Within-host interactions shape virulence-related traits of trematode genotypes
Abstract
Within-host interactions between co-infecting parasites can significantly influence the evolution of key parasite traits, such as virulence (pathogenicity of infection). The type of interaction is expected to predict the direction of selection, with antagonistic interactions favouring more virulent genotypes and synergistic interactions less virulent genotypes. Recently, it has been suggested that virulence can further be affected by the genetic identity of co-infecting partners (G × G interactions), complicating predictions on disease dynamics. Here, we used a natural host–parasite system including a fish host and a trematode parasite to study the effects of G × G interactions on infection virulence. We exposed rainbow trout (Oncorhynchus mykiss) either to single genotypes or to mixtures of two genotypes of the eye fluke Diplostomum pseudospathaceum and estimated parasite infectivity (linearly related to pathogenicity of infection, measured as coverage of eye cataracts) and relative cataract coverage (controlled for infectivity). We found that both traits were associated with complex G × G interactions, including both increases and decreases from single infection to co-infection, depending on the genotype combination. In particular, combinations where both genotypes had low average infectivity and relative cataract coverage in single infections benefited from co-infection, while the pattern was opposite for genotypes with higher performance. Together, our results show that infection outcomes vary considerably between single and co-infections and with the genetic identity of the co-infecting parasites. This can result in variation in parasite fitness and consequently impact evolutionary dynamics of host–parasite interactions.
1 INTRODUCTION
A single host organism is typically co-infected with multiple parasite genotypes and/or species (Cox, 2001; Read & Taylor, 2001) and may thus be seen as an ecosystem of interacting parasites (Graham, 2008; Rynkiewicz, Pedersen, & Fenton, 2015). Theoretical and empirical studies suggest that within-host interactions can have major impacts on parasite traits, such as infectivity, virulence and transmission, and thereby affect parasite evolution (e.g. vanBaalen & Sabelis, 1995; Bell, De Roode, Sim, & Read, 2006; Karvonen, Rellstab, Louhi, & Jokela, 2012; Lello, Boag, Fenton, Stevenson, & Hudson, 2004; May & Nowak, 1995; Poulin, 2001; Read & Taylor, 2001; Telfer et al., 2010). Because the host typically represents a limited resource, co-infecting parasites often face competitive interactions. Within-host competition can be resource-mediated, where high exploitation rates by one parasite limit access for others (exploitation competition), but can also manifest through direct interactions such as the release of toxic compounds (interference competition), or indirectly through the induction of host immune responses by one parasite that inhibits others (apparent competition; reviewed in Read & Taylor, 2001; Mideo, 2009; Bose, Kloesener, & Schulte, 2016). As competition typically induces fitness costs for all parties involved, parasite performance in competitive co-infections is expected to be lower compared to single infections. However, parasites can also benefit from co-infection through cooperation, the level of which should increase with relatedness between co-infecting partners (reviewed in Buckling & Brockhurst, 2008). Further, co-infection can result in synergistic interactions if the higher genetic diversity of infection represents a greater challenge for the host immune system and reduces its effectiveness (Jokela, Schmid-Hempel, & Rigby, 2000; Karvonen et al., 2012; Klemme, Louhi, & Karvonen, 2016; Taylor, Mackinnon, & Read, 1998).
Recent evidence suggests that the intensity and outcome of within-host interactions may depend on the genetic identity of the interacting parasites (genotype by genotype, G × G interactions; reviewed in Seppälä & Jokela, 2016). For example, success of interacting parasite genotypes under competition is typically determined by their relative competitive ability. This has been demonstrated, for example, in co-infecting clones of the rodent malaria Plasmodium chabaudi, where the competitive ability of the strains is predictable from virulence in single infection (Bell et al., 2006; de Roode, Helinski, Anwar, & Read, 2005). In facilitative interactions, on the other hand, parasite success can show marked variation in an unpredictable manner (Louhi, Sundberg, Jokela, & Karvonen, 2015; Seppälä, Karvonen, Rellstab, Louhi, & Jokela, 2012). Such genetic specificity in interactions can lead to variation in parasite fitness across different co-infecting communities, making conclusions on the evolutionary dynamics of parasite traits more challenging (Seppälä & Jokela, 2016).
