Aspirin inhibits adipogenesis of tendon stem cells and lipids accumulation in rat injury tendon through regulating PTEN/PI3K/AKT signalling
Abstract
Tendon injury repairs are big challenges in sports medicine, and fatty infiltration after tendon injury is very common and hampers tendon injury healing process. Tendon stem cells (TSCs), as precursors of tendon cells, have shown promising effect on injury tendon repair for their tenogenesis and tendon extracellular matrix formation. Adipocytes and lipids accumulation is a landmark event in pathological process of tendon injury, and this may induce tendon rupture in clinical practice. Based on this, it is important to inhibit TSCs adipogenesis and lipids infiltration to restore structure and function of injury tendon. Aspirin, as the representative of non-steroidal anti-inflammatory drugs (NSAIDs), has been widely used in tendon injury for its anti-inflammatory and analgesic actions, but effect of aspirin on TSCs adipogenesis and fatty infiltration is still unclear. Under adipogenesis conditions, TSCs were treated with concentration gradient of aspirin. Oil red O staining was performed to observe changes of lipids accumulation. Next, we used RNA sequencing to compare profile changes of gene expression between induction group and aspirin-treated group. Then, we verified the effect of filtrated signalling on TSCs adipogenesis. At last, we established rat tendon injury model and compared changes of biomechanical properties after aspirin treatment. The results showed that aspirin decreased lipids accumulation in injury tendon and inhibited TSCs adipogenesis. RNA sequencing filtrated PTEN/PI3K/AKT signalling as our target. After adding the signalling activators of VO-Ohpic and IGF-1, inhibited adipogenesis of TSCs was reversed. Still, aspirin promoted maximum loading, ultimate stress and breaking elongation of injury tendon. In conclusion, by down-regulating PTEN/PI3K/AKT signalling, aspirin inhibited adipogenesis of TSCs and fatty infiltration in injury tendon, promoted biomechanical properties and decreased rupture risk of injury tendon. All these provided new therapeutic potential and medicine evidence of aspirin in treating tendon injury and tendinopathy.
1 INTRODUCTION
Tendons are anatomical structures connecting muscles to bone which generate transmission of forces, thereby ensuring joint movements. Tendon injuries have become a common clinical disorder due to overuse or age-related degeneration.1 Injury tendons heal slowly and hardly restore the structure integrity and mechanical strength of intact tendon, which often results in clinical and patients' burden.2
TSCs have been reported for self-renewal ability, colony formation ability, multi-differentiation potential, which make them differentiate into adipocytes, tenocytes and osteocytes.3-5 Adipocytes and lipids accumulation is a remarkable pathological process in tendon injury, and this may induce risk of tendon rupture in clinical practice. So to inhibit TSCs adipogenic differentiation is vital to regain tendon structure and function.
The treatment for tendon injury includes NSAIDs oral administration, corticosteroids local injection, extracorporeal shock wave therapy, gene therapy and tissue engineering therapy.6-8 Among that, NSAIDs have been widely used in clinical practice for their anti-inflammation and relieving pain through inhibiting prostaglandin. Aspirin, as the classic representative of NSAIDs, has been used in many clinical fields, such as cardiovascular system,9 central nervous system,10 some cancers11 and tendinopathy/tendon injury. Its anti-inflammation and pain relief effect have been widely accepted, but evidence of its effect on TSCs differentiation is lacking, especially its effect on adipogenic differentiation and injury tendon healing.
The present study aimed to investigate the effect of aspirin on TSCs adipogenic differentiation and fatty infiltration in injury tendon, find out the molecular difference and related signalling through RNA sequencing and provide new therapeutic potential and medicine evidence of aspirin in treating tendon injury and tendinopathy.
2 MATERIALS AND METHODS
2.1 Ethics statement
All animals were treated according to institutional guidelines for laboratory animal treatment and care. All experimental procedures were approved by the Animal Research Ethics Committee of Third Military Medical University, China.
