Psoriasis and metabolic syndrome: implications for the management and treatment of psoriasis
Conflicts of interest
JJW is or has been an investigator for AbbVie, Amgen, Eli Lilly, Janssen, Novartis; a consultant for AbbVie, Almirall, Amgen, Arcutis, Aristea Therapeutics, Bausch Health, Boehringer Ingelheim, Bristol-Myers Squibb, Dermavant, Dr. Reddy’s Laboratories, Eli Lilly, Galderma, Janssen, LEO Pharma, Mindera Novartis, Regeneron, Sanofi Genzyme, Solius, Sun Pharmaceutical, UCB and Zerigo Health; and a speaker for AbbVie, Amgen, Bausch Health, Novartis, Regeneron, Sanofi Genzyme, Sun Pharmaceutical and UCB. AK has conducted clinical research sponsored by and/or consulted for AbbVie, Amgen, Celgene, Eli Lilly, Novartis and Pfizer. MGL is an employee of Mount Sinai and receives research funds from AbbVie, Amgen, Arcutis, Avotres, Boehringer Ingelheim, Dermavant Sciences, Eli Lilly, Incyte, Janssen Research & Development, LLC, Ortho Dermatologics, Regeneron and UCB, Inc., and is a consultant for Aditum Bio, Almirall, AltruBio Inc., AnaptysBio, Arcutis, Aristea Therapeutics, Arrive Technologies, Avotres Therapeutics, BiomX, Boehringer Ingelheim, Bristol-Myers Squibb, Cara Therapeutics, Castle Biosciences, Corrona, Dermavant Sciences, Dr. Reddy’s Laboratories, Evelo Biosciences, Evommune, Inc., Facilitation of International Dermatology Education, Forte Biosciences, Foundation for Research and Education in Dermatology, Helsinn Therapeutics, Hexima Ltd., LEO Pharma, Meiji Seika Pharma, Mindera, Pfizer, Seanergy and Verrica. RG has served on advisory boards and/or received lecture honoraria from AbbVie; Amgen; Celgene; Eli Lilly; Janssen Research & Development, LLC; LEO Pharma; Mallinckrodt; Merck and Novartis. JFM is a consultant and/or investigator for AbbVie; Amgen; Biogen; Bristol-Myers Squibb; Dermavant; Eli Lilly; Janssen; LEO Pharma; Novartis; Pfizer; Regeneron; Sanofi; Sun Pharmaceutical Industries, Inc. and UCB.
Funding source
Medical writing and editorial assistance was provided by Elisabetta Lauretti, PhD, of AlphaBioCom, LLC, and funded by Sun Pharmaceutical Industries, Inc.
Abstract
Psoriasis is a chronic systemic inflammatory disorder associated with several comorbidities in addition to the characteristic skin lesions. Metabolic syndrome (MetS) is the most frequent comorbidity in psoriasis and a risk factor for cardiovascular disease, a major cause of death among patients with psoriasis. Although the exact causal relationship between these two disorders is not fully established, the underlying pathophysiology linking psoriasis and MetS seems to involve overlapping genetic predispositions and inflammatory pathways. Dysregulation of the IL-23/Th-17 immune signalling pathway is central to both pathologies and may be key to promoting susceptibility to metabolic and cardiovascular diseases in individuals with and without psoriasis. Thus, biological treatments for psoriasis that interrupt these signals could both reduce the psoriatic inflammatory burden and also lessen the risk of developing atherosclerosis and cardiometabolic diseases. In support of this hypothesis, improvement of skin lesions was associated with improvement in vascular inflammation in recent imaging studies, demonstrating that the beneficial effect of biological agents goes beyond the skin and could help to prevent cardiovascular disease. This review will summarize current knowledge on underlying inflammatory mechanisms shared between psoriasis and MetS and discuss the most recent clinical evidence for the potential for psoriasis treatment to reduce cardiovascular risk.