One important trait that has received much interest in the co-infection context is virulence, that is, the reduction in host fitness due to infection (for reviews see Alizon, de Roode, & Michalakis, 2013; Bose et al., 2016; Cressler, McLeod, Rozins, van den Hoogen, & Day, 2016). In single infections, selection is expected to favour intermediate virulence, because this optimizes parasite transmission between hosts (trade-off hypothesis, Anderson & May, 1982). In co-infections, such optima can shift to either lower or higher virulence as consequence of within-host interactions (Alizon et al., 2013). For example, if virulence and competitive ability are linked, selection will favour more virulent parasites under exploitation competition (vanBaalen & Sabelis, 1995; Choisy & de Roode, 2010; May & Nowak, 1995; Mosquera & Adler, 1998). Under immune system impairment, on the other hand, any potential costs of competition are outbalanced by the benefit of co-infection, and virulence is expected to evolve to a lower level (Choisy & de Roode, 2010). However, it is important to note that the observed virulence of multiple infections relative to single infections within one generation could be reduced under exploitation competition due to the costs of the competition and increased under immune system impairment due to the benefits of facilitation (Choisy & de Roode, 2010). Thus, to draw inferences on the evolution of virulence, co-infection studies should not only compare virulence between single and multiple infections, but also explore which virulence genotypes are selected for in different co-infection scenarios (Alizon et al., 2013; Choisy & de Roode, 2010).
Here, we study whether virulence of infection is affected by G × G interactions between genotypes of the trematode Diplostomum pseudospathaceum. The parasite has a three-host life cycle with sexual reproduction in the definitive avian host, asexual multiplication in the first intermediate snail host and growth as well as long-term storage in the second intermediate fish host (Chappell, Hardie, & Secombes, 1994). Interactions between clonal parasite larvae within the fish host can take place during the acute invasion, within 24 hours after parasites have penetrated the fish host and migrate towards its eye lenses, where they establish (Chappell et al., 1994). Direct interactions can also occur in the eye lens, where the parasites are protected from host immune responses and develop to larger-sized, long-lived metacercariae (Chappell et al., 1994). Metacercariae damage structures of the host's eye lens, leading to cataracts that obscure host vision (Karvonen, Seppälä, & Valtonen, 2004a; Shariff, Richards, & Sommerville, 1980). This is beneficial for parasite transmission as it increases the fish host's risk of being predated upon by the parasite's final bird host (Seppälä, Karvonen, & Valtonen, 2004, 2005). Because cataract coverage increases linearly with parasite load (Karvonen et al., 2004a) and parasites do not replicate in fish eyes, pathogenicity (here referred to as virulence) of a chronic infection is directly related to infectivity, that is the ability to establish in the eye lenses. This trait shows ample variation among parasite genotypes (Louhi, Karvonen, Rellstab, & Jokela, 2013a; Seppälä et al., 2012). Additionally, genotypes may differ in their per capita ability to induce cataracts (independent of infectivity), but this has not been studied to date.
Natural D. pseudospathaceum infections of fish typically consist of multiple clonal genotypes (Rauch, Kalbe, & Reusch, 2005) and experiments have demonstrated increased infectivity in multiple compared to single-genotype attacks, likely due to host immune system impairment (Karvonen et al., 2012; Klemme et al., 2016, but see Rauch, Kalbe, & Reusch, 2008). Recent co-infection studies with a closely related trematode species (Seppälä, Karvonen, Valtonen, & Jokela, 2009; Seppälä et al., 2012) and with a pathogenic bacterium (Louhi et al., 2015) have revealed genotype-specific interactions between D. pseudospathaceum and the co-infecting species. However, whether interactions among D. pseudospathaceum genotypes at the same infection site (host eye lenses) are genotype-specific is unknown. It is thus unclear whether all genotypes gain equally from host immune system impairment through increased virulence, or whether this is correlated with parasite performance in single infections. To explore these questions, we exposed rainbow trout (Oncorhynchus mykiss) to single- or double-genotype infections using two full co-infection matrices of five genotypes each and estimated two virulence-related traits: infectivity (directly related to cataract coverage) and relative cataract coverage per parasite capita (controlled for infectivity). We also genotyped the established co-infections to assess genotype-specific success in different genotype combinations.
