Helicobacter pylori inhibits autophagic flux and promotes its intracellular survival and colonization by down-regulating SIRT1
Abstract
Helicobacter pylori (H. pylori) is the strong risk factor for a series of gastric pathological changes. Persistent colonization of H. pylori leading to chronic infection is responsible for gastritis and malignancy. Autophagy is an evolutionary conserved process which can protect cells and organisms from bacterial infection. Here, we demonstrated that H. pylori infection induced autophagosome formation but inhibited autophagic flux. SIRT1, a class III histone deacetylase, was down-regulated at both mRNA and protein levels by H. pylori infection in gastric cells. Further investigation showed that the transcriptional factor RUNX3 accounted for down-regulation of SIRT1 in H. pylori-infected gastric cells. SIRT1 promoted autophagic flux in gastric cells and activation of SIRT1 restored the autophagic flux inhibited by H. pylori infection. Furthermore, SIRT1 exerted inhibitory effects on intracellular survival and colonization of H. pylori. And activation of autophagic flux in SIRT1-inhibited gastric cells could significantly reduce intracellular load of H. pylori. Moreover, the relationship between H. pylori infection and SIRT1 expression was identified in clinical specimen. Our findings define the importance of SIRT1 in compromised autophagy induced by H. pylori infection and bacterial intracellular colonization. These results provide evidence that SIRT1 can serve as a therapeutic target to eradicate H. pylori infection.
1 INTRODUCTION
Helicobacter pylori (H. pylori) is one of the most common human pathogens that has colonizes more than 50% of the world’s population. Chronic infection of H. pylori gives rise to a series of well-defined histological stages processing through gastritis, metaplasia, dysplasia and eventually the malignancy, gastric cancer.1 Although H. pylori was previously regarded as an extracellular bacterium, a growing evidence has reported the facultative intracellular nature of H. pylori.2, 3 This bacterium was found to survive and even multiply inside gastric epithelial cells.3, 4 Presence of intracellular H. pylori in progenitor or stem cells serves as a strategy for this invasive pathogen to persist in human stomach.5, 6 Hiding inside the host cells enables H. pylori to escape from the innate immune response.7 Besides, intracellular H. pylori are more resistant to antibiotic treatment and account for repopulation following eradication therapy.2, 8, 9 However, the host factors that affect the intracellular survival and colonization of H. pylori remain poorly understood.
Autophagy is an evolutionary conserved process which may be divided into two steps. First, double-membrane autophagosomes formed sequestrating intracellular components. Second, autophagosome-lysosome fused and eventually degraded the contents. The second step is also named as autophagic flux. Autophagy can be induced by a number of deleterious stimuli and play an important role in maintaining cellular homeostasis and protecting against pathogen infection.10, 11 Intracellular bacteria like Mycobacterium tuberculosis (M. tuberculosis) and Salmonella enterica may evade degradation by autophagy through different mechanisms thereby leading to persistent infection and increased bacterial load. The pathogens may obstruct phagosomal lipid metabolism to inhibit autophagosome maturation or escape from the host cell autophagosomes.12, 13 It has been reported that H. pylori can induce autophagosomes formation, survive and even multiply in autophagosomes before autophagic flux occurs.14, 15 When autophagosomes fused with lysosome, which is referred to autophagic flux, clearance of H. pylori in a lysosome-dependent manner occurred. Therefore, inhibiting autophagic flux may increase intracellular survival and colonization of H. pylori.15 Nevertheless, the mechanism by which H. pylori antagonizes degradation by autophagic flux remains to be elucidated.
