Variation in ecophysiological traits might contribute to ecogeographic isolation and divergence between parapatric ecotypes of Mimulus aurantiacus
Abstract
Many forms of reproductive isolation contribute to speciation, and early-acting barriers may be especially important, because they have the first opportunity to limit gene flow. Ecogeographic isolation occurs when intrinsic traits of taxa contribute to disjunct geographic distributions, reducing the frequency of intertaxon mating. Characterizing this form of isolation requires knowledge of both the geographic arrangement of suitable habitats in nature and the identification of phenotypes involved in shaping geographic distributions. In Mimulus aurantiacus, red- and yellow-flowered ecotypes are incompletely isolated by divergent selection exerted by different pollinators. However, these emerging taxa are largely isolated spatially, with a hybrid zone occurring along a narrow region of contact. In order to assess whether responses to abiotic conditions contribute to the parapatric distribution of ecotypes, we measured a series of ecophysiological traits from populations along a transect, including drought sensitivity, leaf area and the concentrations of vegetative flavonoids. In contrast to the abrupt transitions in floral phenotypes, we found that ecophysiological traits exhibited a continuous geographic transition that largely mirrors variation in climatological variables. These traits may impede gene flow across a continuous environmental gradient, but they would be unlikely to result in ecotypic divergence alone. Nevertheless, we found a genetic correlation between vegetative and floral traits, providing a potential link between the two forms of isolation. Although neither barrier appears sufficient to cause divergence on its own, the combined impacts of local adaptation to abiotic conditions and regional adaptation to pollinators may interact to drive discontinuous variation in the face of gene flow in this system.
1 INTRODUCTION
Considerable progress has been made in understanding the role that ecology plays in the origin of species (Butlin et al., 2012; Coyne & Orr, 2004; Mayr, 1947; Nosil, 2012; Schluter, 2009; Sobel, Chen, Watt, & Schemske, 2010). For example, it is generally accepted that reproductive isolation can evolve as a by-product of adaptation to different environments (Schluter & Conte, 2009). However, an ongoing challenge of modern speciation research is to identify the targets of divergent selection that secondarily result in isolation (Baack, Melo, Rieseberg, & Ortiz-Barrientos, 2015; Rieseberg & Willis, 2007; Schluter, 2001). This has been possible in systems where the connection between traits and isolation is straightforward, such as the relationship between pollinator isolation and variation in flower colour (e.g., Bradshaw & Schemske, 2003). However, not all ecological transitions lend themselves to identifying clear phenotypic targets. For example, environmental gradients may impose multiple selective pressures simultaneously (Nosil, Harmon, & Seehausen, 2009), and the physiological adaptations that result may be cryptic. Although adaptive variation has been characterized in some cases (Lowry, Rockwood, & Willis, 2008; Storz et al., 2009), these ecophysiological traits are underrepresented in the literature relative to their potential importance.
Reproductive barriers often act quantitatively and accumulate over time; therefore, speciation is a continuous process that generally requires the evolution of multiple isolating barriers (Coyne & Orr, 2004; Lowry, Modliszewski, Wright, Wu, & Willis, 2008a). A current major goal of speciation research is to quantify the degree to which multiple forms of reproductive isolation impact overall reductions in gene flow (Sobel & Chen, 2014) in an effort to determine which barriers are most important to the process of divergence (Hendry, 2009; Sobel et al., 2010). Further, whereas geographic separation is viewed as a powerful agent of isolation (Dobzhansky, 1937; Felsenstein, 1981; Mayr, 1942), divergence-with-gene-flow models have demonstrated that conspicuous phenotypic differentiation between emerging taxa can be maintained in the face of gene flow by factors that prevent the break-up of favourable allelic combinations (Bolnick & Fitzpatrick, 2007; Feder et al., 2005; Turelli, Barton, & Coyne, 2001). Thus, an additional current goal is to elucidate the ecological and genetic mechanisms responsible for generating and maintaining the associations between traits involved in multiple forms of reproductive isolation (Servedio, Van Doorn, Kopp, Frame, & Nosil, 2011; Smadja & Butlin, 2011).
Adaptation to different resources or habitats plays a prominent role during divergence (Barrett, Rogers, & Schluter, 2008; Benkman, 1999; Emms & Arnold, 1997), and the traits involved can impact pre- and post-mating isolation in a variety of ways (Bolnick & Fitzpatrick, 2007; Pinho & Hey, 2010; Rundle & Nosil, 2005). For example, over broad scales, the spatial distribution of alternate suitable habitats may result in partially disjunct geographic ranges, where each taxon experiences both exclusive and shared portions of their distributions (i.e., parapatry). Encounter rates between diverging taxa are expected to be diminished in exclusive areas compared to shared regions, generating ecogeographic reproductive isolation (Ramsey, Bradshaw, & Schemske, 2003; Sobel et al., 2010). Although this form of isolation rarely is expected to lead to complete isolation between emerging taxa, it appears to contribute substantially to limiting gene flow between recently diverged pairs of species (e.g., Husband & Sabara, 2004; Kay, 2006; Nakazato, Warren, & Moyle, 2010; Ramsey et al., 2003; Sambatti, Strasburg, Ortiz-Barrientos, Baack, & Rieseberg, 2012; Sobel, 2014). Premating isolation also can arise if individuals from diverging taxa disperse into each other's habitats, but inviability and/or infecundity of immigrants act as barriers to gene flow with locally adapted residents (Nosil, Vines, & Funk, 2005; Porter & Benkman, 2017; Richards & Ortiz-Barrientos, 2016). Further, habitat divergence can result in extrinsic post-mating isolation when hybrids experience reduced fitness in either parental habitat (Hatfield & Schluter, 1999; Melo, Grealy, Brittain, Walter, & Ortiz-Barrientos, 2014). Therefore, identifying the traits that are impacted by habitat divergence can be essential to understanding how gene flow is limited between incipient species.
In this study, we investigate geographic variation in ecophysiological traits that may contribute to incipient speciation in two parapatrically distributed ecotypes of the perennial shrub Mimulus aurantiacus subspecies puniceus (Phrymaceae) (Chase, Stankowski, & Streisfeld, 2017). In San Diego County, California, there is an abrupt phenotypic transition between a red-flowered, hummingbird-pollinated ecotype that occurs in the west and a yellow-flowered, hawkmoth-pollinated ecotype that occurs to the east (Figure 1). Where the ranges of these ecotypes are in contact, a narrow hybrid zone occurs, exhibiting a wide range of segregating phenotypic variation in floral traits (Stankowski, Sobel, & Streisfeld, 2015, 2017). Although the ecotypes are distinguished primarily by flower colour, other traits typical of hummingbird and hawkmoth pollination syndromes also vary with flower colour, suggesting that they are associated via divergent selection by these pollinators (Streisfeld & Kohn, 2005; Tulig, 2000; Waayers, 1996). Experimental evidence and population genetic signatures of selection reveal that flower colour is a target of divergent selection across the hybrid zone (Handelman & Kohn, 2014; Stankowski et al., 2015; Streisfeld & Kohn, 2007), and genetic variation in the transcription factor MaMyb2 is the primary contributor to variation in floral pigmentation (Stankowski & Streisfeld, 2015; Streisfeld, Young, & Sobel, 2013).