2.2 Animal model establishment
We followed the methods as the previous studies.12-15 Twenty-four male Sprague-Dawley rats were randomly divided into three groups: control group, injury model group and aspirin treatment group. Firstly, 30 μl collagenase I (10 mg/ml) were injected into both Achilles tendons of rats in injury group and aspirin treatment group to establish tendinopathy model. One week after collagenase I injection, aspirin (30 mg/d) was given to each rat in aspirin treatment group for 4 weeks.
2.3 Isolation and identification of rat TSCs
We performed the isolation and identification of rat TSCs as our previously studies.12, 16 Briefly, rats in all groups were euthanized, and then, Achilles tendons were carefully collected. The samples were cut into pieces in sterile phosphate buffer saline (PBS) and digested in collagenase I (3 mg/ml; Sigma-Aldrich) for 2.5 hours at 37°C. A single-cell suspension was made through cell strainer (Becton Dickinson). The released cells were washed in PBS and centrifuged at 300 g for 5 minutes, then incubated in Dulbecco's Modified Eagle's Medium (DMEM; Gibco) with 10% foetal bovine serum (Invitrogen). Isolated cells were cultured under conditions of 37°C with 5% CO2 for 2 days, then washed in PBS to discard non-adherent cells. On day 7 of culture, Trypsin-EDTA solution (Sigma-Aldrich) was used to digest TSCs, and TSCs were mixed together and cultured as passage (P) 0 cells. Cells from P1-P3 were used in the next studies.
2.4 Adipogenic differentiation of TSCs
Adipogenic induction medium (Cyagen) was prepared for inducing adipogenesis of TSCs. Throughout the experiments, aspirin (Sigma-Aldrich) was dissolved in dimethyl sulfoxide (DMSO), and induction medium was changed every 3 days. Seeding density of TSCs onto the 6-well plate was 6 × 104 cells/well, and TSCs were treated with 0, 0.25, 0.5, 1 or 2 mM aspirin for 24 hours or with 2 mM aspirin for 3, 7 and 14 days. In inhibition analysis, inhibitors of PI3K/AKT signalling pathway VO-Ohpic (MedChemExpress) and IGF-1 (Beyotime Biotechnology) were added into TSCs with induction medium and aspirin treatment for 3 days.
2.5 RNA-seq and data analysis
Total RNA was collected from TSCs treated with induction medium and induction medium with aspirin through TRIzol Reagent (Takara). Solexa pipeline v1.8 (Off-Line Base Caller software, v1.8 Illumina) was used to perform image analysis and base calling. FastQC software (KangChen Bio-tech) was used to assess total RNA samples. Reference genome using Hisat2 software was used to align the trimmed reads. StringTie was used to estimate the transcript abundances of each sample, R package Ballgown was used to calculate the FPKM value for differentially expressed genes and transcripts. StringTie and Ballgown were used to predict novel genes and transcripts, and CPAT was used to assess the coding potential of those sequences. rMATS.42 Principle Component Analysis was used to detect Alternative splicing events and plots, and gene expression level was used for correlation analysis. The differentially expressed genes in R, Python or shell environment for statistical computing and graphics were used to perform Hierarchical Clustering, Gene Ontology, scatter plots, volcano plots and pathway analysis.17
2.6 Protein extraction and Western blot
Protein extraction and Western blot analysis were performed as our previously study.16 After treatment, the cells were washed twice with ice-cold PBS and lysed in lysis buffer containing a mixture of proteinase inhibitors (Thermo Fisher Scientific Inc). BCA protein assay kit (Thermo Fisher Scientific Inc) was used to measure total protein concentrations, and SDS polyacrylamide gel electrophoresis was performed after adding equal amounts of proteins samples (30 µg/lane) and then proteins were transferred onto polyvinylidene difluoride membranes. After that, the membranes were blocked with 5% non-fat milk containing 0.1% TBSTween at room temperature for 2 hours, and then incubated with primary antibodies at 4°C overnight. We used the following primary antibodies: anti-ap2 (Proteintech, 1:2000), anti-C/EBPα (Cell Signaling Technology, 1:2000), anti-PPARγ (Proteintech, 1:2000), anti-PTEN (Bioss, 1:2000), anti-PI3K (Proteintech, 1:2000), anti-Phospho (P)-PI3K (Proteintech, 1:2000), anti-AKT (Bioss, 1:2000) and anti-P-AKT (Bioss, 1:2000). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Proteintech, 1:5000) was used as internal control. After incubation with primary antibodies, membranes were washed in 0.1% TBST for three times and incubated in goat anti-rabbit secondary antibodies (Proteintech, 1:2000) for 2 hours at room temperature. Enhance chemiluminescence detection kit (GE Healthcare) was used to visualize and capture the protein images.