Sirtuin 1 (SIRT1) belongs to class III histone deacetylases and is the mammalian ortholog of yeast silent information regulator (Sir 2). As Sir 2 was proved to extend lifespan in yeast, research on SIRT1 first concentrated on anti-ageing and then expanded to stress response and metabolism.16-18 By interacting with the energy sensor, AMP-activated protein kinase, SIRT1 was shown to inhibit mammalian target of rapamycin (mTOR) signalling and thus promote formation of autophagosomes.19 SIRT1 can also directly deacetylate ATGs and BECLIN1 and stimulates the initial process of autophagy.20, 21 Moreover, SIRT1 deacetylates and activates transcription factor FOXO1 and its target Rab7, which was both necessary and sufficient for increasing autophagic flux.22 Moreover, SIRT1-dependent autophagy activation was found to reduce bacterial growth and anti-M. tuberculosis responses.21, 23 However, whether SIRT1 and SIRT1-induced autophagy play a role in intracellular survival and colonization of H. pylori has not been elucidated.
In this study, we investigated regulation of SIRT1 expression by H. pylori infection and the underlying mechanism was explored in detail. Then the effects of SIRT1 and SIRT1-induced autophagic flux in intracellular survival and colonization of H. pylori were also explored.
2 MATERIAL AND METHODS
2.1 Cell culture and reagents
Human gastric cell lines AGS, BGC-823, SGC-7901 and GES-1 were obtained from Cell Resource Center, Institute of Biochemistry and Cell Biology at the Chinese Academy of Sciences (Shanghai, China) and cultured in F12 (AGS) or RPMI 1640 (BGC-823, SGC-7901 and GES-1) with 10% foetal bovine serum (Biological Industries, Israel), 100 units/ml penicillin and 100 µg/ml streptomycin. All cells were incubated at 37°C with 5% CO2. Mycoplasma PCR (GeneCopoeia) testing of these cells was performed every month following the protocol of the manufacturer. Reagents used in this study were: Earle’s Balanced Salt Solution (EBSS, HyClone) used for serum starvation to induce autophagic flux, bafilomycin A1 (Baf A1, S1413, Selleckchem) used as inhibitor of autophagic flux, SRT1720 (S1129, Selleckchem) used as activator of SIRT1, and EX 527 (S1541, Selleckchem) used as inhibitor of SIRT1.
2.2 Bacterial culture
H. pylori strains (NCTC 26695 and 11637) were kindly provided by Dr. Jianzhong Zhang (Chinese Disease Control and Prevention Center; Beijing, China). These H. pylori strains were cultured on the Brucella broth plates containing 5% FBS under microaerophilic conditions (5% O2, 10% CO2, and 85% N2) at 37 °C. H. pylori strains were harvested, resuspended with PBS and added to gastric cells at a multiplicity of infection (MOI) of 100.
2.3 Plasmids and siRNAs
The pBabe-myc-RUNX3-R122C plasmid (a mutation located in the conserved Runt domain) was kindly provided by Professor Bae SC (Chungbuk National University, Cheongju 361-763, South Korea). The RUNX3 eukaryotic expression plasmid (pcDNA3.1-RUNX3) was kindly provided by Dr. Zhifang Liu (Shandong University, Jinan, China). The luciferase reporter plasmids containing the promoter sequence of SIRT1 assumed RUNX3 binding sits deleted promoter sequences of SIRT1 were constructed by BioSune Biotechnology (Shanghai, China). The plasmids were delivered into cells by X-tremeGENE HP Transfection Reagent (Roche Applied Science, Basel, Switzerland). The small interfering RNA (siRNA) targeting RUNX3 and FOXO3a, and the negative control siRNA were synthesized by GenePharma (Shanghai, China) and delivered into cells by Lipofectamine 2000 (Invitrogen). The sequences of siRNAs were summarized in Table S1.
2.4 RNA extraction and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted with Trizol, converted into cDNA and amplified by qRT-PCR as previously described.24 Primers were synthesized by BioSune Biotechnology, and the primer sequences were listed in Table S1.