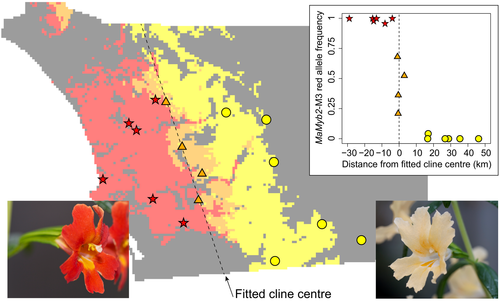
Intrinsic post-mating barriers are weak to nonexistent in this system (Sobel & Streisfeld, 2015), and the incomplete nature of pollinator isolation in the hybrid zone suggests that the ecotypes would not be maintained without some geographic separation (Stankowski et al., 2015). Using geographic distribution modelling, we measured relatively strong ecogeographic isolation between these taxa, with approximately 78% of the range of each ecotype predicted to be exclusive to each taxon (Figure 1; and see Sobel & Streisfeld, 2015). However, the combined impact of dispersal limitation and spatial autocorrelation of habitat characteristics could result in distribution models that predict habitat differences between taxa in the absence of distinct ecological tolerances (Warren, Cardillo, Rosauer, & Bolnick, 2014). Further, the relative roles of biotic and abiotic interactions in driving geographic distributions are unknown. For example, if the ranges are based solely on biotic interactions with pollinators, parapatry may reflect geographic variation in the relative abundance of hummingbirds and hawkmoths (Streisfeld & Kohn, 2007). However, adaptation to the abiotic environment also may drive variation in ecophysiological traits between taxa, contributing to their disjunct distribution. Environmental variation is substantial across the range of these taxa, with the climate of the western red ecotype regulated by its proximity to the cool Pacific Ocean, and the yellow ecotype found in inland regions with more extreme summer and winter temperatures. However, climatic variation in this region is more or less continuous, following the gradual transition between lowland coastal regions to higher elevations inland (Sobel & Streisfeld, 2015).
The process of speciation is characterized by the emergence of discontinuous variation, such as the discrete nature of flower colour (and other floral traits) between the ecotypes. Therefore, variation in ecophysiology could arise in several ways with implications for divergence. For example, ecophysiological traits could vary in a similarly abrupt, step-like manner, transitioning in parallel with floral traits through the hybrid zone. This might be the case if floral traits and ecophysiology share a common genetic basis, or if loci involved in floral and ecophysiological traits experience strong linkage disequilibrium from a substantial period of allopatry followed by recent secondary contact. Under these scenarios, habitat-based forms of reproductive isolation could be of a similar magnitude to pollinator isolation across the distribution of these taxa (i.e., strong isolation in all comparisons between red and yellow populations). Alternatively, ecophysiological traits could vary continuously along a more gradual environmental gradient. Geographic patterns of trait variation and trait-by-environment relationships are predicted outcomes of natural selection (Endler, 1986), so continuous clinal variation in ecophysiological traits could indicate local adaptation to abiotic conditions. In this case, ecophysiology may facilitate divergence by impeding the free movement of alleles across the landscape (Endler, 1977; Stankowski et al., 2017), but the strength of reproductive isolation would not be consistent between ecotypes (i.e., ecologically distant populations would experience stronger isolation than nearby comparisons, regardless of ecotype). Finally, both outcomes could occur, such that ecophysiological traits that share a genetic basis with floral traits exhibit discrete variation, but other traits vary independently of floral traits.
In this study, we examined the contribution of ecophysiological traits to reproductive isolation by carrying out the following analyses: (a) we measured a series of ecophysiological traits from 16 populations across the geographic range of both ecotypes and their hybrid zone. Traits included drought sensitivity, leaf area and vegetative flavonoids, and we employed a mixed modelling approach to detect both continuous clinal variation and discrete differences between the red and yellow ecotypes. (b) On a smaller subset of populations, we measured allocation patterns to above- and below-ground biomass and examined whether red- and yellow-flowered populations varied for these traits. (c) Finally, we tested for a genetic association between ecophysiology and flower colour by measuring a subset of traits in an experimental hybrid population segregating for alleles at the flower colour gene, MaMyb2. These approaches provide insight into the potentially complex interactions between ecological and genetic factors that contribute to the emergence of reproductive barriers in diverging lineages, and they generate additional hypotheses that motivate future genetic mapping and experimental studies.
2 MATERIALS AND METHODS
2.1 Selection of focal populations and establishment of one-dimensional transect
Six red-flowered, six yellow-flowered and four hybrid populations were selected for measurement of the primary ecophysiological traits included in this study. These populations represent a range of geographic locations across the distribution of these ecotypes, from extreme coastal populations, through the middle of the hybrid zone, and to the farthest known inland populations (Figure 1). Previous results from geographic distribution modelling show that these populations occur across a range of predicted suitability scores for each ecotype (Supporting information Figure S1; and see Sobel & Streisfeld, 2015). Therefore, these populations are well suited to reveal ecophysiological differences between taxa if they occur.
Because flower colour is the primary diagnostic trait for inclusion in each ecotype, clinal analyses were performed with reference to the distance each population occurs from the centre of the geographic cline in flower colour. The phenotypic transition that occurs in San Diego County, California, is primarily east–west, creating a contact zone between ecotypes that runs roughly north–south through the study area (Figure 1). Distance across this region was defined independent of climatological conditions. Specifically, position was described relative to the centre of the allele frequency cline of the MaMyb2-M3 marker, as described previously (Stankowski et al., 2015). Position zero represents the centre of the cline in flower colour. Negative values reflect positions to the west, and positive values denote positions east of the centre. Other distance measures, such as distance from the coast or longitude, are highly correlated with the position of the MaMyb2 cline and provide qualitatively similar values (Supporting information Figure S2).
2.2 Seed collection and plant husbandry
Seeds from 189 maternal families (range: 7–17 per population; mean = 11.8; Supporting information Table S1) from the 16 focal populations were sprinkled on moist potting soil in plug trays and placed in a growth chamber under fluorescent light at 23°C on a 16/8-hr light/dark cycle. At the 2- to 4-leaf stage (approximately 2 weeks post-germination), seedlings from each family were assigned randomly to one of two treatment groups. The first group was established to test for differences in drought sensitivity. Three seedlings from each maternal family (567 total seedlings) were transplanted into cone-tainers, randomized into 98-cell racks and sub-irrigated as necessary. The second group was established for the measurement of vegetative secondary compounds. Two seedlings from each maternal family (368 total seedlings) were transplanted into 2.25-inch pots and placed randomly into bottom water trays. All plants were grown under standard conditions in the University of Oregon greenhouses, where they were watered as needed, and fertilized equally.