2 MATERIALS AND METHODS
2.1 Parasite and host sources
Parasites were obtained from naturally infected snail hosts, Lymnaea stagnalis, collected from Lake Vuojärvi (Central Finland, 62° N, 25° E). As this parasite does not show a detectable population genetic structure across a large geographic scale (Louhi, Karvonen, Rellstab, & Jokela, 2010), parasite origin was unlikely to affect the results. Cercariae (free-swimming larval stage) released from infected snails were genotyped to verify single-genotype infection. For this, 20 cercariae were haphazardly collected from each snail and genotyped with four highly polymorphic microsatellite markers (Karvonen et al., 2012; Louhi, Karvonen, Rellstab, Louhi, & Jokela, 2013b; Louhi et al., 2013a; Reusch, Rauch, & Kalbe, 2004). Subsequently, the snails were maintained in standardized conditions in individual containers with 1 l of lake water (4°C) and lettuce ad libitum for two weeks.
Juvenile rainbow trout (average body mass 2.9 g) were obtained from a commercial fish farm in Finland. At the farm, the fish had been maintained in groundwater, which ensured that they were free of D. pseudospathaceum infection. Rainbow trout are highly susceptible to the parasite and have been used in several co-infection studies (Karvonen et al., 2012; Klemme et al., 2016; Seppälä et al., 2009, 2012).
2.2 Experimental exposures
Ten snails carrying ten different parasite genotypes were used for the experimental single-genotype and double-genotype exposures. For double-genotype exposures, the genotypes were randomly assigned to two groups of five, and each genotype was paired with each of the four other genotypes, resulting in 2 × 10 different combinations. To produce cercariae for the exposures, five snails at a time were placed in 200 ml of lake water (17°C) for 3 hr, after which parasite density was estimated from five 1 ml samples for each genotype. Single-genotype exposures (N = 10 genotypes) and each of the double-genotype combinations (N = 20 combinations) were replicated using 20 fish hosts, totalling 600 exposed fish. Rainbow trout were placed individually in containers with 500 ml of water (17°C) and exposed to an estimated number of 50 cercariae (25 of each genotype in co-exposures). After 30 minutes, each group of fish receiving the same exposure treatment (total N = 30 groups, see Table S1), was transferred to a randomly selected flow-through tank (36 × 26 × 22 cm) containing 13 L of lake water (17°C). There was no mortality of fish during or after the exposure. Additionally, four groups containing 20 unexposed rainbow trout each were established in a similar manner to control for possible infections from the incoming lake water. However, none of the control individuals carried infections with D. pseudospathaceum at the end of the study, indicating that there was no uncontrolled exposure during the experiment. The fish were fed daily with commercial fish pellets (3% of body weight).
The groups were kept for 40 days, which is sufficient time for the parasites to fully develop and induce cataracts (Karvonen et al., 2004a). Subsequently, all fish were euthanized with an overdose of MS-222 anaesthetic and measured for length. Cataract coverage was scored for each eye as 10%, 20%,…, 100% of the lens area using slit-lamp microscopy (Karvonen et al., 2004a). Subsequently, the lenses were dissected and parasite numbers were counted under the microscope. From ten randomly chosen fish of each co-exposure treatment (except for one genotype combination, for which only 6 fish remained, see below), ten metacercariae were collected from the right eye. In some cases, the right eye harboured ≤ 10 parasites, in which case the left eye was sampled (N = 40/196 host individuals). The metacercariae were genotyped as described above to determine genotype-specific infectivity of co-infecting genotypes. Genotype identity could be unambiguously assigned to 1909/1948 metacercariae (see Table S1).