2.5 Western blot
Total proteins were extracted with RIPA lysis buffer (Beyotime, Shanghai, China) containing proteinase inhibitor (PMSF, Beyotime). The protein concentrations were measured using a BCA reagent kit (Beyotime). The membranes were probed, and the protein bands were visualized as previously described.24 The primary antibodies used include the following: anti-β-actin (4967, 1: 1000, Cell Signaling), anti-GAPDH (60004-1, 1: 5000, Proteintech), anti-LC3B (3868, 1: 500, Cell Signaling), anti-p62 (ab56416, 1: 2000, Abcam), anti-RUNX3 (ab135248, 1: 1000, Abcam) and anti-SIRT1 (ab110304, 1: 5000, Abcam). Housekeeping gene β-actin or GAPDH served as the internal control.
2.6 Luciferase assay
For luciferase assay, cells were seeded in 24-well plates (5 × 104 cells per well). After transfection with plasmids or siRNAs, appropriate reporter plasmid was transfected into cells. Then, the relative luciferase activities were measured and calculated using the Dual-Luciferase Reporter Assay System (Promega).24
2.7 Chromatin immunoprecipitation (ChIP)
With the SimpleChIP® Enzymatic Chromatin IP Kit (Cell Signaling), ChIP assays were performed with anti-RUNX3 (ab11905, Abcam), and IgG (negative control) in AGS cells transfected with pcDNA3.1-RUNX3 plasmid. The purified DNA served as the template to amplify SIRT1 promoter. Agarose gel electrophoresis was performed to visualize the PCR products. The primers were listed in Table S1.
2.8 The mCherry-EGFP-LC3B fluorescence microscopy assay
Cells were seeded on glass coverslips in 24-well plates (5 × 104 cells per well) and incubated overnight. Then cells were transfected with AdM-CMV-mCherry-EGFP-LC3B adenovirus (MOI = 100, Vigene, Jinan, China) for 24 h. After cells were treated with chemicals or infected with H. pylori, cells were fixed with 4% fixative solution and the coverslips were taken out of the plates for observation. Over 20 cells in one field were randomly selected to examine the numbers of autophagosomes (yellow puncta) and autolysosomes (red puncta) using an Olympus microscope (Tokyo, Japan). Five fields were randomly selected from each group, and the EGFP/mCherry co-localization efficiency was calculated.
2.9 PCR quantitation of bacterial DNA
DNA from gastric cells was extracted using a DNA purification kit (DP302, TIANGEN, Beijing, China) according to the manufacturer’s instruction. H. pylori-specific 16S ribosomal DNA (rDNA) was quantified by real-time PCR using TransStart Top Green qPCR SuperMix. The primer sequences were listed in Table S1. The levels of 16S rDNA in gastric cells were measured and normalized to β-actin based on 2-ΔΔCt method.
2.10 Gentamicin protection assay
Cells were infected with Hp26695 for 12 hours. To remove the nonadherent bacteria, cells were washed three times using warm PBS. Subsequently, the cell monolayers were incubated with gentamicin (E003632, 100 mg/ml, Sigma, St. Louis, MO) for 1 hours at 37°C, washed three times with warm PBS, and then treated with 0.5% saponin (HY-100597, dissolved in PBS, Sigma) at 37°C for 15 minutes. The treated monolayers were resuspended thoroughly, serially diluted (1:30, 1:100, 1:300, 1:900, 1:2700) and plated on Brucella broth containing 10% FBS. After 5 days of incubation, the colony formation of intracellular bacteria was calculated as CFU per well of cells.
2.11 Immunofluorescence stain
Cells (2.5 × 104) were seeded onto coverslips in 24-well plates and incubated overnight. After infected with Hp26695 for 10 h, extracellular H. pylori were killed using 100 µg/ml gentamicin for 1 hour at 37°C and then washed with PBS two times. Next, cells were fixed with 4% fixative solution and permeabilized with 0.2% Triton X-100. Then, after blocking, cells were incubated with anti-H. pylori antibody (ab7788, 1: 100, Abcam) and the fluorescent secondary antibody. Nuclei were stained with 4’, 6-diamidino-2-phenylindole (DAPI, Beyotime). Slides were observed under microscope (Olympus), and photographs were taken using CellSens Dimension software. Red fluorescence intensities for the H. pylori-infected group were calculated by randomly selecting over 30 cells in one field, and three fields were detected for each group (Image J).