2.3 Drought sensitivity
We performed a terminal drought experiment to test for differences in drought sensitivity across focal populations. When plants reached the 8- to 10-leaf stage (sub-adult), we saturated the soil of each plant with water and ceased watering (experimental day 0). This stage of development mimics the size of young plants at the time when the southern California seasonal drought commences (M. A. Streisfeld, pers obs). Each day for the next 22 days, plants were assigned a score from 0 to 4 to assess their condition. A score of 0 indicated plants exhibiting no signs of stress, 1 represented the initial signs of drought stress (i.e., leaves curl under slightly), 2 reflected the first true wilting, 3 showed severe systemic wilting, and 4 was assigned to plants that were dead (Supporting information Figure S3). To avoid bias, drought scores were collected blindly with respect to plant identity by a single observer at the same time each day.
We used a three-parameter exponential function, y = y0 + a(1 − e−dx), to approximate the change in drought score over time x for each individual, where y approaches the upper asymptote, y0 + a, as x tends towards infinity. The upper asymptote was fixed at y0 + a = 4, as this represents the score assigned once plants were dead. Therefore, d provides an estimate of the rate at which individuals are negatively affected by drought as they approach this terminal asymptote. Estimation of d was conducted by a nonlinear least-squares iterative approach in R (R Core Development Team, 2013). In preliminary work, we found that ecotypes varied for early-stage leaf area, which we hypothesized to be a potential mechanism for drought sensitivity differences. We therefore estimated total leaf area (cm2) for each plant using ImageJ from overhead digital photographs taken on day 0 of the drought experiment.
In order to analyse drought sensitivity across the distribution of focal populations, we applied a restricted maximum likelihood mixed modelling approach using the R package lme4 (Bates, Maechler, Bolker, & Walker, 2015). Square root transformations improved normality for d, leaf area and residuals (Supporting information Figure S4), and Box–Cox transformation using the BoxCoxTrans command in the R package caret (Kuhn et al., 2012) confirmed that this was a reasonable approximation of the best transformation (Supporting information Table S2). Construction of mixed models varied depending on the specific hypothesis being tested. To test for variation in d across populations, population was treated as a fixed effect, with family nested within population included as a random factor. Similarly, a simplified model was constructed to test for the fixed effect of ecotype (red, hybrid or yellow), with population nested within ecotype and family nested within population included as random factors. To determine if differences in d among ecotypes and populations could be explained by distance along the flower colour transition, another model was constructed that included the continuous variable distance (km) from the MaMyb2-M3 cline centre (see above), along with an interaction between fixed effects.
Finally, to determine if leaf area impacted d, models were constructed that included leaf area as a continuous variable. To test if leaf area explained variation in d, models were constructed first with the fixed effects of ecotype and distance excluded, but with the random effects of population and family retained. Subsequently, ecotype and distance were included in the model (along with interactions with leaf area) to determine if their effects on d remained once corrected for variation in leaf area. In all cases, significance of fixed effects was assessed by a Wald chi-square test using the Anova function in the car package in R (Fox & Weisberg, 2011), and random effects were assessed by elimination of the variable of interest followed by likelihood ratio tests between full and reduced models.
2.4 Vegetative flavonoids
Vegetative anthocyanins and their associated flavonoid compounds are well known for their roles in protecting plants from biotic and abiotic stresses (Chalker-Scott, 1999; Gould & Lee, 2002; Winkel-Shirley, 2002), and variation has been noted in leaf and stem anthocyanin and resin content among M. aurantiacus populations (Han & Lincoln, 1994; Hare, 2002; Streisfeld & Rausher, 2009). Leaf geranylflavanone resins in M. aurantiacus can constitute up to 30% of the dry mass of leaves (Hare, 2002, 2008; Lincoln, 1980), and these compounds have been hypothesized to protect against UV damage and desiccation (Hare, 2002). Vegetative resin and anthocyanin were measured in both unstressed and stressed conditions. Stress was imparted by withholding nutrients and pulsing sub-lethal drought conditions. We extracted total leaf resins by taking single-hole punches from two young, fully emerged leaves on each plant. Leaf discs were placed in 1 ml of methanol and stored overnight in the dark, and resin concentration was estimated from absorbance of the extract at 292 nm (Lincoln, 1980). Anthocyanins from unstressed leaves were measured from two-hole punches per leaf, sampled from the pair of leaves at the mid-point node of the main stem of each plant. Anthocyanins were extracted in 500 μl of acidic methanol (1% HCl) overnight. Stressed leaf anthocyanins were visibly more intense, so extractions were performed on a single-hole punch per leaf in 1 ml acidic methanol. Anthocyanin concentrations were quantified by absorbance of the extract at 530 nm (Harborne, 1998), and the unstressed absorbance was divided by 4 to place it on the same relative scale as the stressed samples.
As above, a separate restricted maximum likelihood mixed modelling approach was used to assess variation in each of the four response variables: unstressed leaf anthocyanin (ULA), unstressed leaf resin (ULR), stressed leaf anthocyanin (SLA) and stressed leaf resin (SLR). Both ULA and ULR were treated as untransformed variables (Supporting information Figure S5), as Box–Cox transformations were unable to improve normality. SLR showed a mild positive skew, and a square root transformation improved normality. SLA exhibited a highly positive skew, and Box–Cox transformation suggested the multiplicative inverse of the square root (SLA−0.5) gave the best approximation of a normal distribution (Supporting information Table S2). Mixed modelling was performed in a similar manner to the drought sensitivity analyses presented above. Family was treated as a random factor nested within population in all iterations. Fixed effects of population and ecotype were examined in separate simplified models to assess whether the four flavonoid traits varied at these levels. Subsequently, in cases where population and/or ecotype exhibited significant impacts on the dependent variables, models were tested that included the one-dimensional distance to the MaMyb2-M3 cline centre as a continuous fixed effect.
2.5 Assessing overall variation in ecophysiological traits
All statistical tests were performed in R, with packages and commands indicated below (R Core Development Team, 2013). Pearson product–moment correlations among all ecophysiological variables were calculated with the cor command. To summarize relationships among the ecophysiological phenotypes, principal components analysis (PCA) was performed on correlation coefficients using the prcomp command. Due to the destructive nature of data collection for the drought sensitivity test, d and leaf area were measured on one set of individuals, and vegetative flavonoids were measured on another set of individuals. However, the same maternal families were used in both experiments. Therefore, individual phenotypes were calculated at the level of family for these analyses. To visualize clustering of traits by ecotype, the first two principal components (PC1ecophys and PC2ecophys) were examined in a bivariate plot, and geographic variation in ecophysiology was assessed with linear models between each PC and distance across the cline.
In order to examine the relationship between ecophysiological traits and environmental conditions experienced by these populations, we employed two-table comparison approaches. Data from 30-arc-second grids (~1 km2) for eight climatic variables from the publicly available WORLDCLIM v1.4 data set (Hijmans, Cameron, Parra, Jones, & Jarvis, 2005) were extracted from geographic coordinates of the focal populations, and procrustean rotations were used to examine the association between climate and population-level ecophysiological traits. Procrustes analysis is well established in morphometric analysis (Bookstein, 1991), but it can be employed whenever comparisons are made between two data matrices with equivalent row values. Although it has been slow to be adopted by ecologists, the approach has demonstrated advantages over traditional matrix comparison methods (Peres-Neto & Jackson, 2001). The procedure involves performing PCA on individual data tables and rescaling and rotating the two outcomes to minimize distances between points in a least-squares framework (Gower, 1971). The analysis provides a visualization of similarities between two multivariate data sets collected from the same populations, enabling examination of the relationship between putative agents (climatic conditions) and targets (phenotypic traits) of selection. Procrustes analysis was applied between ecophysiological traits (ecophys) and climatic data (bioclim) using the R package ade4 (Dray & Dufour, 2007), and PROTEST (Jackson, 1995) and RV (Heo & Gabriel, 1998) randomization procedures were used to compare the association between ecophys and bioclim to a null expectation where no relationship exists between traits and the environment.