Introduction
Although psoriasis most prominently affects the skin and joints, it is a systemic inflammatory disorder characterized by alteration of adaptive and innate immunity with consequences extending beyond the obvious lesions.1, 2 In psoriasis, circulating proinflammatory mediators result in a widespread inflammatory response frequently accompanied by comorbidities such as cardiovascular disease (CVD) and metabolic syndrome (MetS).3-6 The prevalence of MetS, a complex disorder characterized by the combination of several conditions that increase the risk of cardiovascular disease, ranges from 20% to 50% in patients with psoriasis and increases with increasing psoriasis severity.3 According to the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) guidelines, the diagnosis of MetS requires the presence of ≥3 of the following: waist circumference >102 cm (men) or >88 cm (women); triglycerides ≥150 mg/dL; high-density lipoprotein (HDL) <40 mg/dL (men) or <50 mg/dL (women); blood pressure ≥130/85 mm Hg; and fasting plasma glucose ≥110 mg/dL.7 Given its rising prevalence throughout Western Europe and in the USA, MetS is an increasingly significant public health problem8 and a serious concern in patients with psoriasis.9-12
Although no definitive causal relationship has been identified, a combination of genetics, common signalling pathways and environmental factors may contribute to metabolic abnormalities in patients with psoriasis.13 These links and the chronic inflammatory nature of both psoriasis and MetS may have ramifications for clinical and therapeutic psoriasis management. The impacts of the systemic anti-inflammatory activity of biologics used to treat psoriasis on the incidence and progression of MetS and of concomitant MetS on efficacy and safety of these drugs for psoriasis treatment are under investigation.14 This review will summarize current knowledge on underlying inflammatory mechanisms shared between psoriasis and MetS and discuss the most recent clinical evidence for the potential for psoriasis treatment to reduce cardiovascular risk.
Epidemiological, genetic and mechanistic studies examining the relationship between psoriasis and metabolic syndrome
Epidemiological studies
A higher prevalence of MetS among patients with psoriasis, including children and adolescents, compared with the general population has been consistently observed worldwide.3-5, 8, 15-23 A large population-based study in the UK found a greater prevalence of MetS among participants with psoriasis compared with controls (odds ratio [OR]: 1.50, 95% confidence interval [CI]: 1.40–1.61) independent of age and gender. This study was the first to highlight the existence of a dose–response relationship between psoriasis severity and incidence of MetS, with an increase in the odds of developing MetS relative to controls of 22%, 56% and 98% in patients with mild, moderate and severe psoriasis respectively.5
Among the individual components of MetS, obesity and diabetes had the highest observed prevalence in patients with psoriasis.21, 24-26 In a meta-analysis of 44 studies, the pooled OR for diabetes in patients with vs. without psoriasis was 1.76 (95% CI: 1.59–1.96), with even higher OR for patients with severe psoriasis (OR: 2.10, 95% CI: 1.73–2.55).24 Moreover, the link between diabetes and psoriasis was found to be independent of age, sex, smoking and body mass index (BMI).27 Many but not all cross-sectional studies identify alterations of the plasma lipid profile in patients with psoriasis.21, 28 A meta-analysis of 24 studies reported increased blood pressure in patients with psoriasis (pooled OR for hypertension: 1.58, 95% CI: 1.42–1.76) compared with healthy controls.29 Likewise, in a large cross-sectional study in the UK, OR for hypertension was 1.16 (95% CI: 1.14–1.18) for patients with mild psoriasis and 1.25 (95% CI: 1.13–1.39) for those with severe psoriasis compared with patients without psoriasis.26 In contrast, there was no significant association between hypertension and psoriasis in another set of studies.8, 26 Despite some inconsistency, due in part to the heterogeneity of the criteria for MetS among different studies, the preponderance of evidence supports an association between psoriasis and MetS as well as between psoriasis and individual MetS components.