An unexpected bacterial infection (Flavobacterium columnare, identified by visual symptoms on the skin and gills) made it necessary to supplement the fish feed with antibiotics (oxytetracycline,30 g/kg feed), starting on day 7 after exposure and lasting for 10 days. Due to the bacterial infection, 202/600 (33.7%) of the experimentally infected individuals and 24/80 (30.0%) of the control individuals were lost. Thus, at the end of the experiment, numbers of fish in the exposure treatment groups varied between 6 and 19 (see Table S1). Survival of treatment groups was not correlated with average infectivity of D. pseudospathaceum in each group (rS = −0.133, p = 0.485, N = 30) or relative cataract coverage later in the experiment (rS = −0.114, p = 0.550, N = 30).
2.3 Data analysis
All analyses were conducted using SAS v. 9.4 (SAS Institute, Cary, NC, USA). Infectivity was calculated as sum of the parasites established in the left and right eye lens of each infected fish. As expected, there was a positive relationship between log-transformed infectivity and logit-transformed cataract coverage (R2 = 0.423, F1, 395 = 289.6, p < 0.001, see Figure S1). To gain a measure of the ability of parasites to cause cataracts at a given infectivity, relative cataract coverage was estimated for each fish as the residual deviation from the regression line across all experimentally infected fish (single- and double-genotype infections combined, see Figure S1). Here, negative and positive residuals represent relatively smaller cataracts (lower ability) and larger cataracts (higher ability), respectively, for a given infectivity.
Variation in parasite genotype performance in single-genotype infections was assessed with generalized linear models (GLMs). Infectivity (negative binomial error, log link) and relative cataract coverage (normal error) were entered as response variables, genotype identity as a fixed factor and fish host length as a covariate. G × G interactions in infectivity and relative cataract coverage were explored by first calculating mean trait values in single infections for each of the genotype combinations, using randomly chosen pairs of single-infected fish from both genotypes (Bose & Schulte, 2014). These means, together with their corresponding trait values in co-infections, were entered as response variable in a GLM. Infection treatment (single vs. co-infection), genotype combination (nested within matrix), their interaction and fish length were included as factors. A significant interaction would indicate G × G interactions, that is that a change in parasite performance from single- to double-genotype exposures depends on the specific genotype combinations (Bose & Schulte, 2014). For each genotype combination, significant differences in both traits between single and co-infection were determined by calculating the 95% confidence intervals (CI) for co-infection values and examining whether the single infection values lie within these intervals (Louhi et al., 2015).
Infectivity in co-infection was also explored on the genotype level to study possible variation in genotype-specific success across different co-infecting combinations. This was done by testing whether genotype-specific infectivity in co-infection deviated from the infectivity expected from single-genotype infections. For each host, the total number of parasites of each genotype was extrapolated from the proportions determined through genotyping. Subsequently, it was examined whether the expected value (½ infectivity in single infections) was outside the 95% CI of the observed infectivity in double infection.