2.7 Real-time quantitative PCR
The mRNA expression levels of adipogenesis related gene were determined by qRT-PCR. TRIzol regent was used to extract total RNA from cells, according to the protocol provided by the manufacturer (Takara). Superscript III first-strand synthesis kit (TaKaRa) was used to synthesize cDNA from total RNA. SYBR Green RT-PCR kit (TaKaRa) and an ABI Prism 7900 Sequence Detection System (PE Applied Biosystems) were used to perform qPCR. Expression of the housekeeping gene GAPDH was used as relative expression control.
2.8 Immunostaining
Achilles' tendons were cut coronally to prepare serial frozen sections (5 μm) as previously study.16, 18 Briefly, sections were rewarmed at room temperature for 30 minutes and washed thrice in PBS every 5 minutes. 0.1% Triton 100 and 5% BSA were used to punch and block the samples for 1 hour, and sections were incubated with adipogenic differentiation markers ap2 (Abcam, 1:200) and PPARγ (Abcam, 1:200) at 4°C overnight. After that, sections were incubated with goat anti-rabbit IgG H&L (Dylight-594; Proteintech, 1:200) for 2 hours in the darkroom. After washed 5 minutes for four times, sections were counterstained with DAPI for 5-8 minutes, and then, fluorescence quencher was used for the sections.
2.9 Biomechanical testing
We performed the biomechanical test as previous study.19 Briefly, the Achilles' tendons with upper and lower bony ends were firstly isolated. The two bony ends of Achilles' tendon were fixed on a custom-made testing jig with two clamps. The calcaneus end was fixed on the lower clamp site while the tibia end was fixed on the upper clamp site. The mechanical testing machine was then connected to testing system of computer. After the operating parameters were entered, the biomechanical testing started on. Six to eight samples were used for each group.
2.10 Statistical analysis
Mean ± standard deviation (SD) was used to express all values. Comparison between two groups was calculated by the Student's t test. Multiple comparisons were calculated by one-way analysis of variance followed by Fisher's tests. P < .05 was considered to be significantly different.
3 RESULTS
3.1 Aspirin inhibits adipogenic differentiation of TSCs
To testify effect of aspirin on TSCs adipogenic differentiation, TSCs were treated by gradient concentration of aspirin with adipogenic induction medium. Oil red O staining showed that aspirin significantly inhibited lipid droplets formation with increasing concentration of aspirin (Figure 1A,B). qRT-PCR showed that aspirin significantly lowered the gene expression of ap2, PPARγ and C/EBPα at 3, 7 and 14 days (Figure 1C-E). Western blot also showed that protein expression of ap2, PPARγ and C/EBPα was inhibited by aspirin at 3, 7 and 14 days (Figure 1F).