Genetic studies
Family members of patients with psoriasis are at greater risk for developing psoriasis compared with the general population, implying at least some genetic basis for psoriasis pathogenesis.30 Of particular interest is whether a distinct genetic background may also contribute to the increased susceptibility of patients with psoriasis to develop MetS.30-35
Many single-nucleotide polymorphisms (SNPs) associated with psoriasis are located on genes implicated in adaptive and innate immune responses essential to T helper 17 (Th17) cell activation, including genes encoding human leucocyte antigen (HLA)-C, interleukin (IL)-23 receptor (R), IL-23A, IL-12B and TRAF3-interacting protein 2 (TRAF3IP2).30 In a single-population study, IL-12B, IL-23R and IL-23A gene variants were linked not only to psoriasis susceptibility but also to increased risk for developing type II diabetes (TIID).36 When 363 SNPs selected for their known association with MetS and CVD were analysed for potential association with psoriasis in four genome-wide association study (GWAS) patient cohorts, dyslipidaemia, hypertension and CVD-related genes were linked to increased risk of psoriasis.33
CDKAL1, which is linked to TIID, is also a putative susceptibility gene for psoriasis. However, the rs6908425 SNP conferring susceptibility to psoriasis is not the one associated with TIID, suggesting distinct mechanisms for the two conditions.37-39 Apolipoprotein E (Apo E), a key glycoprotein in lipid metabolism, is expressed as three isoforms differing by a single amino acid substitution: Apo E2, Apo E3 and Apo E4. Apo E4 is a risk factor for heart disease, diabetes, hypertriglyceridaemia and hypercholesterolaemia and is significantly more prevalent in patients with vs. without psoriasis, potentially explaining the high incidence of dyslipidaemia in these patients.40 The Apo E4 allele but not the Apo E2 allele was significantly associated with non-pustular psoriasis in a largely white population, while the Apo E2 allele was associated with psoriasis vulgaris in a Japanese population, implying the existence of distinct genetic forms of psoriasis.40, 41 However, other genetic studies did not detect shared genetic components between psoriasis, CVD and MetS.32, 34
In a GWAS analysis including epidemiological data from the population-based KORA (Cooperative Health Research in the Region Augsburg) study and German Health insurance beneficiaries records, there was only a modest effect for the HLA-Cw6 allele. However, this study did not consider rare genetic variants.34 Similarly, only the major histocompatibility complex locus at 6p21.33 and at 2p16.1 overlapped among individuals with MetS and psoriasis in the GWAS analysis from Gupta et al.32 Given the complexity of psoriasis and degree of variability among populations, further genetic analyses are required to fully unravel the relevance of disease-associated variants and their intricate relationship with MetS.
Potential mechanistic links between psoriasis and metabolic syndrome
Adipokines
Adipokines are cytokines secreted by adipose tissue capable of regulating glucose, lipid and insulin metabolism, and inflammation.42 Because patients with psoriasis are more likely to be overweight or obese relative to the general population, alterations in adipokine levels may contribute to the development of both metabolic disorders and cutaneous inflammation in psoriasis.43, 44 Low levels of the anti-inflammatory adipokine adiponectin are associated with the development of diabetes and MetS45; however, reported adiponectin levels in different studies of patients with psoriasis were increased, decreased or unchanged compared with healthy controls.42, 45 The proinflammatory adipokine leptin, also expressed by endothelial cells, regulates keratinocyte proliferation, angiogenesis and adhesion molecule expression and can promote Th1 and Th17 proliferation.46, 47 Excess leptin production is associated with increased intima-media thickness (IMT) of the common carotid artery (CCA) relative to individuals with lower leptin levels, and IMT thickness of the CCA is notably higher in patients with psoriasis relative to controls.48, 49 Serum leptin concentrations are consistently elevated and positively correlated with increased IMT in patients with psoriasis relative to controls.42, 45 Additionally, serum and tissue (lesional skin) levels of leptin and leptin receptor were higher in patients with greater psoriasis severity relative to those with less severe disease and healthy controls in one small study (patients with psoriasis, n = 43; healthy controls, n = 10; diseased controls, n = 10; all with normal BMI).47