2.12 Immunohistochemistry (IHC) stain
We obtained paraffinized gastric tissue sections from Jinan Central Hospital. H. pylori infection was examined by rapid urease test and pathological examination using methylene blue staining. These paraffinized gastric tissue sections were divided into two groups: H. pylori-positive (n = 15) and H. pylori-negative (n = 15). For each group, there were three types of pathological changes: superficial gastritis (n = 5), atrophic gastritis (n = 5) and dysplasia (n = 5). To investigate SIRT1 expression levels in these sections, IHC analysis was performed. Paraffin-embedded sections were deparaffinized, rehydrated and antigen-retrieved. The remaining steps were performed as previously described.24 The primary antibody anti-SIRT1 (ab32441, 1: 100, Abcam) was used. The images were obtained under microscope (Olympus) using CellSens Dimension software. The intensity of SIRT1 staining was scored as follows: 0, no staining; 1, light brown; 2, medium brown; and 3, dark brown. The expression score was calculated by multiplying the staining intensity score and the positive percentages.
2.13 Statistical analysis
All experimental data were analysed with GraphPad Prism 8.0 software. Data were presented as mean ± SD. Comparisons between different groups were determined by Student’s t test or one-way ANOVA. A value of P < 0.05 was considered statistically significant.
3 RESULTS
3.1 H. pylori infection induces autophagosome formation but inhibits autophagic flux leading to increased colonization
We first verified increased autophagosome formation induced by H. pylori infection (Hp26695 and Hp11637). As revealed in Figure 1A and Figure S1A,B, H. pylori infection significantly increased protein levels of LC3B-II (a hallmark of autophagy) in gastric cell lines (AGS and SGC-7901). Additionally, another autophagy marker, SQSTM1/p62 was also examined. Similar to LC3B-II, protein levels of SQSTM1/p62 significantly increased in H. pylori-infected gastric cell lines (Figure 1A, Figure S1A,B). Concomitantly increased levels of both LC3B-II and SQSTM1/p62 indicate that autophagic flux is impaired by H. pylori infection. The impairment of autophagic flux induced by H. pylori infection was further confirmed by expressing mCherry-EGFP-LC3B fusion protein in AGS and SGC-7901 cells. Fluorescence from EGFP but not mCherry is quenched in late acidic autophagosomes, so autophagic flux appears as red dots. For the early autophagosomes, EGFP signals overlap with mCherry and appear as yellow dots. Using this method, we found that in cells treated with EBSS (serum starvation to induce autophagic flux), most of the autophagosomes retained only mCherry signals indicating autophagic flux occurred (Figure 1B,C, Figure S1C,D). In contrast, in cells infected with H. pylori, we found increased retention of EGFP signals (yellow dots) and very few single mCherry signals (red dots) indicating compromised autophagy in H. pylori-infected cells (Figure 1B,C).

Then, we assessed the effects of autophagic flux on intracellular survival and colonization of H. pylori. Colony formation assays, bacterial rDNA quantitation and immunofluorescence staining of H. pylori were performed to evaluate intracellular load of H. pylori. Results from the above assays revealed that inducing autophagic flux using EBSS decreased colonization of H. pylori whereas compromised autophagy induced by Baf A1 (an inhibitor of autophagic flux) exerted the opposite effects (Figure 1D-I). Taken together, we confirmed that H. pylori infection inhibited autophagic flux which led to increased intracellular survival and colonization.
3.2 H. pylori infection inhibits expression levels of SIRT1 in gastric cells
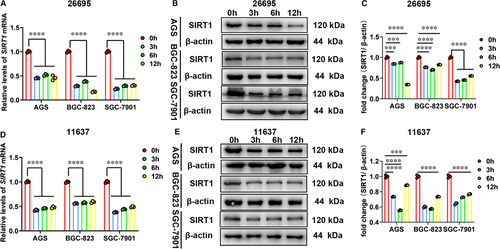
Since SIRT1 can activate autophagy to eliminate bacterial load,21, 23 we have been suggested that SIRT1 might be the downstream target of H. pylori infection and play a role in intracellular survival and colonization of H. pylori. Then, we attempted to determine whether SIRT1 levels could be affected by H. pylori infection in several gastric cell lines. The qRT-PCR and Western blot analysis were performed to examine levels of SIRT1, and two different H. pylori strains (Hp26695 and Hp11637) were used to infect gastric cells. We found that SIRT1 was significantly down-regulated at both mRNA and protein levels in H. pylori-infected cells (Figure 2, Figure S2).