To further investigate the relationship between traits and climatic conditions, associations between the first principal component from ecophysiological traits (PC1ecophys), climatic data (PC1bioclim) and geographic location were examined via full and partial Mantel correlation tests (Legendre, 2000) using the R package vegan (Oksanen et al., 2017). Euclidian distances were calculated among all pairs of populations for PC1ecophys and PC1bioclim data sets. Geographic distance was calculated in both one dimension (i.e., total distance from cline centre) and two dimensions (i.e., direct X–Y distance between populations). Genetic distance (FST) between all pairs of these 16 populations was calculated from a previously published analysis of 5,382 SNPs generated using RADseq (Stankowski et al., 2015). Pairwise Mantel correlations were performed between all relevant sets of distance matrices, and partial Mantel tests examined the correlation of each distance matrix with PC1ecophys while removing the effect of FST. This removes the effects of the shared evolutionary history among populations and evaluates the overall contribution of geography and/or abiotic environment to variation in ecophysiological traits. Statistical significance was tested using 10,000 permutations per comparison.
2.6 Biomass allocation
Previous observations indicated that red- and yellow-flowered plants varied in leaf area at early stages of development (also see “Drought sensitivity” in the Results section). Given the importance of above- and below-ground allocation strategies to the establishment and survival of plants (Enquist & Niklas, 2002), these differences may reflect variation between the ecotypes with important impacts on their ecogeographic distribution. Therefore, to test for differences in allocation to above- and below-ground biomass, we grew seeds from the same six populations used in a previous study of reproductive isolation: red ecotype (UCSD, LH, ELF) and yellow ecotype (PCT, POTR, LO; see Sobel & Streisfeld, 2015). Seeds from four families per population were combined and germinated on moist soil, and 252 total seedlings were transplanted into cone-tainers at the 2- to 4-leaf stage (N = 42 per population; family information was not retained). Plants were grown under fluorescent lighting for an additional 4 weeks after transplantation. Surviving plants (37–42 plants per population) were removed from cone-tainers, soil was washed from the roots, and above- and below-ground tissues were separated. Tissue was dried and subsequently weighed to the nearest 0.1 mg. We performed general linear mixed modelling individually on square-root-transformed root mass and shoot mass, using the packages described above. Ecotype was treated as a fixed effect, and population nested within ecotype was treated as a random factor. The ratio of above- to below-ground tissue also was calculated, but this ratio was not used in statistical testing. As these phenotypic measurements were performed only on a subset of populations outside of the hybrid zone, geographic distance was not included as a factor.
2.7 Co-segregation of vegetative and floral anthocyanins
As discussed above, the primary gene responsible for the ecotypic transition between yellow and red flowers has been identified previously (Streisfeld et al., 2013). The MaMyb2 gene encodes a transcription factor that regulates expression of several enzymes associated with the anthocyanin biosynthetic pathway. To investigate whether floral and vegetative anthocyanins share a common genetic basis, we took advantage of a genetic cross that was developed previously (Stankowski et al., 2015). From 96 F2 plants generated from a cross between the ecotypes, we genotyped the MaMyb2-M3 marker, which is tightly linked to the mutation affecting flower colour (see Streisfeld et al., 2013). As expected, plants in this population segregated approximately 1:2:1 for the three possible genotypes: 25 Red/Red, 50 Red/Yellow and 21 Yellow/Yellow (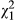 = 0.5; p = 0.779). We then surveyed the plants for the presence of visually detectable leaf and stem anthocyanins and performed a Fisher's exact test to determine whether the presence of vegetative anthocyanins was related to MaMyb2 genotype.
= 0.5; p = 0.779). We then surveyed the plants for the presence of visually detectable leaf and stem anthocyanins and performed a Fisher's exact test to determine whether the presence of vegetative anthocyanins was related to MaMyb2 genotype.
3 RESULTS
3.1 Drought sensitivity
Drought sensitivity (d) is significantly impacted by experimental variables under a variety of mixed modelling conditions (Table 1). For example, when other predictor variables are excluded from the model, the fixed effect of population explains significant variation in d (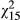 = 33.76, p = 0.004; Table 1; also see Figure 2a). Similarly, d varies significantly among ecotypes (in models where ecotype is a fixed effect but population and family are nested random variables), with the red ecotype experiencing a more rapid onset of drought symptoms than the yellow ecotype, and hybrids were intermediate (
= 33.76, p = 0.004; Table 1; also see Figure 2a). Similarly, d varies significantly among ecotypes (in models where ecotype is a fixed effect but population and family are nested random variables), with the red ecotype experiencing a more rapid onset of drought symptoms than the yellow ecotype, and hybrids were intermediate (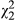 = 14.37, p < 0.001). Significant variation in population and ecotype revealed in simplified models appears to be driven by distance across the one-dimensional cline, as both of these variables and their interactions are not significant in models that include distance as an explanatory factor (Table 1). Indeed, d appears to be highest in western populations, and sensitivity to drought declines to the east (Figure 2a;
= 14.37, p < 0.001). Significant variation in population and ecotype revealed in simplified models appears to be driven by distance across the one-dimensional cline, as both of these variables and their interactions are not significant in models that include distance as an explanatory factor (Table 1). Indeed, d appears to be highest in western populations, and sensitivity to drought declines to the east (Figure 2a; 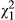 = 4.65, p = 0.031). Visual analysis of residual variation suggests that a linear fit for distance is appropriate. Finally, models that include leaf area suggest that it is primarily responsible for variation in d (
= 4.65, p = 0.031). Visual analysis of residual variation suggests that a linear fit for distance is appropriate. Finally, models that include leaf area suggest that it is primarily responsible for variation in d (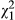 = 925.5, p < 0.001). Indeed, all other variables except family become nonsignificant when corrected for differences in leaf area (Table 1; p > 0.05). Large-leaved plants experienced the onset of drought symptoms much more quickly than small-leaved plants (Figure 2b), consistent with drought sensitivity being driven by elevated rates of transpirational water loss.
