Amelanotic/hypomelanotic lentigo maligna: Dermoscopic and confocal features predicting diagnosis
Abstract
Background
Amelanotic/hypomelanotic lentigo maligna and lentigo maligna melanoma (AHLM/LMM) may be very difficult to diagnose at an early stage.
Objectives
To quantify the predictive value of dermoscopic and reflectance confocal microscopy (RCM) features for AHLM/LMM.
Methods
Dermoscopic and RCM images of histopathologically diagnosed AHLM/LMM, amelanotic/hypomelanotic benign lesions (AHBL), and amelanotic/hypomelanotic basal and squamous cell carcinomas (AHBCC/AHSCC) of the head and neck from consecutive patients were retrospectively collected and blindly evaluated by three observers to assess presence or absence of dermoscopic and RCM criteria.
Results
Overall, 224 lesions in 216 patients including LM/LMM (n = 55, 24.6%), AHBL (n = 107, 47.8%) and AHBCC/AHSCC (n = 62, 27.7%) were analysed. Multivariable analysis showed that milky-red areas (OR = 5.46; 95% CI: 1.51–19.75), peripheral light brown structureless areas (OR = 19.10; 4.45–81.96), linear irregular vessels (OR = 5.44; 1.45–20.40), and asymmetric pigmented follicles (OR = 14.45; 2.77–75.44) at dermoscopy, and ≥3 atypical cells in five fields (OR = 10.12; 3.00–34.12) and focal follicular localization of atypical cells at dermo-epidermal junction (DEJ) (OR = 10.48; 1.10–99.81) at RCM were significantly independent diagnostic factors for AHLM/LMM vs. AHBL. In comparison with AHBCC/AHSCC, peripheral light brown structureless area (OR = 7.11; 1.53–32.96), pseudonetwork around hair follicles (OR = 16.69; 2.73–102.07), and annular granular structures (OR = 42.36; 3.51–511.16) at dermoscopy and large dendritic (OR = 6.86; 3.15–38.28) and round pagetoid cells (OR = 26.78; 3.15–227.98) at RCM led to a significantly increased risk of diagnosing AHLM/LMM.
Conclusions
Amelanotic/hypomelanotic lentigo maligna and lentigo maligna melanoma may have the same dermoscopic features of AHM on other body sites, such as milky red areas, peripheral light brown structureless areas and linear irregular vessels. These features, asymmetric pigmented follicles and at RCM ≥ 3 atypical cells in five fields and focal follicular extension of atypical cells at DEJ may help in recognizing AHLM/LMM even when LM conventional features (e.g., obliteration of hair follicles under dermoscopy and large pagetoid cells under RCM) are absent or present only in very small areas of the lesion.
INTRODUCTION
Lentigo maligna (LM) is an in situ melanoma arising on chronically sun-exposed skin, usually the face, in middle-aged and older patients, representing 79%–83% of all in situ melanomas.1 LM is characterized by a variable long radial growth phase before progressing into invasive lentigo maligna melanoma (LMM) with a 3.5% annual risk of progression.1 Clinically, LM presents as a slowly growing macule/patch with irregular, ill-defined borders, variable shades of brown and black, often as pseudonetwork with hypopigmented areas making a discontinuous appearance.2 The dermoscopic diagnosis of LM may be difficult because it may show clinical and dermoscopic features of benign skin lesions and because of the frequent presence of collisions arising on sun-damaged skin.
Lentigo maligna may also be amelanotic or hypomelanotic presenting as ill-defined erythematous sometimes scaly macule/patch, mimicking eczematous dermatitis, Bowen's disease, inflamed solar lentigo, actinic keratosis, naevus depigmentosus and basal cell carcinoma (BCC), very difficult to diagnose in its early stage.3-5 In amelanotic/hypomelanotic LM/LMM (AHLM/LMM), non-pigmented or hypopigmented areas extends beyond the visible margins of the lesion hindering the identification of the peripheral margin.2
Although several dermoscopic features have been described for pigmented LM/LMM, the literature on dermoscopy and AHLM/LMM is scanty.6, 7 Reflectance confocal microscopy (RCM) is an additional imaging technique that can help the diagnosis and definition of the margins of LM/LMM.8-11
Reflectance confocal microscopy resulted especially helpful for AHLM because in the latter, malignant melanocytes may appear very bright under RCM because they still have melanosomes, even if not dermoscopically visible.11
In this retrospective study, 224 amelanotic/hypomelanotic skin lesions of the head and neck were examined by dermoscopy and RCM to quantify by a multivariable analysis the predictive value of dermoscopic and RCM features for AHLM/LMM.