3 RESULTS
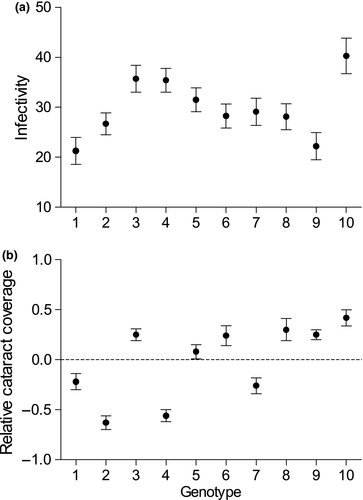
3.1 Infectivity
Infectivity varied among the ten parasite genotypes in single-genotype exposures (GLM, N = 133, 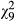 = 43.0, p < 0.001; covariate length:
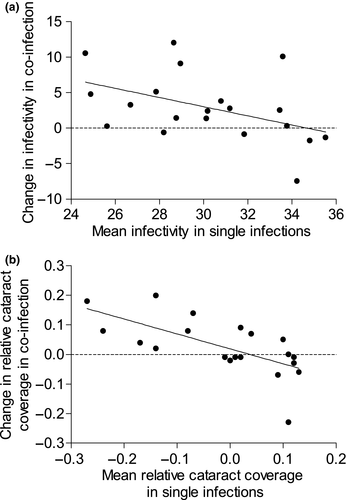
= 43.0, p < 0.001; covariate length: 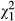 = 0.39, p = 0.535; Figure 1a). In co-infections, infectivity was affected by G × G interactions, as indicated by a significant interaction between infection treatment (single vs. double) and genotype combination (Table 1, Figure 2a). For 7/20 combinations, infectivity increased significantly from single infection to co-infection and decreased in one combination. Overall, the average infectivity in single exposures was 30.0 ± 0.5 and in co-infections 32.9 ± 0.5, representing an increase of 9.84% (Figure 2a). There was a negative relationship between the mean (combination-specific) infectivity in single-genotype infections and the change in infectivity from single infection to co-infection (N = 20, r = −0.451, p = 0.046, Figure 3a). More specifically, the result indicates that combinations including genotypes of low infectivity in single infection showed the highest increase in infectivity in co-infection. However, this change was negative in the combinations including genotypes of high infectivity, suggesting that these genotypes suffered from co-infection.
= 0.39, p = 0.535; Figure 1a). In co-infections, infectivity was affected by G × G interactions, as indicated by a significant interaction between infection treatment (single vs. double) and genotype combination (Table 1, Figure 2a). For 7/20 combinations, infectivity increased significantly from single infection to co-infection and decreased in one combination. Overall, the average infectivity in single exposures was 30.0 ± 0.5 and in co-infections 32.9 ± 0.5, representing an increase of 9.84% (Figure 2a). There was a negative relationship between the mean (combination-specific) infectivity in single-genotype infections and the change in infectivity from single infection to co-infection (N = 20, r = −0.451, p = 0.046, Figure 3a). More specifically, the result indicates that combinations including genotypes of low infectivity in single infection showed the highest increase in infectivity in co-infection. However, this change was negative in the combinations including genotypes of high infectivity, suggesting that these genotypes suffered from co-infection.
| Factors | Infectivity | Relative cataract coverage | ||||
|---|---|---|---|---|---|---|
| df | X 2 | p | df | X 2 | p | |
| Infection treatment | 1 | 18.13 | <0.001 | 1 | 5.08 | 0.024 |
| Genotype combination(matrix) | 19 | 90.21 | <0.001 | 19 | 467.29 | <0.001 |
| Treatment × combination(matrix) | 19 | 45.56 | 0.001 | 19 | 118.73 | <0.001 |
| Length | 1 | 0.22 | 0.639 | 1 | 82.68 | <0.001 |
Genotype-specific infectivity (estimated separately for each of the co-infection genotypes) in co-infections also depended on the specific genotype combination, showing either higher, unaltered or lower infectivity than expected from single infection (Table 2). Average genotype-specific infectivity across different co-infection (“averages” in Table 2) was independent of infectivity in single-genotype exposures (N = 10, r = 0.455, p = 0.187).