= 925.5, p < 0.001). Indeed, all other variables except family become nonsignificant when corrected for differences in leaf area (Table 1; p > 0.05). Large-leaved plants experienced the onset of drought symptoms much more quickly than small-leaved plants (Figure 2b), consistent with drought sensitivity being driven by elevated rates of transpirational water loss.
| Model category (hypothesis) | Fixed effects | χ 2 | p-Value | Random factors | χ 2 | p-Value |
|---|---|---|---|---|---|---|
| Population variation in d | Population | 33.76 | 0.004 | Family | 64.56 | <0.001 |
| Ecotypic variation in d | Ecotype | 14.37 | <0.001 | Population | 0 | 1 |
| Family | 74.41 | <0.001 | ||||
| Impact of distance on d | Distance | 4.65 | 0.031 | Population | 0 | 1 |
| Ecotype | 0.866 | 0.649 | Family | 72.71 | <0.001 | |
| Distance × Ecotype | 1.415 | 0.493 | ||||
| Impact of leaf area on d | Leaf area | 925.5 | <0.001 | Population | 0 | 1 |
| Family | 60.28 | <0.001 | ||||
| All variables on d | Leaf area | 913.7 | <0.001 | Population | 0 | 1 |
| Distance | 0.213 | 0.645 | Family | 62.58 | <0.001 | |
| Ecotype | 0.314 | 0.855 | ||||
| Leaf area × Distance | 1.566 | 0.211 | ||||
| Leaf area × Ecotype | 0.952 | 0.621 |
Note
- Significant factors in bold at p < 0.05.
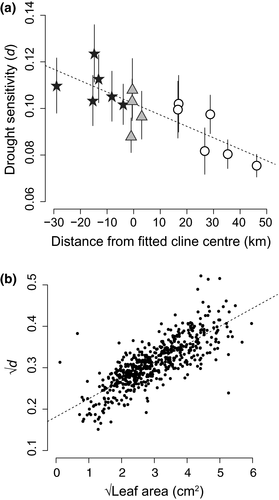
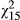 = 4.65, p = 0.031). Symbols for exclusively red ecotype populations are filled stars, hybrid populations are grey triangles, and yellow ecotype populations are unfilled circles. Population means are plotted, and ±1 SE bars are included. (b) Drought sensitivity is highly correlated with the potentially mechanistic trait, leaf area (r = 0.80)
= 4.65, p = 0.031). Symbols for exclusively red ecotype populations are filled stars, hybrid populations are grey triangles, and yellow ecotype populations are unfilled circles. Population means are plotted, and ±1 SE bars are included. (b) Drought sensitivity is highly correlated with the potentially mechanistic trait, leaf area (r = 0.80)3.2 Vegetative flavonoids
Vegetative flavonoids show a variety of responses in focal populations in both stressed and unstressed conditions (Table 2). In unstressed leaf tissue, vegetative anthocyanins (ULA) are typically expressed at very low levels (Figure 3a), and population does not have a significant impact on the concentration of pigment produced (p = 0.155). However, ecotype has a marginal impact on ULA in a simplified model where it is the only fixed effect (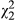 = 5.36; p = 0.069; Table 2), with the red ecotype and hybrid populations exhibiting slightly higher concentrations than yellow. When distance is added to the model, there is a significant distance × ecotype interaction (
= 5.36; p = 0.069; Table 2), with the red ecotype and hybrid populations exhibiting slightly higher concentrations than yellow. When distance is added to the model, there is a significant distance × ecotype interaction (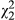 = 6.70; p = 0.035), but the marginal effect of ecotype disappears. Under stress (SLA), differences in vegetative anthocyanins among groups become more apparent. For example, in simplified models, population (
= 6.70; p = 0.035), but the marginal effect of ecotype disappears. Under stress (SLA), differences in vegetative anthocyanins among groups become more apparent. For example, in simplified models, population (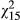 = 69.9; p < 0.001) and ecotype (
= 69.9; p < 0.001) and ecotype (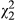 = 20.8; p < 0.001) are highly significant. Distance is a significant predictor of anthocyanin concentration (
= 20.8; p < 0.001) are highly significant. Distance is a significant predictor of anthocyanin concentration (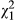 = 4.54; p = 0.033), with SLA declining in a roughly linear manner from coastal to inland populations (Figure 3b). In the full model, there is a weak effect of ecotype (
= 4.54; p = 0.033), with SLA declining in a roughly linear manner from coastal to inland populations (Figure 3b). In the full model, there is a weak effect of ecotype (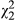 = 5.22; p = 0.073), with red and hybrid populations continuing to show marginally higher anthocyanin concentrations despite being corrected for the effect of distance.
= 5.22; p = 0.073), with red and hybrid populations continuing to show marginally higher anthocyanin concentrations despite being corrected for the effect of distance.
| Response variable | Model category (hypothesis) | Fixed effects | χ 2 | p-Value | Random factors | χ 2 | p-Value |
|---|---|---|---|---|---|---|---|
| Unstressed leaf anthocyanin (ULA) | Population variation | Population | 20.5 | 0.155 | Family | 6.65 | 0.001 |
| Ecotypic variation | Ecotype | 5.36 | 0.069 | Population | 0 | 1 | |
| Family | 9.54 | 0.002 | |||||
| Impact of distance | Distance | 0.02 | 0.895 | Population | 0 | 1 | |
| Ecotype | 1.01 | 0.604 | Family | 8.36 | 0.004 | ||
| Distance × Ecotype | 6.70 | 0.035 | |||||
| Stressed leaf anthocyanin (SLA) | Population variation | Population | 69.9 | <0.001 | Family | 0 | 1 |
| Ecotypic variation | Ecotype | 20.8 | <0.001 | Population | 2.18 | <0.001 | |
| Family | 0.21 | 0.648 | |||||
| Impact of distance | Distance | 4.54 | 0.033 | Population | 0 | 1 | |
| Ecotype | 5.22 | 0.073 | Family | 0.23 | 0.629 | ||
| Distance × Ecotype | 1.37 | 0.504 | |||||
| Unstressed leaf resin (ULR) | Population variation | Population | 42.0 | <0.001 | Family | 3.00 | 0.083 |
| Ecotypic variation | Ecotype | 5.36 | 0.069 | Population | 0 | 1 | |
| Family | 9.54 | 0.002 | |||||
| Impact of distance | Distance | 8.25 | 0.004 | Population | 0 | 1 | |
| Ecotype | 3.15 | 0.207 | Family | 5.26 | 0.022 | ||
| Distance × Ecotype | 0.37 | 0.831 | |||||
| Stressed leaf resin (SLR) | Population variation | Population | 27.7 | 0.023 | Family | 0.82 | 0.364 |
| Ecotypic variation | Ecotype | 1.04 | 0.594 | Population | 0 | 1 | |
| Family | 2.01 | 0.156 | |||||
| Impact of distance | Distance | 0.22 | 0.636 | Population | 0 | 1 | |
| Ecotype | 0.17 | 0.921 | Family | 2.10 | 0.147 | ||
| Distance × Ecotype | 1.94 | 0.379 |
Note
- Bold indicates statistical significance, p < 0.05; italics indicate marginal significance, 0.05 < p < 0.1.