PATIENTS AND METHODS
We collected consecutive cases of histopathologically confirmed AHLM/LMM, amelanotic/hypomelanotic melanoma (AHM) not of LM/LMM subtypes, amelanotic/hypomelanotic basal and squamous cell carcinoma (AHBCC), (AHSCC), and amelanotic/ hypomelanotic benign lesions (AHBL) of the head and neck evaluated with dermoscopy and RCM at four participating centres in Italy between January 2010 and December 2019. AHBL included both amelanotic/hypomelanotic benign melanocytic (e.g., melanocytic nevus) and non-melanocytic lesions (e.g., solar lentigo, actinic keratosis, seborrhoeic keratosis, liken-planus-like keratosis). In this study, only flat amelanotic/hypomelanotic lesions were included, lacking any trace of melanin or partially pigmented (melanin pigmented area ≤25%) or lightly coloured showing only tan, light grey-blue pigmentation that occupied >25% of the lesion.12
Clinical, dermoscopic and RCM images (Vivascope 1500 or Vivascope 3000, for lesions localized on areas not accessible with the Vivascope 1500, Mavig, Munich, Germany), together with patients' clinical information and histopathological diagnosis were collected.
We assessed the lesions using dermoscopic and RCM features associated with LM/LMM, melanoma, BCC, SCC, melanocytic nevus, actinic keratosis, solar lentigo, seborrhoeic keratosis, and liken-planus-like keratosis.6, 7, 11 Dermoscopic images were evaluated together with the RCM images by a panel of three blinded observers; the evaluation of the dermoscopic and RCM criteria was assessed when 3/3 or 2/3 observers agreed. LM score was also computed according to Guitera et al.11
In virtue of the Italian research regulations (D.Lgs. 101/2018), the approval by the Board of Ethics is waived for retrospective studies, as the patients give their consent to the use of clinical data for research purposes at hospitalization. The signed consent is stored, according to Italian regulations under the responsibility of the principal investigator of each participating centre.
Statistical analysis
Continuous variables were reported as median values and interquartile range (IQR); differences across groups were evaluated through Kruskal–Wallis test. The prevalence of dermoscopic and RMC features was reported as a percentage with corresponding 95% Clopper–Pearson confidence interval (CI); differences were tested through Fisher's exact test. For each dermoscopic feature, the univariate odds ratio (OR) and corresponding 95% CI of AHLM/LMM versus either AHBL and AHBCC/AHSCC were estimated through unconditional logistic regression models. To account for potential feature association, the independent effect of each factor was evaluated through a multivariable stepwise logistic regression model which included all dermoscopic and RCM features significantly associated with AHLM/LMM in the univariate analysis. Statistical significance was claimed for p-values < 0.05 (two-sided). The statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC) statistical software.
RESULTS
Patients demographics and classification of the lesions
The study evaluated 224 lesions in 216 patients (116 men, 100 women) with a median age of 67 years (range:19–96 years). The series included 55 melanomas (43 LMs, eight LMMs, two invasive melanomas, and two in situ melanomas), 62 non-melanocytic skin cancers (44 BCC, 15 in situ SCC, two invasive SCC, and one case of metatypical carcinoma) and 107 benign lesions (51 actinic keratoses, 25 solar lentigines, 16 melanocytic nevi seven seborrhoeic keratoses and one lichen-planus-like keratosis). Regarding the histogenetic melanoma subtypes, the proportion of LM/LMM was enormously bigger than SMM. These results mirrored the literature data as LM/LMM was reported to be the most frequent histogenetic subtype (47.4%) of head and neck melanoma, followed by superficial spreading melanoma (34.5%), and nodular melanoma (15.8%) in a very large series of 5702 patients.13 Compared with AHBL (Table 1), AHLM/LMM was more frequent in men (N = 69; 69%; 95% CI: 55–81%) and at an older age (median: 70 years; IQR: 61–78). Conversely, AHLM/LMM and AHBCC/AHSCC showed similar distribution by sex and similar median age. The three groups significantly differed according to site of presentation (p = 0.03). AHLM/LMM and AHBL was diagnosed more frequently on the cheek (N = 22, 40% [27–54%] and N = 41, 38% [29–48]%, respectively) and nose (N = 15, 27% [16–41%] and N = 18, 17% [10–25]%, respectively); conversely, AHBCC/AHSCC mainly occurred on frontal (N = 15, 24% [14–37%]) and nose (N = 23, 40% [13–35%]), (Table 1).