| Genotype | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1/2 single | 10.7 | 13.4 | 17.9 | 17.7 | 15.8 |
| (a) | |||||
| With 1 | 22.5 | 25.3 | 23.4 | 22.4 | |
| With 2 | 13.7 | 15.6 | 18.4 | 18.6 | |
| With 3 | 11.5 | 18.9 | 21.4 | 19.4 | |
| With 4 | 13.3 | 12.6 | 12.8 | 20.5 | |
| With 5 | 12.3 | 20.3 | 16.6 | 22.6 | |
| Average | 12.7 | 19.2 | 17.6 | 21.8 | 20.2 |
| Genotype | 6 | 7 | 8 | 9 | 10 |
| 1/2 single | 14.2 | 14.6 | 14.0 | 11.1 | 20.2 |
| b) | |||||
| With 6 | 17.5 | 14.6 | 17.2 | 14.7 | |
| With 7 | 12.7 | 19.2 | 10.8 | 16.3 | |
| With 8 | 15.4 | 21.5 | 13.2 | 17.7 | |
| With 9 | 14.5 | 20.5 | 15.6 | 14.9 | |
| With 10 | 13.7 | 15.4 | 20.2 | 18.2 | |
| Average | 14.1 | 18.7 | 17.4 | 14.8 | 15.9 |
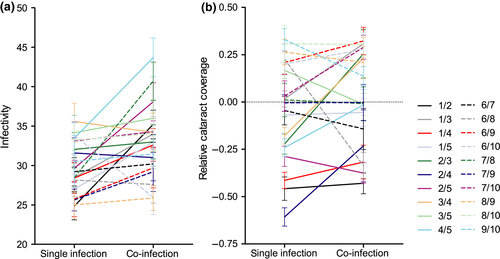
3.2 Relative cataract coverage
Parasite genotypes also showed variation in relative cataract coverage (N = 133, 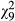 = 293.7, p < 0.001, covariate length:
= 293.7, p < 0.001, covariate length: 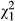 = 26.0, p < 0.001, Figure 1b), but this trait was not related to infectivity in single-genotype exposures (N = 10, r = 0.203, p = 0.575). There was also evidence for G × G interactions in this trait (Table 1, Figure 2b), with 6/20 genotype combinations showing a significant increase in relative cataract coverage from single infection to co-infection and 3/20 combinations showing a significant decrease. Overall, the average relative cataract coverage increased slightly from −0.03 ± 0.02 in single infections to 0.02 ± 0.01 in co-infections (Figure 2b). Similarly to infectivity, the change in relative cataract coverage induced by each genotype combination was negatively correlated with the mean relative cataract coverage in single-genotype infections (N = 20, r = −0.662, p = 0.001, Figure 3b).
= 26.0, p < 0.001, Figure 1b), but this trait was not related to infectivity in single-genotype exposures (N = 10, r = 0.203, p = 0.575). There was also evidence for G × G interactions in this trait (Table 1, Figure 2b), with 6/20 genotype combinations showing a significant increase in relative cataract coverage from single infection to co-infection and 3/20 combinations showing a significant decrease. Overall, the average relative cataract coverage increased slightly from −0.03 ± 0.02 in single infections to 0.02 ± 0.01 in co-infections (Figure 2b). Similarly to infectivity, the change in relative cataract coverage induced by each genotype combination was negatively correlated with the mean relative cataract coverage in single-genotype infections (N = 20, r = −0.662, p = 0.001, Figure 3b).
4 DISCUSSION
The genetic identity of co-infecting parasites may shape the direction (antagonistic, neutral, facilitative) and intensity of within-host interactions and, consequently, virulence of infection (Seppälä & Jokela, 2016). To understand how selection shapes virulence, it is therefore important to take such G × G interactions into account and explore fitness of virulence genotypes across different co-infection communities (Alizon et al., 2013; Choisy & de Roode, 2010). We assessed two virulence-related traits across 10 different parasite genotypes in single infections and 20 genotype combinations in co-infections in a natural host–parasite system—a fish host and the fluke D. pseudospathaceum. We found that both infectivity and relative cataract coverage (degree of pathogenicity independent of infectivity) were associated with parasite G × G interactions, including both increases and decreases from single infection to co-infection.
Parasite traits typically show marked genetic variation (e.g. Carius, Little, & Ebert, 2001; Tack, Thrall, Barrett, Burdon, & Laine, 2012; Vale & Little, 2009), which is a paradox, as selection is expected to drive fitness-related alleles to fixation (Roff, 1997). One possible mechanism maintaining genetic heterogeneity in parasite traits is co-infection with other genotypes of the same or another parasite species (Seppälä & Jokela, 2016). For example, in the present system, co-infections of D. pseudospathaceum genotypes with other taxonomically closely related species (Seppälä et al., 2009, 2012) or even with completely unrelated pathogens (Louhi et al., 2015) can result in variable outcomes of infection depending on the genotype combinations. Further, in accordance with the present results, co-infections between genotypes of D. pseudospathaceum can result in facilitation in infectivity, likely following an additional challenge to the host immune system (Karvonen et al., 2012; Klemme et al., 2016), which can be beneficial to both co-infecting genotypes. However, our results suggest that some genotype combinations benefitted from co-infection, whereas others showed lower infectivity and relative cataract coverage compared to single-genotype exposures. In particular, combinations where both genotypes had low virulence in single infections benefited the most from co-infection, while the pattern was opposite for combinations with a higher virulence. This suggests that the direction and intensity of within-host interactions in co-infection could depend on the particular virulence genotypes involved.