The IL-23/Th17 axis as a driver of skin and vascular inflammation
The IL-23/Th17 axis, central to psoriasis and MetS pathogenesis,50 provides a mechanistic explanation for the high incidence of metabolic and cardiovascular disorders observed in patients with psoriasis.22, 51-54 In psoriasis, the inflammatory cascade is initiated in the skin, where keratinocytes activate dendritic cells (DCs). Activation of DCs and IL-23 expression in the presence of transforming growth factor beta, IL-6 and IL-1β stimulate Th17 differentiation and phenotype maintenance. Thereafter, the activated Th17 cells secrete IL-17A, IL-22 and tumour necrosis factor (TNF)-α, leading to the formation of the psoriatic plaques and development of a proinflammatory feedback loop.50, 51
The IL-23/Th17 axis is also recognized to be involved in the pathogenesis of atherosclerosis. Circulating levels of Th17 and IL-17 are significantly higher in patients with atherosclerosis compared with patients with stable angina or non-cardiac chest pain.55 Moreover, levels of IL-23 and IL-23R are higher in atherosclerotic plaques compared with unaffected vessels.56 Locally, IL-17 produced by vascular endothelial and smooth muscle cells is hypothesized to stimulate production of proinflammatory molecules in an autocrine-paracrine IL-17 regulatory loop, contributing to the initiation and maintenance of an inflammatory cascade leading to the development of atherothrombotic vascular disease.57 Additionally, a SNP (A > G rs11209026) in the IL-23R gene was significantly associated with the risk and severity of atherosclerosis in a case–control study.58
IL-17 is also implicated in the components of MetS. IL-17 appears to be necessary for the maintenance of hypertension and vascular dysfunction in animal models and inflammation in cultured human cells.59 Angiotensin II induces IL-17 production; furthermore, excessive stretch of the endothelial wall is proposed to stimulate release of IL-6 and IL-23 with activation of signal transducer and activation of transcription-3, which may ultimately promote differentiation of T cells into the Th17 phenotype.59, 60 Serum concentrations of IL-23 and IL-17 were significantly increased in obese women (BMI: 30–48 kg/m2) compared with normal-weight women (BMI: 18–25 kg/m2) in a cross-sectional study.61 Serum levels of IL-23 and IL-17A were also elevated in patients with type 1 diabetes, and serum levels of IL-17A and IL-22 were elevated in patients with TIID compared with controls, further supporting the diabetogenic potential of this signalling pathway.59, 62, 63 The common underlying immunological mechanisms of psoriasis and CVD are summarized in Figure 1.
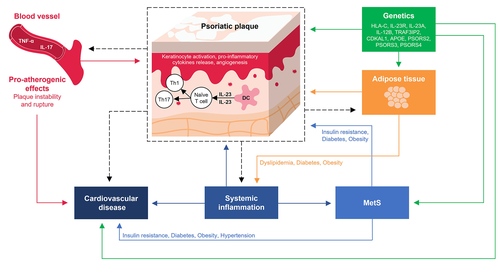
Relationship between psoriasis severity, concomitant metabolic syndrome and cardiovascular health
Incidence of myocardial infarction (MI), ischaemic heart disease and severe vascular accidents is higher in patients with vs. without psoriasis, and the risk increases with increasing psoriasis severity.64-68 In a large study population from the General Practice Research Database in the UK, patients with severe psoriasis had a clinically significant 57% increased risk of CVD compared with controls.12 Ischaemic heart disease (3.3% vs. 1.8%; OR: 1.87) and vascular-cerebral accidents (1.8% vs. 1.2%; OR: 1.55) were more prevalent in patients with psoriasis vs. healthy controls in an observational and cross-sectional study in Spain.10 Another study in Denmark showed that the risk of MI was increased only in patients with severe psoriasis – but not those with mild psoriasis – relative to the general population (hazard ratio: 1.21, 95% CI: 1.07–1.37).69