| AHLM/LMM | AHBL | AHBCC/AHSCC | p | ||||
|---|---|---|---|---|---|---|---|
| No. | % (95% CI) | No. | (%) | No. | (%) | ||
| Total diagnoses | 55 | 107 | 62 | ||||
| Sexa | |||||||
| Man | 38 | 69 (55–81) | 43 | 40 (31–50) | 39 | 63 (50–75) | <0.001b |
| Woman | 17 | 31 (19–45) | 64 | 60 (50–69) | 23 | 37 (25–50) | |
| Age (years) | |||||||
| Median (IQR) | 70 (61–78) | 63 (53–74) | 69 (56–75) | 0.02c | |||
| Sitea | |||||||
| Cheek | 22 | 40 (27–54) | 41 | 38 (29–48) | 10 | 16 (8–28) | 0.03b |
| Nose | 15 | 27 (16–41) | 18 | 17 (10–25) | 14 | 23 (13–35) | |
| Frontal | 4 | 7 (2–18) | 17 | 16 (10–24) | 15 | 24 (14–37) | |
| Peri-auricular | 1 | 2 (0–10) | 3 | 3 (1–8) | 4 | 6 (2–16) | |
| Peri-orbital | 4 | 7 (2–18) | 8 | 7 (3–14) | 9 | 15 (7–26) | |
| Peri-oral | 0 | 0 (0–6) | 4 | 4 (1–9) | 1 | 2 (0–9) | |
| Neck | 2 | 4 (0–13) | 3 | 3 (1–8) | 1 | 2 (0–9) | |
| Scalp | 1 | 2 (0–10) | 5 | 5 (2–11) | 6 | 10 (4–20) | |
| Other | 6 | 11 (4–22) | 8 | 7 (3–14) | 2 | 3 (0–11) | |
- Abbreviations: AHBCC/AHSCC, amelanotic/hypomelanotic basal and squamous cell carcinoma; AHBL, amelanotic/hypomelanotic benign lesion; AHLM/LMM, amelanotic/hypomelanotic lentigo maligna/lentigo maligna melanoma; IQR, interquartile range.
- a The sum does not add up to total because of some missing values.
- b Estimated from Fisher's exact test.
- c Estimated from Kruskal–Wallis test.
Multivariable analyses of dermoscopic and RCM features
Dermoscopic and RCM features significantly associated with LMM in the univariate analysis in comparison with AHBL and AHBCC/AHSCC were presented in Tables S1–S4. The independent effect of each feature was evaluated through a multivariable model, and significant associations with AHLM/LMM vs. AHBL and vs. AHBCC/AHSCC are reported in Table 2. Milky-red areas (OR = 5.46; 95% CI: 1.51–19.75), peripheral light brown structureless areas (OR = 19.10; 95% CI: 4.45–81.96), linear irregular vessels (OR = 5.44; 95% CI: 1.45–20.40) and asymmetric pigmented follicles (OR = 14.45; 95%: CI 2.77–75.44) at dermoscopy, and ≥3 atypical cells in five fields (OR = 10.12; 95% CI: 3.00–34.12) and focal follicular localization of atypical cells at DEJ (OR = 10.48; 95% CI: 1.10–99.81) at RCM led to a significantly increased risk of diagnosing AHLM/LMM vs. AHBL (Table 2).