There are several possible mechanisms to explain these results. First, parasite genotypes may compete during migration in the host body and in the eye lens. The intensity of competition could depend on the particular virulence genotypes in the co-infection and predict the change in infectivity and cataract formation per parasite from single to co-infection. For example, parasite-induced cataracts likely result from metabolic excretions and mechanical destruction of the lens structure due to parasite movement (Shariff et al., 1980), both of which could increase with competition. Overall, however, competition in the fish host is expected to be low. This is because the host is mainly used as transmission vehicle to the final bird host, which likely includes relatively little resource exploitation (Karvonen et al., 2012). Second, the strength of the innate host response could be affected by the parasite genotypes involved. Previous work in this system has demonstrated evidence for parasite genotype-specific innate host defence in single-genotype infections (Rauch, Kalbe, & Reusch, 2006). In a similar manner, host defence in co-infections could be combination-specific and affect different combinations with varying intensity. In turn, the strength of the host immune response could potentially affect the ability of parasites to induce cataracts in the eye lens, but only if the effects came about during the tissue migration towards the eye (parasites are protected from host immune responses in the lens). Independent of the mechanism, however, these G × G interactions led to high variation in genotype-specific infectivity across different co-infection partners. Moreover, average infectivity in co-infection was not predictable from infectivity in single infection. Together, these findings suggest significant variation in parasite fitness-related traits, not only from single to co-infection, but also across different co-infection communities. This makes predictions on the effect of co-infection on virulence evolution difficult.
It is important to note that each parasite genotype originated from a different snail host, which could potentially influence the phenotypic expression of parasite traits. For example, nutritional status of snails infected with D. pseudospathaceum has been found to affect cercarial output and longevity (Seppälä, Liljeroos, Karvonen, & Jokela, 2008). However, we minimized possible variation in the condition of the snails by providing ad libitum feeding and identical housing conditions for two weeks before the experiment commenced (Louhi et al., 2013a). Moreover, although genotype-specific infectivity has been found to vary over time, it is still highly repeatable within genotypes (Louhi et al., 2013a). There was also an unexpected outbreak of F. columnare (Declercq, Haesebrouck, Van den Broeck, Bossier, & Decostere, 2013) during the study, which required administration of antibiotics. However, the bacterial disease broke out one week after the exposures to D. pseudospathaceum, when the parasites were already well established in the eye lenses (establishment takes place within 24 h from exposure). Moreover, all fish groups received the same antibiotic treatment, which is why the treatment is unlikely to explain the results.
In conclusion, we demonstrate complex G × G interactions between genotypes of the trematode parasite D. pseudospathaceum that have important implications for infection virulence. Both observed traits, infectivity (number of parasites in the host) and relative cataract coverage (damage per parasite), together affected the severity of eye cataracts and, thus, overall virulence. As absolute cataract coverage associates positively with the vulnerability of fish to predation by the parasite's final bird host (Seppälä et al., 2005), parasites should gain higher transmission probability by increasing it. Thus, the observed G × G interactions in co-infections may directly affect parasite fitness and, consequently, the evolution of virulence and host–parasite evolutionary dynamics. In natural conditions, this complexity is likely further increased, because co-infections often consist of more than two genotypes (Rauch et al., 2005) and occur not only simultaneous, but also sequentially (Karvonen, Seppälä, & Valtonen, 2004b; Klemme et al., 2016). These aspects can make predictions of virulence and estimations of host–parasite dynamics increasingly challenging.