A recent review suggests that patients with psoriasis have a higher incidence of carotid artery plaque and vascular inflammation compared with controls in the preponderance of studies.31, 70, 71 Moreover, psoriasis severity seems to be associated with vascular inflammation independently from cardiovascular risk factors.72 In a systematic literature review of 12 studies from the Medline database and Ovid SP up to 2012, six studies showed increased carotid IMT, a marker of subclinical atherosclerosis; three showed decreased flow-mediated dilation, a measure of endothelial dysfunction; and two reported increased mean coronary artery calcification (CAC) in patients with psoriasis relative to controls.73 The IMT of the CCA was higher and strongly associated with Psoriasis Area Severity Index (PASI) score in patients with psoriasis vs. controls in a systematic meta-analysis including all studies between the year 2000 and 2018.74 Moreover, higher epicardial adipose tissue volume was observed in patients with psoriasis compared to the general population and linked to increased CAC and carotid IMT.75-77 A recent study involving 83 patients with moderate-to-severe psoriasis found a significant positive association between vascular inflammation and F-18 fluorodeoxyglucose (FDG) uptakes in visceral, subcutaneous and pericardial adipose tissue, spleen and bone marrow in patients with/without prior CVD, suggesting that the chronic inflammatory state in psoriasis might affect adipose and haematopoietic tissues as well as the arteries.78
Even young patients with psoriasis show signs of coronary microvascular dysfunction, the presence of which is associated with greater psoriasis severity based on PASI score.79-81 More important, quantitative coronary flow reserve and myocardial blood flow showed prognostic value for major adverse cardiovascular events (MACE) in patients with psoriasis.80, 82 Finally, patients with psoriasis have a higher endothelial microparticles (EMPs)/endothelial progenitors (EPCs) ratio, indicating endothelial injury and vascular competence dysfunction, compared with people without psoriasis.83 Because imbalance of the EMPs:EPCs ratio is linked to atherosclerosis and increased cardiovascular risk, the results of this study further support the association between preclinical atherosclerosis and psoriasis.
Given the common inflammatory component, it is intuitive to hypothesize a synergistic action of MetS on CVD risk in patients with psoriasis.10, 21, 84 A recent cross-sectional study involving 260 participants with psoriasis, 80 of whom had MetS, revealed that participants with both conditions had more CVD risk factors, systemic inflammation and coronary plaque burden compared with patients with psoriasis without MetS.85
Potential for psoriasis treatment to improve CVD risk
Cardiovascular risk factors are under-recognized and under-treated in patients with psoriasis. Although there is a lack of consensus among clinicians and scientists on the impact of systemic treatments for psoriasis on vascular inflammation and coronary disease, the hypothesis is that reducing the psoriatic and systemic inflammatory burden would consequently lessen the risk of developing MetS and CVD.11, 86, 87
Imaging studies
Imaging studies have suggested an association between improvements in psoriasis skin disease severity and vascular inflammation.88 The size of the lipid-rich necrotic core (LRNC) in carotid plaques is a predictive factor for plaque rupture and future cardiovascular events. A prospective observational study enrolling biological-naïve patients with psoriasis, using novel validated software on coronary computed tomography angiography (CCTA) images, demonstrated both that LRNC positively correlated with psoriasis severity and cardiovascular risk factors and that LRNC size decreased in patients with psoriasis after 1 year of anti-TNF-α, anti-IL-12/23 or anti-IL-17 biological therapy compared with patients who did not receive biological treatment.87
