paedomorphs almost 25 times as many eggs than in the presence of fish. However, time had no significant effect on the number of eggs laid (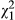 = 0.741, p = 0.389).
= 0.741, p = 0.389).
4 DISCUSSION
The experimental approach carried out in this study on a polymorphic newt species brings new insights to our understanding of the adaptive value of a paedomorphic process, progenesis, across sexes and environmental contexts. The present results demonstrate that reproductive fitness pay-offs of progenesis differ across the sexes, giving an empirical support of the male escape hypothesis (Mathiron et al., 2017; Whiteman, 1997) and more broadly on the possible contrasted evolution of progenesis across the sexes (McKinney & McNamara, 1991). Moreover, these results agree with the theory predicting that progenesis should bring reproductive cost due to size limitations (Stearns, 1992) and that it is affected in risky, predator-rich environments (McNamara, 1990). Altogether, these results show that ultimate causes may contribute to explain the patterns of sex-specific occurrence of progenesis in natural populations.
4.1 The male escape hypothesis
Although progenesis is recognized to be an efficient way to create abrupt morphological changes, its adaptive value for each sex remains poorly understood (Giard, 1887; Gould, 1977; McKinney & McNamara, 1991). On the basis of classical theory, progenesis is thought to release morphology from selective forces whose first target becomes breeding at an early stage of life (Gould, 1977). Accordingly, both sexes should be progenetic. However, this view is nuanced by the fact that several groups have evolved with progenesis occurring in only one sex, giving sex-specific competitive mating advantages (Adamson, 1990; Giard, 1887). Our experiment supports this hypothesis because progenesis came with a higher cost in progenetic males than in progenetic females. Their reproductive activity was proportionally three times lower when compared to the respective metamorphosed phenotypes of each sex. This indicates that being progenetic is more favourable in females than in males and that the natural morph ratio should reflect this pattern in natural populations of newts. Field-based research on newts showed that this is effectively the case as the sex ratio is typically female-biased in paedomorphs, whereas it is not so, on average, in populations of metamorphs (Gabrion, 1976; Gill, 1978; Kalezić & Džukić, 1986; Mathiron et al., 2017; Whiteman, 1997). This is also the case in the studied population of palmate newts where progenetic females are twice as numerous as progenetic males (Denoël, Drapeau, et al., 2019). We previously showed that a sex-biased metamorphosis explains this lower abundance of progenetic males (Denoël, Drapeau, et al., 2019; Mathiron et al., 2017), but, here, we identify ultimate causes of their phenotypic change (cf. the male escape hypothesis). These results along with those on other paedomorphic polymorphisms (Clarkson & Beachy, 2015; Ryan & Hopkins, 2000; Whiteman, 1997) suggest that sex-specific pay-offs should not be neglected in considering the success of alternative developmental pathways. The causes of persistence of progenesis in male newts remain yet to be explored. Several scenarios may be proposed in this perspective (see also Gould, 1977). For instance, because body size difference is an important factor of trophic niche differentiation (Wilson, 1975), progenesis might allow a better exploitation of resources. Then, because metamorphosis is likely a costly event that imposes habitat change and late gonadal maturation (Denoël & Joly, 2000; Mathiron et al., 2017), progenesis could allow newts to capitalize on small reproductive gains at an early life stage. On the other hand, studying the sex-specific role of hormones in the regulation of metamorphosis could help in understanding the mechanistic bases of biased sex ratio in paedomorphs (Bonett, 2016).
4.2 The reproductive cost hypothesis of progenesis
On the basis of sexual and fecundity selection, it is expected that smaller-sized individuals, such as those produced by progenesis, have a lower reproductive output than larger-sized individuals (Gould, 1977; Pincheira-Donoso & Hunt, 2017). Here, our results clearly show that progenesis induces a lower reproductive fitness in both sexes in comparison with the larger metamorphosed newts (i.e., metamorphs). We found that progenetic males court females less often than do metamorphic males and that progenetic females lay fewer eggs than metamorphic females. By displaying less often and having less conspicuous sexual morphological traits, palmate newt males therefore reduce mating opportunities (Cornuau, Rat, Schmeller, & Loyau, 2012; Winandy & Denoël, 2015b). Because the tail filament is used by males during courtship to attract females and because females show preference for males with a long filament (Cornuau et al., 2012), we can therefore consider progenetic males at a double disadvantage, both in terms of reduced courtship activity and in reduced attractiveness. This is particularly relevant in terms of sperm competition and, therefore, individual success because newts are long-term promiscuous breeders (Griffiths, 1987; Halliday, 1977; Verrell & McCabe, 1988). Similarly, in ambystomatids, smaller paedomorphic males sire fewer progeny than larger-sized individuals (Whiteman, Krenz, & Semlitsch, 2006). These results therefore indicate that the advantages of an early maturation may be balanced by its costs in terms of immediate reproductive outputs. This contrasts with the highest fecundity found in some progenetic groups (McNamara, 1988) but fits well with the consideration that progenesis could impact fecundity (Gould, 1977). These ideas are not opposed because both earlier maturation and higher fecundity are adaptations of r-strategists, which are considered progenetic organisms. Indeed, producing progenies earlier in life is considered to overcome a decrease of clutch size; therefore, the benefits of an earlier breeding can be balanced with the cost of a reduced fecundity (Denoël & Joly, 2000; Miaud, 1991; Ryan & Semlitsch, 1998; Snyder & Bretsky, 1971; Stearns, 1992). Future work should examine life-term fitness in both natural and experimental populations (Whiteman et al., 2012). Yet, this is a difficult and long-term task given the small size of progenetic newts and the difficulties of keeping them in captivity over years.