| AHLM/LMM (No. = 55) | Comparison group | |||
|---|---|---|---|---|
| AHBL (No. = 107) | AHBCC/AHSCC (No. = 62) | |||
| No. | % (95% CI) | OR (95% CI)a | OR (95% CI)a | |
| Dermoscopic features | ||||
| Milky-red areas/>1 shade of pink | 42 | 76 (63–87) | 5.46 (1.51–19.75) | n.s. |
| Peripheral light brown structureless area | 39 | 71 (57–82) | 19.10 (4.45–81.96) | 7.11 (1.53–32.96) |
| Pseudonetwork | 33 | 60 (46–73) | n.s. | 16.69 (2.73–102.07) |
| Annular granular structures | 28 | 51 (37–65) | n.s. | 42.36 (3.51–511.16) |
| Linear irregular vessels | 27 | 49 (35–63) | 5.44 (1.45–20.40) | n.s. |
| Asymmetric pigmented follicles | 18 | 33 (21–47) | 14.45 (2.77–75.44) | n.s. |
| Reflectance confocal microscopy features: epidermis | ||||
| Large dendritic pagetoid cells | 34 | 62 (48–75) | n.s. | 6.86 (3.15–38.28) |
| Large round pagetoid cells | 18 | 33 (21–47) | n.s. | 26.78 (3.15–227.98) |
| Reflectance confocal microscopy features: dermal/epidermal junction | ||||
| ≥3 atypical cells in 5 fields | 41 | 75 (61–85) | 10.12 (3.00–34.12) | n.s. |
| Focal follicular localization of atypical cells | 12 | 22 (12–35) | 10.48 (1.10–99.81) | n.s. |
- Abbreviations: AHBCC/AHSCC, amelanotic/hypomelanotic basal and squamous cell carcinoma; AHBL, amelanotic/hypomelanotic benign lesion; AHLM/LMM, amelanotic/hypomelanotic lentigo maligna/lentigo maligna melanoma; CI, confidence interval; n.s., not significant (the feature was not selected by the stepwise regression model); OR, Odds ratio.
- a Estimated from stepwise unconditional logistic regression model including all dermoscopic and confocal features emerging from univariate analysis.
In addition, the multivariable analysis of the dermoscopic and RCM features of AHLM/LMM vs. AHBCC/AHSCC showed that a peripheral light brown structureless area (OR = 7.11; 95% CI: 1.53–32.96), pseudonetwork around hair follicles (OR = 16.69; 95% CI: 2.73–102.07) and annular granular structures (OR = 42.36; 95% CI: 3.51–511.16) at dermoscopy and large dendritic (OR = 6.86; 95% CI: 3.15–38.28) and large round (OR = 26.78; 95% CI: 3.15–227.98) pagetoid cells in the epidermis on RCM were significantly associated with an increased risk of AHLM/LMM (Table 2).
DISCUSSION
The most striking results of our study were that the only significant dermoscopic and RCM features discriminating AHLM/LMM from AHBL at the multivariable analysis were milky-red areas, peripheral light brown structureless areas, linear irregular vessels and asymmetric pigmented follicles at dermoscopy and at RCM ≥ 3 atypical cells in five fields and focal follicular localization of the atypical cells at the DEJ.
Milky-red areas, also known as more than one shade of pink, probably corresponding to areas with increased vascularity14 (Figures 1a and 2a), and the peripheral light brown structureless areas15 (Figure 3a) have already been associated with AHM on other body sites.12, 16 The peripheral light brown structureless areas seemed to occur in the early phases of the neoplastic invasion in both melanoma on other body sites17, 18 and AHLM. Linear vessels, significantly found in our cases of AHLM/LMM (Figures 1a, 2a and 3a), have also been reported in some cases of AHLM6 as well as in AHM.12, 19 Therefore, milky-red areas, peripheral light brown structureless areas and linear irregular vessels can be useful to identify not only AHM on other body sites but also AHLM/LMM. Asymmetric pigmented follicles are considered a first sign of invasion/outgrow20 of the follicular unit still respecting the basement membrane,7, 21 which seem to characterize not only pigmented LM but also AHLM/LMM; this feature is due to neoplastic melanocytes surrounding follicular openings and to their uneven descent within hair follicles.22, 23 It is interesting to note that hyporeflective and weakly reflective atypical cells in a single unit or aggregated in dense and sparse nests and/or cord-like nests (Figures 1b–d, 2b and 3c,d) among follicles as well as in the follicular adnexa and/or perifollicular distribution in the epidermis and at DEJ were found in our cases of AHLM.