Likewise, treatment with anti-TNF-α, anti-IL-12/23 or anti-IL-17 agents for 1 year improved coronary inflammation from baseline as assessed by reduced perivascular fat attenuation index (FAI) – a novel imaging biomarker that traces spatial changes of perivascular fat composition using CCTA – in patients with psoriasis.89 In a prospective observational study, anti-TNF-α, anti-IL-12/23 and anti-IL-17 therapies were also associated with a 6% reduction in non-calcified plaque burden in LRNC compared with no biological treatment, with the greatest effect exerted by anti-IL-17 agents as assessed by CCTA.90 In a single-centre prospective trial, 13 months of treatment for severe psoriasis with adalimumab, etanercept, infliximab or ustekinumab reduced coronary artery disease progression, as well as the severity of luminal abnormalities and the vessel wall volume, compared with control patients not receiving systemic therapy.91 Another study indicated that the extent of cardiovascular risk was greater in patients with psoriasis relative to those with hyperlipidaemia. Furthermore, anti-TNF-α, anti-IL-12/23 or anti-IL-17 therapy for 1 year significantly reduced non-calcified burden of coronary plaque and high-risk plaque in patients with psoriasis compared with healthy or hyperlipidaemic controls.92 In the randomized, double-blind, placebo-controlled CARIMA trial, secukinumab treatment of patients with moderate-to-severe plaque psoriasis was associated with significant improvement of endothelial function at Week 52, measured by flow-mediated dilation (FMD), but a neutral effect on vessel wall characteristics and other CVD biomarkers. Although the selection of patients with a low baseline cardiovascular risk profile may have hindered detection of additional benefits of IL-17 inhibition, as reduced FMD is an early marker of subclinical atherosclerosis, this study suggests that secukinumab might lessen CVD risk.93
Finally, in a small prospective study in patients with psoriasis, treatment with anti-IL-12/23 agents significantly reduced carotid IMT from baseline.94 Increased whole body and aortic inflammation measured by fluorodeoxyglucose (18F) positron emission tomography/computed tomography (18-FDG PET/CT) imaging was found in patients with psoriasis compared with controls after adjustment for traditional cardiovascular risk factors and BMI.95 Overall, these data suggest a beneficial effect of biological agents as well as great potential for imaging techniques to unmask inflammatory risk factors not usually detected by the traditional cardiovascular risk assessments routinely used in clinical practice. However, larger and longer term studies should be performed to validate these results.97, 99, 100
Vascular inflammation studies in psoriasis
Based on data from preliminary research and given the critical role of inflammation in all phases of atherosclerosis and psoriasis, a series of clinical studies, known as the Vascular Inflammation in Psoriasis (VIP) trials, was specifically designed to investigate the effect of psoriasis treatment on vascular inflammation in patients with moderate-to-severe psoriasis. The first VIP trial (NCT01553058) evaluated changes from baseline in total vascular inflammation of five aortic segments as assessed by FDG PET/CT in patients receiving adalimumab compared to phototherapy or placebo. Despite substantial improvement in psoriasis severity from baseline to Week 12, Week 52 and in the open-label extension following both adalimumab and phototherapy, neither treatment decreased vascular inflammation relative to placebo.96 Likewise, in the VIP-S (NCT02690701) trial, there was no change in aortic vascular inflammation at the end of 52 weeks of secukinumab treatment in patients with psoriasis compared with baseline.97 In the VIP-U trial (NCT02187172), a transient improvement in aortic vascular inflammation was observed after 12 weeks of ustekinumab treatment in patients with psoriasis compared to placebo. However, this beneficial effect was not sustained at the 52-week follow-up.98 Finally, the phase 4 VIP-A study is ongoing (NCT03082729) to determine the effect of apremilast, a phosphodiesterase-4 inhibitor, on aortic vascular inflammation and CVD biomarkers in patients with moderate-to-severe psoriasis.99 Imaging studies reporting effects of biologics on markers of vascular inflammation in patients with psoriasis are summarized in Table 1.