3.3 RUNX3 can regulate expression of SIRT1 in gastric cells
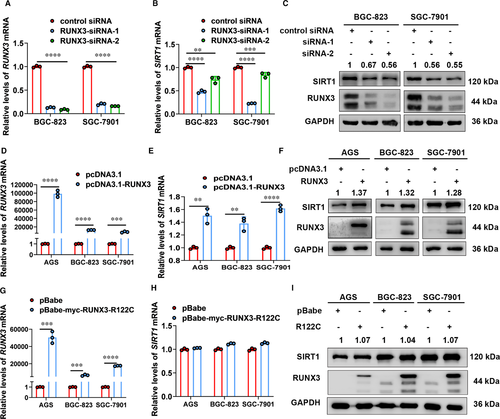
Next, we screened for transcriptional factors responsible for regulation of SIRT1 expression in gastric cells. We searched the promoter of SIRT1 in JASPAR database for transcriptional factors that are closely related to H. pylori infection and found putative binding sites of FOXO3 and RUNX3 (Figure 4A, Figure S3A). Our results from small interference experiments showed that FOXO3 did not regulate SIRT1 expression in gastric cells (Figure S3B,C). In contrast, cells with RUNX3 knockdown showed significantly decreased SIRT1 expression (Figure 3A-C). To further confirm regulation of SIRT1 by transcriptional factor RUNX3, we transfected cells with the RUNX3-expressing vector (pcDNA3.1-RUNX3) and the mutant vector (pBabe-myc-RUNX3-R122C, the mutation located in the conserved Runt domain), respectively. Results from qRT-PCR and Western blot analysis showed that overexpression of RUNX3 up-regulated expression of SIRT1 whereas expression of mutant RUNX3 did not exert this positive regulation in gastric cells (Figure 3D-I). These results suggested that RUNX3 can regulate expression of SIRT1 in gastric cells and this regulation is Runt domain-required.
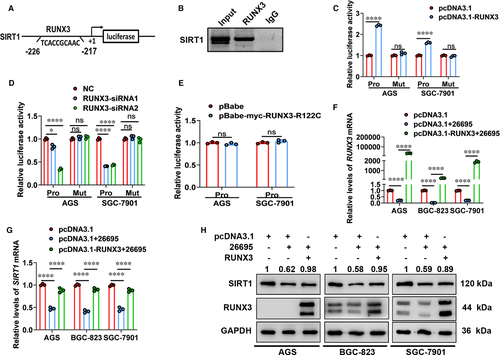
Furthermore, we performed ChIP assays in RUNX3-expressing vector-transfected AGS cells and demonstrated direct occupancy of RUNX3 on SIRT1 promoter (Figure 4B). Additionally, luciferase assays were used to verify the functional significance of this binding. We constructed luciferase reporter plasmids containing SIRT1 intact promoter (Pro) and mutant SIRT1 promoter (Mut). Overexpression of RUNX3 significantly increased promoter activities, while inhibition of RUNX3 exhibited the opposite effects (Figure 4C,D). Nevertheless, mutation of RUNX-binding site abolished the changes in promoter activities (Figure 4C,D). Moreover, expression of mutant RUNX3 did not regulate SIRT1 promoter activities (Figure 4E). Therefore, in gastric cells SIRT1 was a direct target gene of RUNX3.