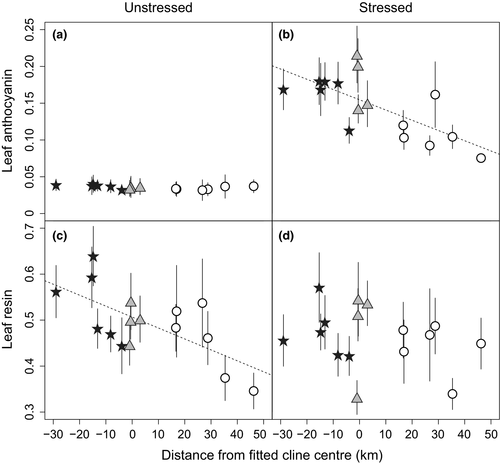
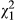 = 4.54, p = 0.033). Ecotype also has an impact on stressed leaf anthocyanin (SLA) in both simplified models (
= 4.54, p = 0.033). Ecotype also has an impact on stressed leaf anthocyanin (SLA) in both simplified models (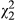 = 20.8, p < 0.001), and a marginal impact remains when distance is included (
= 20.8, p < 0.001), and a marginal impact remains when distance is included (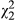 = 5.22, p = 0.073; see Table 2). (c) Unstressed leaf geranylflavanone resin exhibits significant clinal variation (
= 5.22, p = 0.073; see Table 2). (c) Unstressed leaf geranylflavanone resin exhibits significant clinal variation (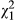 = 8.25, p = 0.004). However, under stress, resins do not vary with distance across the transect (p = 0.636). Symbols as in Figure 2
= 8.25, p = 0.004). However, under stress, resins do not vary with distance across the transect (p = 0.636). Symbols as in Figure 2In contrast to leaf anthocyanins, geranylflavanone resins in unstressed conditions but not stressed conditions vary across the transect (Figure 3c,d). Specifically, ULR varies significantly according to population (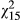 = 42.0; p < 0.001), and ecotype is marginally significant (
= 42.0; p < 0.001), and ecotype is marginally significant (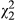 = 5.36; p = 0.069) in simplified models. ULR also exhibits a significant linear relationship with distance (Figure 3c;
= 5.36; p = 0.069) in simplified models. ULR also exhibits a significant linear relationship with distance (Figure 3c; 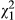 = 8.25; p = 0.004), but the effect of ecotype is not significant when distance is included in the model (p = 0.207). However, under stressful conditions, resins (SLR) exhibit significant variation only at the population level (
= 8.25; p = 0.004), but the effect of ecotype is not significant when distance is included in the model (p = 0.207). However, under stressful conditions, resins (SLR) exhibit significant variation only at the population level (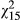 = 27.7; p = 0.023), but no other factor is significant under any modelling conditions (Figure 3d; Table 2).
= 27.7; p = 0.023), but no other factor is significant under any modelling conditions (Figure 3d; Table 2).
3.3 Overall variation in ecophysiological traits in focal populations
There are positive correlations among all ecophysiological traits. As noted above, d and leaf area are strongly correlated; however, these variables also show positive associations with anthocyanins and resins in both stressed and unstressed conditions (Supporting information Figure S6). Vegetative anthocyanins and resins also are positively correlated with each other in stressed and unstressed conditions (Supporting information Figure S6). The first two principal components (PC1ecophys and PC2ecophys) explain 41.4% and 18.4% of the variation, respectively, and a bivariate plot shows moderate ecotypic clustering along these axes (Supporting information Figure S7A). Given the significant clinal variation found in several underlying traits, we had an a priori expectation of associations between principal components and distance along the one-dimensional transect. Indeed, PC1ecophys exhibits a strong pattern of clinal variation (r14 = 0.84; p < 0.001; Figure 4a), but PC2ecophys does not co-vary with distance along the one-dimensional transect (p = 0.166).
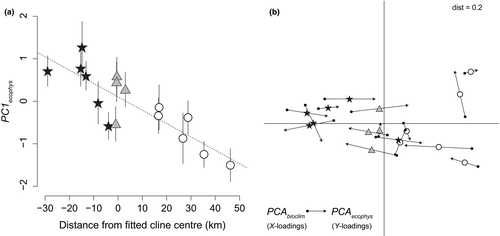
Climate varies more or less continuously across the distribution of focal populations (Supporting information Figure S7C), and significant relationships are found between climate and ecophysiological traits. For example, visualization via procrustean rotation indicates high degrees of overlap between PCAs of traits (PCAecophys) and climate (PCAbioclim; Figure 4b and Supporting information Figure S7B), and randomization approaches show these data tables are more similar than expected by chance (p < 0.01; Supporting information Figure S8). PC1ecophys varies with the eight bioclim layers used in distribution modelling (Sobel & Streisfeld, 2015), including particularly strong relationships with seasonality in both temperature and precipitation (Supporting information Figure S9). Moreover, the first principal component for ecophysiological traits (PC1ecophys) and that for climate data (PC1bioclim) are significantly correlated (r14 = 0.385, p = 0.0043; Supporting information Figure S7B). In addition, pairwise Mantel tests indicate positive correlations between PC1ecophys and geographic and genetic distances (Table 3). In partial Mantel tests that correct for genetic differentiation due to a shared evolutionary history, significant relationships between PC1ecophys and climatic and geographic distance remain (Table 3).
| Variable | Pairwise mantel | Partial mantel (FST corrected) | ||
|---|---|---|---|---|
| r | p-Value* | r | p-Value* | |
| 1D geographic distancea | 0.628 | 0.0001 | 0.512 | 0.0008 |
| 2D geographic distanceb | 0.540 | 0.0002 | 0.393 | 0.0023 |
| PC1bioclim | 0.385 | 0.0043 | 0.208 | 0.0402 |
| F ST | 0.423 | 0.0065 | N/A | N/A |
Notes
- a1D geographic distance refers to physical distance between populations along the one-dimensional fitted cline centre. b2D geographic distance refers to Euclidian X–Y straight-line distance between populations.
- * p-Values based on 10,000 permutations.
3.4 Biomass allocation
Above-ground biomass differed significantly between ecotypes, with red-flowered plants exhibiting significantly higher mass than yellow (Figure 5; red = 16.1 mg ± 0.80 SE; yellow = 12.4 mg ± 0.63 SE; 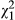 = 6.38; p = 0.012). However, root biomass did not differ significantly between the ecotypes (p = 0.982). In both response variables, the random effect of population was not significant (above-ground: p = 0.643; below-ground: p = 0.858). Overall, red-flowered plants produced a higher above-ground:below-ground biomass ratio (red: 1.84 ± 0.14 SE; yellow = 1.46 ± 0.11 SE).
= 6.38; p = 0.012). However, root biomass did not differ significantly between the ecotypes (p = 0.982). In both response variables, the random effect of population was not significant (above-ground: p = 0.643; below-ground: p = 0.858). Overall, red-flowered plants produced a higher above-ground:below-ground biomass ratio (red: 1.84 ± 0.14 SE; yellow = 1.46 ± 0.11 SE).