| Study | Agent | Result |
|---|---|---|
| Randomized controlled trials | ||
| Gelfand et al., 202098 | IL-12/23 inhibitor (ustekinumab) | Reduction in aortic vascular inflammation (−6.58%, 95% CI: −13.64% to 0.47%) at Week 12 and no change in aortic vascular inflammation (0.84%, 95% CI: −4.38% to 6.07%) at Week 52 in patients treated with ustekinumab compared with baseline |
| Gelfand et al., 202097 | IL-17 inhibitor (secukinumab) | No discernible changes in aortic inflammation at Weeks 12 and 52 in patients treated with secukinumab compared with baseline and at Week 12 compared with placebo |
| Mehta et al., 201896 | TNF-α inhibitor (adalimumab) | No effect on vascular inflammation at Weeks 12 and 52 in patients treated with secukinumab compared with placebo and baseline respectively |
| Von Stebut et al., 201893 | IL-17 inhibitor (secukinumab) | FMD was significantly higher at Week 52 in patients treated with adalimumab compared with baseline |
| Bissonnette et al., 201795 | TNF-α inhibitor (adalimumab) | No significant change in vascular inflammation at Week 16 in patients treated with adalimumab or placebo and a modest increase in vascular inflammation in carotids at Week 52 in patients treated with adalimumab compared with baseline |
| Bissonnette et al., 2013113 | TNF-α inhibitor (adalimumab) | No significant difference in change in vascular inflammation from baseline to Week 15 in the vessel with the highest level of inflammation at baseline between patients treated with adalimumab vs. non-biological therapies |
| Observational studies | ||
| Abrahao-Machado et al., 2020114 | MTX and TNF-α inhibitors (adalimumab, infliximab) | Moderate-to-strong positive correlation (r = 0.617; P < 0.001), between Framingham score values and IMT |
| Choi et al., 202087 | TNF-α inhibitors (adalimumab, etanercept), IL-12/23 inhibitor (ustekinumab) and IL-17 inhibitors (ixekizumab and secukinumab) | Reduction in LRNC (−0.22 mm2 vs. 0.14 mm2; P = 0.004) at 1 year in patients receiving biologics compared with non-biologics-treated group |
| Elnabawi et al., 201989 | TNF-α inhibitors, IL-12/23 inhibitors and IL-17 inhibitors | Reduced coronary inflammation assessed by perivascular FAI at 1 year in patients receiving biologics (median FAI −71.22 HU [IQR], −75.85 to −68.11 HU at baseline vs. −76.09 HU [IQR], −80.08 to −70.37 HU at 1 year; P < 0.001); no change in FAI in the non-biologics-treated group. |
| Elnabawi et al., 201990 | TNF-α inhibitors (adalimumab, etanercept), IL-12/23 inhibitor (ustekinumab) and IL-17 inhibitor (secukinumab, ixekizumab) | 6% reduction in non-calcified plaque burden at 1 year in patients receiving biologics compared with non-biologics-treated group (Δ, −0.07 mm2 vs. 0.06 mm2; P = 0.02) |
| Lerman et al., 201892 | MTX, TNF-α, IL-12/23 and IL-17 inhibitors | Correlation between PASI and reduction in total (β: 0.45, 95% CI: 0.23–0.67; P < 0.001) and NCB (β: 0.53, 95% CI: 0.32–0.74; P < 0.001) at 1 year in patients receiving biologics |
| Martinez-Lopez et al., 201894 | MTX, TNF-α inhibitors (adalimumab, etanercept and infliximab) and IL-12/23 inhibitor (ustekinumab) | Reduced IMT at 8 months in patients receiving MTX (P = 0.045) and ustekinumab (P = 0.005) compared with baseline |
| Hjuler et al., 201691 | TNF-α inhibitors (adalimumab, etanercept and infliximab) and IL-12/23 inhibitor (ustekinumab) | Reduced coronary artery disease progression in patients receiving biologics compared with non-biologics-treated group (mean CAC difference between groups: 29.9, 95% CI: 5.6–54.1; P = 0.02; and mean difference in the progression of the vessel soft wall component: 0.4, 95% CI: 0.1–0.7; P = 0.02) |
- β, beta coefficient; Δ, change; CAC, coronary artery calcification; CI, confidence interval; FAI, fat attenuation index; FMD, Flow-Mediated Dilation; HU, Hounsfield unit; IL, interleukin; IMT, intima-media thickness; IQR, interquartile range; LRNC, lipid-rich necrotic core; MTX, methotrexate; NCB, non-calcified plaque burden; PASI, Psoriasis Area and Severity Index; r, linear correlation coefficient of Pearson; TNF, tumour necrosis factor.