3.4 RUNX3 accounts for down-regulation of SIRT1 in H. pylori-infected gastric cells
Then, we assessed whether RUNX3 was involved in SIRT1 expression inhibited by H. pylori infection. As shown in Figure 4F-H, Hp26695 infection decreased expression of transcriptional factor RUNX3 and its target gene SIRT1 at both mRNA and protein levels. In RUNX3-overexpressing gastric cells, expression of RUNX3 increased significantly even upon Hp26695 infection. And overexpression of RUNX3 rescued expression of SIRT1 in gastric cells infected with Hp26695. Taken together, H. pylori infection inhibited expression of SIRT1 in gastric cells in a RUNX3-dependent manner.
3.5 Activation of SIRT1 rescues autophagic flux inhibited by H. pylori infection
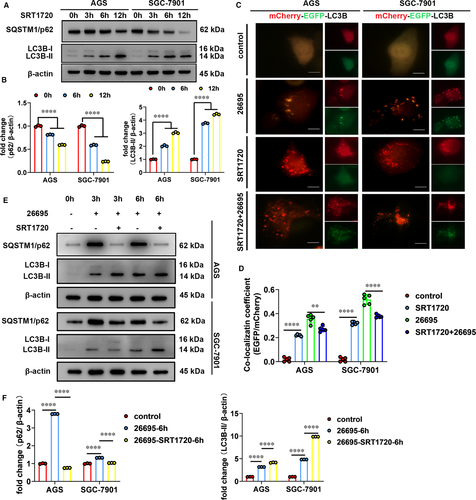
Next, we tested whether increasing SIRT1 activity could restore the autophagic flux inhibited by H. pylori infection. To this end, we activated SIRT1 using SRT1720 (a small molecule compound specific to activate SIRT1) and assessed autophagic flux by detecting levels of autophagy marker and the mCherry-EGFP-LC3B fluorescence microscopy assays. We showed that upon SIRT1 activation, protein levels of LC3B-II increased but SQSTM1/p62 decreased over time indicating induction of autophagic flux (Figure 5A,B). In addition, cells with SIRT1 activation showed that most of the LC3B-positive autophagosomes lost EGFP signal but retained mCherry signal (Figure 5C,D). This is consistent with the results from serum-starved cells shown in Figure 1B and confirmed that activation of SIRT1 leads to increased autophagic flux. Then, we investigated the effects of SIRT1 activator on autophagic flux in H. pylori-infected cells. Following SRT1720 administration, H. pylori-infected cells showed decrease in SQSTM1/p62 protein levels and less EGFP-mCherry overlapping LC3B-positive puncta (Figure 5C-F). This indicated that autophagic flux in H. pylori-infected cells was restored by SRT1720. Therefore, these data indicated that activation of SIRT1 rescued autophagic flux that was inhibited by H. pylori infection.
3.6 SIRT1 inhibits intracellular colonization of H. pylori by activating autophagic flux
Then we investigated the effects of SIRT1 on intracellular survival and colonization of H. pylori. We pretreated H. pylori-infected cells with SIRT1 activator (SRT1720) and then determined intracellular survival and colonization of H. pylori by bacterial DNA measurement, colony formation assays and immunofluorescence staining. As shown in Figure 6A, upon SIRT1 activation, gastric cells infected with Hp26695 showed significantly decreased 16S rDNA levels and less colonies on agar plates indicating reduced H. pylori colonization. Additionally, immunofluorescence staining of H. pylori demonstrated less H. pylori colonization pretreated with SIRT1 activator (Figure 6D,E). To further confirm the effect of SIRT1 on H. pylori colonization, we pretreated H. pylori-infected cell with SIRT1 inhibitor (EX 527). In contrast, SIRT1 inhibitor led to increased colonization of H. pylori in gastric cells (Figure 6B,D,E). Combined with our results that H. pylori infection inhibited expression levels of SIRT1, we demonstrated here that H. pylori infection inhibited SIRT1 expression and thus promoted its colonization in gastric cells. Next, we assessed whether the increase in H. pylori colonization can be reversed by autophagic flux. Serum starvation was used to induce autophagic flux in SIRT1 inhibitor-treated gastric cells. Results from bacterial quantitation and immunofluorescence staining experiments showed that activation of autophagic flux significantly reduced the intracellular load of H. pylori in SIRT1-inhibited cells (Figure 6C-E). In brief, inhibition of SIRT1 increased intracellular survival and colonization of H. pylori which can be reversed by activation of autophagic flux.