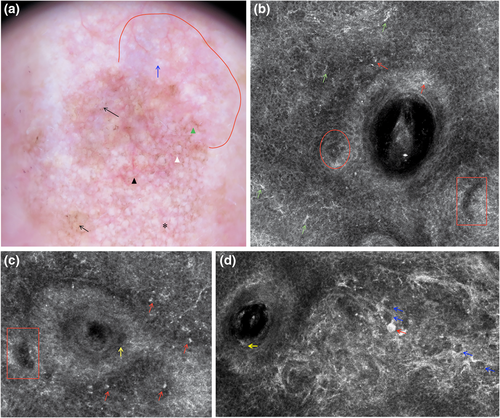
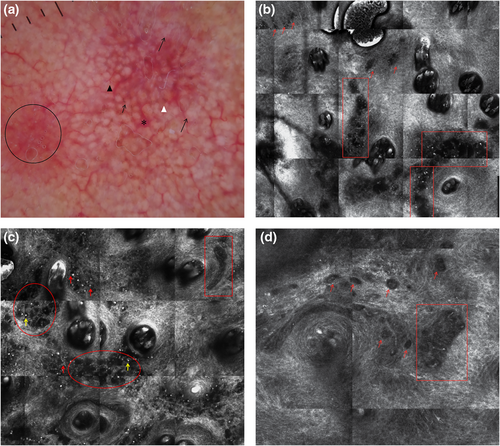
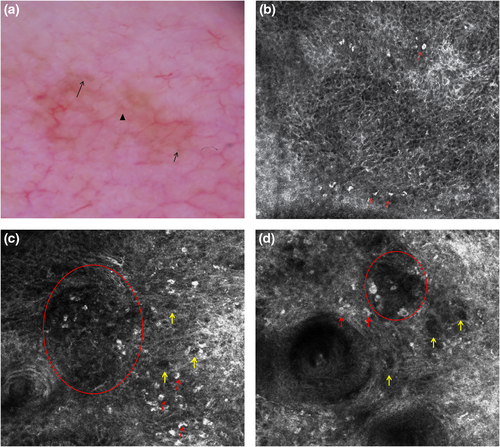
Hyporeflective atypical cells in the epidermis correlated with pagetoid infiltration of hypomelanotic/amelanotic malignant melanocytes were also reported in AHM.24
Notably, hyporeflective atypical cells in single unit and aggregated in cord-like nests among follicles, in follicular and perifollicular distribution in the epidermis and at DEJ, have not yet been described and could be a clue for the diagnosis of AHLM. In agreement with Guitera et al.,11 we found also that ≥3 atypical cells, including both hyper-reflective and hyporeflective ones, in five fields at DEJ (Figures 1d, 2d and 3d) were significantly associated with the diagnosis of AHLM/LMM. At the DEJ, large hyper-reflective dendritic and roundish cells as well as hyporeflective atypical cells among hair follicles were visible in our AHLM cases (Figures 1d and 3c). Hyper-reflective dendritic and roundish cells could be probably correlated with pigmented rhomboidal structures since hyper-reflective dendritic and roundish cells were found in 66.7% of cases having pigmented rhomboidal structures and in 20.2% of cases without pigmented rhomboidal structures (p < 0.0001). Likewise, weakly and hyporeflective atypical cells among hair follicles could be probably correlated with red rhomboidal structures because of a significant association between these structures and atypical cells among hair follicles; red rhomboidal structures were found in 11.1% of cases with atypical cells among hair follicles, and in only 2.2% of cases without atypical cells among hair follicle (p = 0.018). Although Pralong et al.7 have found that red rhomboidal structures could be associated with invasive LM in their series,7 we did not find red rhomboidal structures in any of our eight invasive LM cases.
In the study by Guitera et al.,11 other positive correlating LM features were found at multivariate analysis, such as large pagetoid cells >20 μm in diameter in the epidermis and non-edged papillae at DEJ, which we found significantly associated with AHLM/LMM only at univariate analysis.
The differences between the two studies could depend on the fact that the study by Guitera et al.11 also included pigmented LM in addition to different size and type of sample (81 LMs and 203 benign macules).