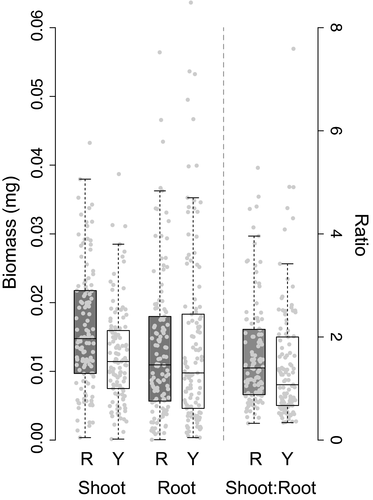
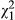 = 6.38; p = 0.012), whereas root biomass was not significantly different (p = 0.858). To the right of the dashed line, overall shoot:root biomass ratio is given for both ecotypes
= 6.38; p = 0.012), whereas root biomass was not significantly different (p = 0.858). To the right of the dashed line, overall shoot:root biomass ratio is given for both ecotypes3.5 Co-segregation between MaMyb2-M3 and vegetative anthocyanins
We detected significant co-segregation between alleles of MaMyb2 and visible anthocyanins in leaf and stem tissue of F2 plants (Supporting information Figure S10). Among 25 RR plants, 44% showed visible stem anthocyanins and 80% showed visible leaf anthocyanins. In contrast, none of the 21 YY plants showed stem anthocyanins and only 43% showed visible leaf anthocyanins. In both tissues, heterozygotes had intermediate frequencies of visible anthocyanins compared to each homozygote. Differences in the frequencies of visible anthocyanins among genotypes were statistically significant by Fisher's exact test (leaf: p = 0.031; stem: p < 0.001).
4 DISCUSSION
The role of ecological barriers in the speciation process has received considerable attention in recent years. However, there is much to learn about how natural selection produces reproductive isolation in the face of recurrent gene flow (Pinho & Hey, 2010). Our previous results indicated that pollinator fidelity maintains differentiation of the red- and yellow-flowered ecotypes despite evidence for gene flow across the hybrid zone (Sobel & Streisfeld, 2015; Stankowski et al., 2015, 2017; Streisfeld & Kohn, 2005; Streisfeld et al., 2013). However, populations of each ecotype are arranged in a highly nonrandom fashion, with the red ecotype residing in coastal regions and populations of the yellow ecotype found farther inland (Sobel & Streisfeld, 2015; Streisfeld & Kohn, 2005). Our primary goal in this study was to determine if nonfloral phenotypes exhibited geographically structured variation that could be responsible for the parapatric geographic ranges of these taxa. We found that a number of ecophysiological traits differed, confirming that ecogeographic differences exist. However, rather than finding discontinuous variation between taxa, the ecophysiological traits examined here generally exhibit a gradual clinal transition across the entire geographic distribution. Nevertheless, these traits change coincidently with variation in abiotic conditions, suggesting that trait differences are a direct or indirect product of local adaptation to the environment. Below, we discuss these results with regard to the combined impact of ecogeographic and pollinator isolation on divergence between these two taxa.
4.1 Evidence for variation in ecophysiological phenotypes
The environmental conditions experienced by the red- and yellow-flowered ecotypes differ in a number of ways that are likely to influence water availability (Figure 1; Supporting information Figure S1). Coastal regions are warmer on an annual basis, but they experience less severe seasonal fluctuations in temperature compared to inland sites (Sobel & Streisfeld, 2015). In addition, whereas overall precipitation is slightly higher for inland regions, elevated spring temperatures and the lack of a recurrent coastal marine layer make inland habitats drier during seedling establishment (M. Streisfeld, pers. obs.). Consistent with these conditions, drought sensitivity exhibits linear clinal variation across the distribution, with the lowest drought sensitivity (d) scores found in populations located farthest from the coast (Figure 2a). Although physiological traits are typically associated with dehydration avoidance (Chapin, Autumn, & Pugnaire, 1993; Knight et al., 2006; Voltas, Chambel, Prada, & Ferrio, 2008), it appears that leaf area is the primary driver of differences in drought sensitivity among populations studied here (Figure 2b; Table 1). Indeed, whereas inland drought is a compelling putative agent of selection, these results motivate further exploration of alternate hypotheses. For example, in response to inter- and/or intraspecific competition, coastal populations could be under selection to increase investments in above-ground biomass (Figure 5), and drought sensitivity may occur as an indirect physiological trade-off. Field measurements of selection or manipulative experiments are needed to examine these potential scenarios.
Although the role that anthocyanins play in attracting pollinators is well known (Rausher, 2008), their physiological effects in vegetative tissues are much less understood. However, increased vegetative anthocyanin concentrations are a common response to a variety of stressful conditions (Chalker-Scott, 1999; Winkel-Shirley, 2002). Consistent with this pattern, our experiments showed that stress revealed variation in vegetative anthocyanin, and pigment concentrations decreased with distance along the transect (Figure 3b). These results can be interpreted in a number of different ways. For example, higher production of vegetative anthocyanins under stress may indicate that red ecotype populations are better at mobilizing anthocyanins, resulting in a higher tolerance to stress. Alternatively, increased vegetative anthocyanins may be an indicator of plant stress, revealing that coastal populations experienced stress more quickly than inland populations. Correlations between ecophysiological traits favour the latter interpretation, as the families that exhibit the most sensitivity to drought also make the most vegetative anthocyanin (Supporting information Figure S6).
Interestingly, vegetative resins show the opposite pattern to anthocyanins in response to stress, where a clear geographic cline is observed only for unstressed plants (Figure 3c,d). Like anthocyanins, these resins are products of the flavonoid pathway, so competition for precursors may affect the relative production of these two compounds (e.g., Yuan, Rebocho, Sagawa, Stanley, & Bradshaw, 2016). However, we find a weak positive correlation between vegetative resins and anthocyanins (Supporting information Figure S6), suggesting that coastal populations may experience increased flux of precursors impacting the entire pathway. Vegetative resins are implicated in a variety of responses to abiotic conditions, including dehydration avoidance and protection from UV damage (Lincoln & Walla, 1986); however, the lack of clinal variation under abiotic stress runs counter to these predictions. Indeed, previous studies in M. aurantiacus have shown the highest concentrations of resins in coastal regions (Hare, 2002). Therefore, biotic agents of selection may play a role in shaping constitutive expression of these resins. For example, coastal populations of M. aurantiacus in northern California are host to larva of the checkerspot butterfly, Euphydryas chalcedona, and resins appear to reduce the growth rate of these herbivores (Lincoln, 1985).
Although all plants were raised in a common environment, trait measurements were obtained from individuals collected as seeds from natural populations. As a relatively long-lived woody shrub with substantial inbreeding depression, we did not perform a generation of greenhouse propagation prior to conducting this experiment. Therefore, maternal effects could play some role in shaping the phenotypic variation observed. However, we expect impacts of maternal effects to be minimal for several reasons. First, in a small number of populations from each ecotype where we have grown multiple generations in the greenhouse, we see consistent differences in early-stage leaf area and visible vegetative anthocyanins. Further, Mimulus seeds are tiny, allowing little maternal provisioning that would be expected to impact plant traits past its earliest stages. Thus, even though maternal effects remain a possibility, their impacts on our overall conclusions would appear to be minimal.