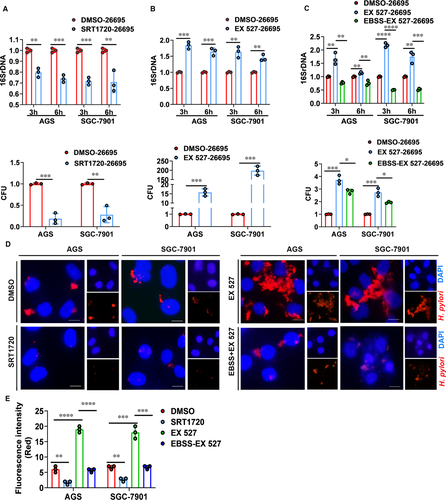
3.7 Validation of relationship between SIRT1 expression levels and H. pylori infection in clinical specimens
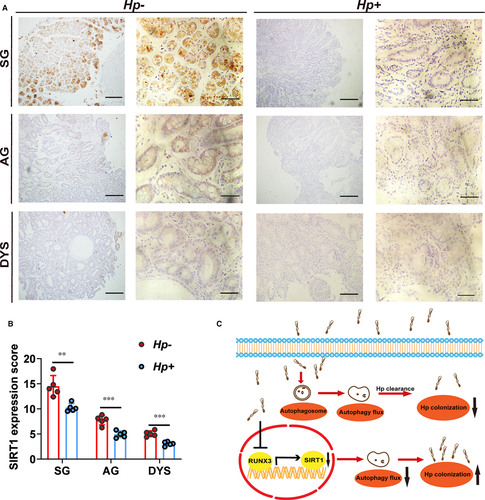
To determine the relationship between SIRT1 expression levels and H. pylori infection, we performed IHC staining using clinical specimens. We collected gastric tissues diagnosed as superficial gastritis, atrophic gastritis and dysplasia. For each pathogenic stage, both H. pylori-positive and H. pylori-negative specimens were included. For each histologic stage (superficial gastritis, atrophic gastritis or dysplasia), expression levels of SIRT1 in H. pylori-positive patients were significantly lower than those in patients H. pylori-negative (Figure 7A,B). These results confirmed the relationship between SIRT1 expression and H. pylori infection in clinical specimen.
4 DISCUSSION
H. pylori is the strong risk factor for a series of gastric disorders, including gastritis and gastric cancer. Failure of our immune system to eliminate this bacterium leads to persistent colonization of H. pylori in gastric tissues and chronic infection. On the other hand, antibiotic treatment often cannot achieve a satisfactory result which makes it hard for H. pylori eradication.25, 26 Besides standardizing treatment regimen, exploring the mechanism underlying H. pylori colonization is critical to eradicate bacterial infection. In this study, we show that autophagic flux plays an important role in eliminating intracellular survival and colonization of H. pylori. Autophagy has emerged as an essential protective strategy employed by the host to eliminate intracellular bacteria since 2004.27, 28 On the other hand, intracellular bacteria develop diverse strategies to reprogram or stall autophagy process and thus escape from autophagic degradation.29-33 H. pylori, which was previously thought only survive on the surface of gastric mucosa, have recently been found to invade epithelial cells and colonize intracellularly. Although the intracellular part represents a small percentage of overall bacteria, this proportion is strongly resistant to antibiotic treatment and play a critical role in infection recrudescence post-therapy.3, 8, 9 Here, we show that in gastric cells, H. pylori infection induced formation of autophagosomes but failed to induce autophagic flux. And the inhibited autophagic flux helps H. pylori to colonize inside the cells successfully.