4.3 The predator-risk hypothesis
Metamorphosis is a morpho-functional transformation that is usually associated with habitat change (Ebenman, 1992; Laudet, 2011). In pond-breeding amphibians, it is associated with a colonization of the terrestrial environment after an aquatic larval development (Wilbur & Collins, 1973). This transition depends on various environmental variables, such as drying and predation risks (Ebenman, 1992; Semlitsch, 1987; Werner, 1986). Consequently, progenesis, and more generally a prolonged aquatic life, in newts and salamanders is thought to be favoured in permanent and predator-free aquatic environments (Denoël & Ficetola, 2014; Whiteman, 1994). However, natural or anthropogenic invasions of predators can also negatively affect populations where progenesis has evolved in their absence (Denoël & Winandy, 2015). The present results are supportive of this hypothesis because progenetic newts considerably reduced their courtship activity and egg laying in the presence of fish. Although an anti-predator response was also found in metamorphic individuals of the studied and other species (Cabrera-Guzmán, Díaz-Paniagua, & Gomez-Mestre, 2019; Winandy, Darnet, & Denoël, 2015; Winandy & Denoël, 2013), here we show that this response was much higher in progenetic than in metamorphic newts. This is in concordance with previous experimental research which determined that paedomorphs react more strongly to fish than do metamorphs in respect to other behavioural patterns such as foraging (Winandy & Denoël, 2015a). Altogether, these results of a cost of progenesis agree with field-based data that indicate that fish negatively impact newt populations (see e.g., Cabrera-Guzmán, Díaz-Paniagua, & Gomez-Mestre, 2017; Miró, Sabás, & Ventura, 2018; Orizaola & Braña, 2006) and, more particularly, those of progenetic newts (Denoël & Ficetola, 2014). In contrast, progenesis has been interpreted as an anti-predator adaptation in some fossorial groups that can escape predators by living in the sediment (Gould, 1977; McKinney & McNamara, 1991; McNamara, 1990). As progenetic palmate newts are not fossorial organisms, this indicates that a similar selective pressure can differently affect developmental pathways in meiofauna and in epigeous organisms.
5 CONCLUSIONS
Although progenesis is considered to be an important evolutionary process, resulting in the specialization of many lineages, there is a need for further demonstration of its adaptive value across environments and, therefore, the role of environmental drivers on its evolution (Gould, 1977; McKinney & McNamara, 1991). The present study shows that polymorphisms are valuable models to explore such specific scenarios because fitness parameters can be evaluated on progenetic versus metamorphic phenotypes. Specifically, paedomorphic newts and salamanders could be used to test how developmental changes impact individual success (see also Whiteman, 1997) and how it relates to global change (Bonett, Steffen, Lambert, Wiens, & Chippindale, 2014). This is timely as new anthropogenic pressures, such as climate change and massive introduction of invasive species, are threatening the last remaining populations of paedomorphic newts and salamanders across the globe (Denoël, Ficetola, et al., 2019; Turvey et al., 2018; Voss, Woodcock, & Zambrano, 2015; Walls, Barichivich, Brown, Scott, & Hossack, 2013).
ACKNOWLEDGMENTS
We wish to thank Ken McNamara and an anonymous reviewer for their constructive comments on our manuscript, J.L. Soulié for allowing access to the pond and N. Oromi for her help in the field. M.D. is a Research Director and L.W. was a PhD fellow at the Fonds de la Recherche Scientifique (F.R.S.—FNRS). This study was funded by F.R.S.—FNRS grant numbers J.008.13, J.0112.16 and T.0070.19 to MD.