Observational studies
Data describing the impact of biological therapies on cardiovascular risk factors and MetS are very limited.11, 13, 86, 100, 101 In a longitudinal Danish nationwide cohort study from 2007 to 2011, systemic treatment with methotrexate was associated with significantly lower rates of cardiovascular events and cardiovascular mortality during 5 years of follow-up in patients with psoriasis compared with other anti-psoriatic therapies including biologics, cyclosporine and retinoids.102 Likewise, in several observational studies, lowering inflammation with anti-TNF-α agents significantly reduced the risk of MACE and MI in patients with psoriasis compared with other anti-psoriatic treatments.103 TNF-α inhibitors were associated with a statistically significant 50% reduction (adjusted HR, 0.50; 95% CI, 0.32–0.79) in MI risk compared with patients treated with topical agents.103 Moreover, data extracted from the Truven Health Analytics MarketScan Databases (MarketScan) revealed that anti-TNF-α agents were more effective in decreasing the risk of MACE relative to methotrexate (1.45% vs. 4.09%, P < 0.01) and that cumulative TNF-α inhibitor exposure further reduced the risk (11% cardiovascular risk reduction).68 These findings are consistent with a similar study in which patients with psoriasis treated with TNF-α inhibitors experienced lower cardiovascular event risk (including MI, stroke and unstable angina) compared with patients treated with phototherapy.104 Although a more recent retrospective cohort study conducted on a population level (18154 patients vs. 8845 patients in the original study) reported a much lower percentage of reduction in the risk of all MACE (20% vs. 50% in the original study) in patients receiving TNF-α inhibitors compared to topical therapy, the results provided further evidence of the beneficial effect of this group of drugs in reducing cardiovascular outcomes.105
No effect of IL-17 inhibitor treatment on MACE rate or CVD risk has been observed in patients with psoriasis.97, 106 Overall, clinical trials and observational studies reported no significant change in the rate of MACE in patients with psoriasis treated with ustekinumab compared with placebo.107-109 One real-world data study suggested that in patients with psoriasis with high baseline cardiovascular risk, ustekinumab promoted severe cardiovascular events within 6 months of initiation compared with placebo treatment; no similar effect was observed among patients with low baseline cardiovascular risk.110 However, interpretation of the study was limited by a short 6-month treatment window and a very low number of reported cardiovascular events, calling into question the clinical relevance of the results and the validity of the statistical model.111
Conclusions
Overlapping inflammatory pathways and genetic predisposition may link the pathophysiology of MetS and psoriasis. Given this overlap, it is reasonable to infer that the conditions are related and that activity in one may be accompanied by activity in the other; likewise, effective treatment of one condition may result in improvement of the other. Within the limitations of small sample sizes and heterogeneity of baseline metabolic parameters, comorbidities and severity of psoriasis among recruited patients, current findings suggest that reduction of systemic inflammation achieved by biological treatments may improve cardiovascular outcomes in patients with psoriasis. Likewise, adequate control of MetS through medication or lifestyle changes may lead to improvement in the signs and symptoms of psoriasis. Systematic screening for MetS in patients with psoriasis, as recommended by treatment guidelines,112 is thus imperative for reasons beyond the need to identify patients at risk for poor cardiovascular outcomes. Treatment plans should also encompass evaluation of the patient’s lifestyle and risk factors for co-existing comorbidities; monitoring body weight, blood pressure and cholesterol and triglyceride levels, as well as screening for diabetes mellitus, should not be overlooked. A timely diagnosis of comorbid MetS may prompt consideration for early systemic treatment for psoriasis, as well as helping to identify patients in need of cardiovascular preventive measures, resulting in more successful clinical management for both pathologies. To guide treatment optimization, additional research to elucidate drug-specific effects of biological treatment for psoriasis on MetS components and CVD risk – as well as the effects of treating MetS components on psoriasis disease activity – is strongly recommended.
Open Research
Data availability statement
Data sharing is not applicable to this article as no data sets were generated or analysed during the current study.