4.2 Clinal variation, adaptation and reproductive isolation
In his classic work, Endler (1977) attributed clinal variation in phenotypes to the impacts of natural selection along environmental gradients. Moreover, associations between environmental conditions and phenotypes often reveal suites of traits that vary along geographic transects (Chapin et al., 1993). Clinal variation has long been regarded as an indication of local adaptation (Endler, 1977, 1986; Haldane, 1948) that presumably results in the negative relationship between gene flow and distance across a cline (i.e., “isolation by adaptation”; Nosil, Egan, & Funk, 2008). Indeed, the red and yellow ecotypes display clinal variation in ecophysiological traits (Figure 4a), consistent with a scenario in which phenotypic variation is shaped by adaptation across its geographic distribution. However, much like neutral genetic variation, clinal patterns in phenotypic traits also may be the result of stochastic processes (Whitlock, 1999; Wright, 1943). Indeed, determining the relative roles of genetic drift and natural selection in shaping biological diversity remains a central challenge in evolutionary biology (Luo et al. 2015). Although our family-level replication was insufficient to formally address this question (i.e., QST–FST analysis; Whitlock, 2008; Leinonen, McCairns, O'Hara, & Merila, 2013), we favour adaptive explanations for trait diversity for a number of reasons. For example, these traits exhibit significant associations with distance and climate, even when the shared evolutionary history among populations is taken into account in Mantel tests (Table 3). Moreover, the consistent pattern of trait variation with distance suggests the action of a deterministic force, such as natural selection. Three of the ecophysiological traits examined here show significant clinal variation: drought sensitivity (Figure 2a), stressed leaf anthocyanin (Figure 3b) and ULR (Figure 3c). In previous work using these same 16 populations, we identified six floral traits that varied across this geographic transect (Stankowski et al., 2015). Although floral traits exhibit a more step-like cline, transitions in all nine traits occur along the same coast-to-inland geographic axis. Whereas the hybrid zone could represent a recently formed point of contact following allopatric separation, our previous analyses of genomic variation are inconsistent with this scenario (Stankowski et al., 2015, 2017). Therefore, the common transition of multiple quantitative traits (with somewhat independent genetic bases) seems unlikely to result from stochastic processes alone.
The observed differences in cline shapes between the ecophysiological and floral traits likely occur due to variation in the nature of selection on these ecological targets. Because climate varies continuously, traits respond accordingly. By contrast, hummingbird and hawkmoth pollinators likely exert correlated selection pressures on a suite of pollination syndrome traits (Fenster, Armbruster, Wilson, Dudash, & Thomson, 2004). Therefore, pollinator-mediated selection appears capable of producing the discontinuous variation characteristic of incipient speciation. However, pollinator preferences do not result in complete reproductive isolation, with hummingbirds especially likely to visit yellow ecotype flowers, despite their strong preference for red (Handelman & Kohn, 2014; Sobel & Streisfeld, 2015; Streisfeld & Kohn, 2007). The leaky nature of pollinator isolation, no evidence for intrinsic post-mating isolation (Sobel & Streisfeld, 2015) and no evidence for suppressed recombination (Stankowski et al., 2015) suggest that the nearly complete differentiation of red and yellow populations would not be maintained if these two ecotypes were fully sympatric. Further, the geographic distribution of pollinators is unlikely to fully explain ecotypic differentiation. For example, although hummingbirds appear to be somewhat more abundant in coastal areas, there is no difference in hummingbird visitation rates in coastal versus inland sites (Streisfeld & Kohn, 2007). Further, whereas relative abundance data are lacking for the primary hawkmoth (Hyles lineata) pollinator of the yellow ecotype, this species has been observed routinely in both coastal and inland locations (Streisfeld & Kohn, 2007). We therefore propose that divergence in ecophysiological traits may be a necessary component of speciation driven by pollinators. Intriguingly, many well-studied examples of pollinator divergence occur along environmental gradients [e.g., Mimulus lewisii and Mimulus cardinalis (Ramsey et al., 2003), Aquilegia formosa and Aquilegia pubescens (Hodges, Fulton, Yang, & Whittall, 2004), Ipomopsis aggregata and Ipomopsis tenuituba (Campbell, 2004)]. Whereas variation in ecophysiological traits may not impede gene flow between red and yellow ecotypes in a discontinuous manner, gene flow is likely to be slowed between them due to the position of the two morphs on the landscape. Therefore, the average red ecotype population appears ecogeographically isolated from the average yellow ecotype population (Sobel & Streisfeld, 2015). At present, it is unclear whether interactions between these continuous and discontinuous forms of selection would be sufficient to maintain distinct taxa in the face of gene flow, but this scenario could be modelled in the future to determine the scenarios that would support their maintenance.
One factor that could contribute to the observed relationships between ecogeographic and pollinator isolation is a genetic correlation between ecophysiological and floral traits. Recent studies have revealed that nonpollinator agents of selection, including abiotic factors, may drive flower colour divergence in nature (Arista, Talavera, Berjano, & Ortiz, 2013; Schemske & Bierzychudek, 2007; Strauss & Whittall, 2006; Wessinger & Rausher, 2012). Conversely, when pollinators are responsible for divergence in flower colour, correlated physiological changes that impact interactions with the abiotic environment may evolve indirectly (Sobel & Streisfeld, 2013). In a segregating F2 population of plants made from crosses between red- and yellow-flowered parents, we found a significant association between the genotype at MaMyb2 and the occurrence of floral and vegetative anthocyanins (Supporting information Figure S10; Streisfeld et al., 2013). This co-segregation could be due to pleiotropic effects of MaMyb2, or via the effects of linkage disequilibrium between MaMyb2 and another linked locus. In stressed conditions, there is a modest difference in the concentration of vegetative anthocyanins between ecotypes even when corrected for the effect of distance (Figure 3b, Table 2). Therefore, an intriguing possibility is that genetic correlations between floral and vegetative anthocyanins provided an initial physiological change between ecotypes that resulted in a largely parapatric distribution, followed by subsequent local adaptation that resulted in clinal variation of other ecophysiological traits. Alternatively, selection on vegetative pigmentation across the distribution could have produced a cline in flower colour, with positive frequency-dependent selection by pollinators subsequently stabilizing initial differences. Further work aimed at understanding the degree to which each of these traits impacts geographic distributions in natural populations will be needed to help distinguish between these scenarios.
5 CONCLUSIONS
Early-acting ecological barriers, such as ecogeographic and pollinator isolation, have the first opportunity to limit gene flow between emerging taxa (Ramsey et al., 2003; Sobel et al., 2010). Whereas the impact of pollinator isolation is well established between the red- and yellow-flowered ecotypes, little has been known about variation in traits that contribute to the ecogeographic distribution of these emerging species. In this study, we found evidence that ecophysiological traits exhibit significant clinal variation, confirming that there are intrinsic biological differences between these incompletely isolated taxa. However, these traits alone would be unlikely to produce the discontinuous variation commonly associated with speciation. Nevertheless, the ecophysiological traits may provide an additional barrier to gene flow that helps maintain differentiation in the face of incomplete pollinator preferences. Intriguingly, genetic linkage between loci controlling floral and vegetative anthocyanin expression may be partially responsible for associations between these forms of isolation, and ongoing genetic mapping and field experiments will help to test these hypotheses further.
ACKNOWLEDGMENTS
The authors would like to thank W. Young and L. Poasa for laboratory and greenhouse assistance. In addition, a number of undergraduate students contributed to data collection for this project. We would especially like to thank N. Nnatubuego, C. Brocious and B. Winkelman for their efforts. We are also grateful to Associate Editor Porcher and three anonymous reviewers for their comments and suggestions throughout the review process. The project was supported by the University of Oregon and by a National Science Foundation grant to MAS: DEB-1258199.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare regarding this publication.