However, in the epidermis, large dendritic and round pagetoid cells were not found significantly associated with AHLM at multivariable analysis. As we have previously described in some cases of AHM not of lentigo maligna type,25 RCM features for malignancy can be more difficult to identify because large hyper-reflective pagetoid cells could be less numerous and hyporeflective pagetoid cells could be difficult to identify; these cells were visible only by zooming the mosaic images and carefully examination, often missed at first glance.25 In addition, few small dendritic cells can be challenging to discriminate from Langherans dendritic cells seen in the epidermis of benign lesions such as actinic keratosis and liken-planus-like keratosis. Moreover, in the epidermis of AHLM/LMM, we found small hyper-reflective irregular cells without evident nucleus, difficult to discriminate from keratinocytes, probably corresponding to neoplastic melanocytes. This could explain why pagetoid cells did not allow to discriminate AHLM/LMM from AHBL and why the integration of dermoscopy with RCM could improve the diagnosis not only of pigmented lentigo maligna8 but also of AHLM.
Atypical cells of different reflectivity aggregated in dense and sparse nests surrounding adnexal openings at the DEJ, and/or just under it were seen in our cases, which may be correlated with irregular brown and grey dots significantly associated with AHLM/LMM at univariate analysis. In agreement with our results, other authors found in LM/LMM dense and sparse nests of pleomorphic atypical cells surrounding the adnexal opening at the DEJ, correlated with brown dots/globules surrounding the adnexal opening observed at dermoscopy.26, 27
At the multivariable analysis, peripheral light brown structureless area, pseudonetwork, annular granular structures, large dendritic and large round pagetoid cells were the significant features distinguishing AHLM/LMM from AHBCC/AHSCC. Pseudonetwork has also been reported in both pigmented LM and solar lentigo/seborrhoeic keratosis and pigmented actinic keratosis.28 Therefore, pseudonetwork did not allow to discriminate AHLM/LMM from AHBL, whereas this feature and annular granular structures, peripheral light brown structureless area, large dendritic and large round pagetoid cells were more frequent in AHLM/LMM than AHBCC/AHSCC. Pseudonetwork and annular granular structures are related to a folliculotropism of the LM without a complete invasion of the hair follicles, whereas AHBCC/AHSCC directly destroys hair follicles. As expected, large roundish and dendritic cells in the epidermis were found in LM/LMM and a few epithelial cancers because they are quite specific of malignant melanocytes in RCM.
Interestingly, LM score according to Guitera et al.11 was reported significantly more frequently in LM than in AHBCC/AHSCC, and AHBL; LM score ≥2 was found in 89.1% of AHLM/LMM, in 58.1% of AHBCC/AHSCC, and in 29.0% of AHBL (p ≤ 0.001; data not shown). Therefore, LM score may help in discriminating LM from head and neck malignant non-melanocytic and benign lesions. Nonetheless, diagnostic performance of LM score in AHBCC/AHSCC should be investigated.
Finally, our study found that AHLM/LMM may have the same dermoscopic features of AHM on other body sites, such as milky red areas, peripheral light brown structureless areas and linear irregular vessels. These features, asymmetric pigmented follicles and at RCM ≥ 3 atypical cells in five fields and focal follicular extension of atypical cells at DEJ may be useful in improving the detection of this difficult to diagnose subtype of melanoma even when LM conventional features (e.g., obliteration of hair follicles under dermoscopy and large pagetoid cells under RCM) are absent or present in very small areas of the lesion.
ACKNOWLEDGEMENT
The authors wish to thank Ms Luigina Mei for editorial assistance and Nancy Michielin and Francesca Battistella for technical support. Open access funding provided by BIBLIOSAN.
FUNDING INFORMATION
This work was partially funded by Italian Ministry of Health (Ricerca Corrente).
CONFLICT OF INTEREST
IZ reports consulting fees from Sanofi Genzyme and honoraria from MSD, Novartis, Sunpharm, Sanofi Genzyme, La Roche Posay, Philogen, Biogena, Almiral Hermal, outside the submitted work. FP reports grants from AstraZeneca, Eisai, Roche and honoraria from AstraZeneca Daiicki Sankyo, Eli Lilly, Novartis, Pfizer, Pierre Fabre, Gilead, Seagen, and Roche, outside the submitted work. All the remaining authors declared no conflict of interests.
Open Research
DATA AVAILABILITY STATEMENT
The data are available upon reasonable request to the corresponding author.