Until now, the mechanisms underlying that intracellular bacteria initiate autophagy have not been completely elucidated. Nevertheless, compelling evidence suggests that intracellular pathogens may trigger nutritional deprivation and energy stress both of which were generally regarded as initial factors of autophagy.29, 33-35 SIRT1, a NAD+-dependent deacetylase, acts as an energy and nutrition sensor. It has been shown that SIRT1 plays an essential role in calorie restriction-induced longevity and starvation-activated autophagy.36-38 By treating gastric cells with SIRT1 activator or inhibitor, we showed that SIRT1 can induce not only autophagosomes formation but also autophagic flux occurrence. The following rescue experiment indicated that activation of SIRT1 restored the autophagic flux inhibited by H. pylori infection. Through deacetylation of autophagy-related proteins and promotion of phagosome-lysosome fusion, activation of SIRT1 induces effective antimicrobial responses against mycobacterial infection and significantly reduces intracellular growth of both drug-susceptible and drug-resistant strains.21, 23 Consistent with the data of M. tuberculosis infection, by bacterial quantification and immunofluorescence staining, we demonstrated the inhibitory effects of SIRT1 on intracellular colonization of H. pylori. Furthermore, stimulation of autophagic flux by serum starvation significantly reduced H. pylori colonization in SIRT1-inhibited gastric cells. Therefore, our findings indicate that the inhibitory effects of SIRT1 on H. pylori colonization are autophagy-dependent. And these results collectively raise the possibility that activation of SIRT1 may serve as a potential therapeutic target in eradicating H. pylori infection.
H. pylori infection may induce complicated changes in gastric epithelial cells. Previous studies have found that H. pylori infection could increase SIRT2 expression levels whereas decrease SIRT3 expression levels.39, 40 Here using H. pylori-infected cell models, we showed that H. pylori infection significantly inhibited expression levels of SIRT1 at both mRNA and protein levels. Next, we identified tumour-suppressive transcriptional factor RUNX3 could regulate expression levels of SIRT1 in gastric cells. It has been revealed that H. pylori infection can inhibit expression of RUNX3 at mRNA levels through CpG island methylation or Src/MEK/ERK and p38 MAPK pathways.41, 42 Work from Tsang et al. even showed that H. pylori CagA targets RUNX3 for proteasome-mediated degradation.43 The cytoplasmic mislocation of RUNX3 induced by H. pylori infection can also suppress transcriptional activities of RUNX3.44 Although we did not go further into the mechanism underlying H. pylori infection regulating RUNX3 expression, we identified down-regulation of RUNX3 at both mRNA and protein levels after H. pylori infection. Overexpressing RUNX3 in H. pylori-infected cells restored expression levels of SIRT1 suggested that inhibition of SIRT1 expression by H. pylori infection is RUNX3-dependent.
In summary, we demonstrated that H. pylori infection induces autophagosomes formation but suppresses autophagic flux. H. pylori infection inhibited SIRT1 expression at both mRNA and protein levels. And transcriptional factor RUNX3 was identified to account for down-regulation of SIRT1 by H. pylori infection. Inhibition of SIRT1 leads to decrease in autophagic flux induced by H. pylori infection and thus increased intracellular colonization of H. pylori. We further confirmed inhibition of SIRT1 expression by H. pylori infection in clinical samples. Our results provide evidence that SIRT1 can serve as a therapeutic target to eradicate H. pylori infection (Figure 7C).
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (No. 81472270, 81772151, 81971901), the Natural Science Foundation of Shandong Province (ZR2016HM04, ZR2020MH298) and the Shandong Province Major Science and Technology Innovation Project (2018CXGC1208).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHORS CONTRIBUTIONS
Xin Wang, Bo Wang and Qing Yang: Study conception and design, Xin Wang, Bo Wang, Wei Gao, Yifei An and Guoying Dong: Performing the experiments, Xin Wang, Jihui Jia and Qing Yang: Data analysis and interpretation, Jihui Jia and Qing Yang: Writing the manuscript. All the authors had final approval of the submitted version.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