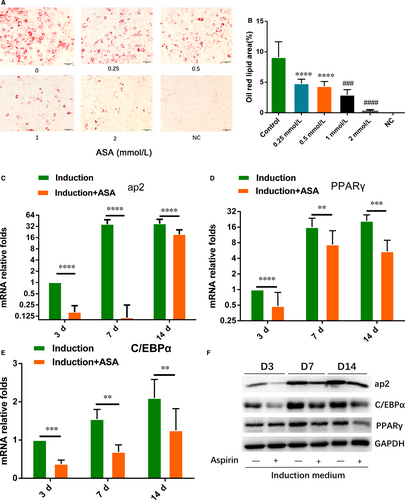
3.2 RNA-seq analysis of gene expression profile of ASA-treated and untreated TSCs induced by adipogenic differentiation culture
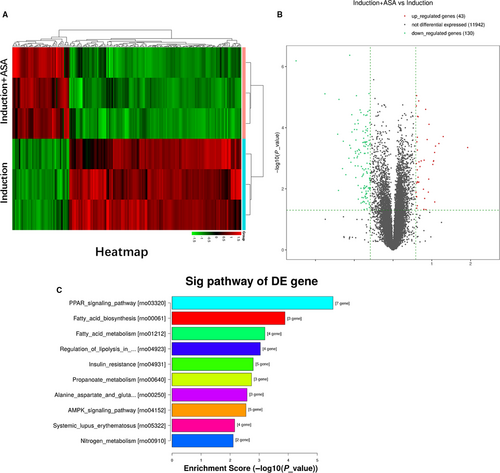
In order to analyse the underlying mechanism related to the changes of adipogenic differentiation between aspirin treatment group and induction group, RNA sequencing was used to observe changes of gene expression profiles of TSCs. From results of the heatmap and volcano map (Figure 2A,B), 43 genes and 130 genes with log2 ratio above 2 were up- and down-regulated, respectively, in the two groups. Kyoto Encyclopedia of Genes and Genomes (KEGG) showed that top ten down-regulated signalling pathways in the two groups were enriched as shown (Figure 2C). The results showed that top four pathways, including PPARγ signalling, fatty acid biosynthesis, fatty metabolism and regulation of lipolysis, are all related to decreasing formation of fatty acids. The results above showed that fatty synthesis metabolism was inhibited in aspirin treatment group. From the results, we found that gene expression of PTEN was up-regulated in aspirin treatment group, and PTEN was the upstream of PI3K/AKT signalling. So PTEN/PI3K/AKT signalling was enrolled into the next studies.
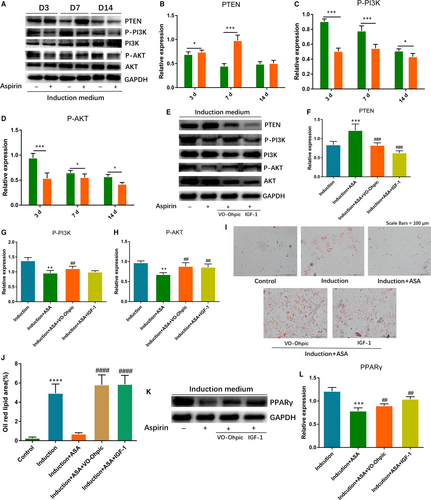
3.3 Aspirin inhibited adipogenesis of TSCs through inhibiting PTEN/PI3K/AKT signalling pathway
To verify the effect of aspirin on PTEN/PI3K/AKT pathway, we firstly observed expression changes of PTEN, P-PI3K and P-AKT through Western blot. According to the results, we found that expression of P-PI3K and P-AKT decreased significantly at 3, 7 and 14 days. PTEN, which was the upstream inhibitor of PI3K/AKT pathway, was significantly elevated at 3 and 7 days, and was slightly up-regulated at 14 days (Figure 3A-D). To investigate if PTEN/PI3K/AKT signalling was engaged in aspirin-inhibited adipogenesis, we added the activators of the signalling, VO-Ohpic and IGF-1, into TSCs before adding aspirin. Firstly, we verified the activation effect of VO-Ohpic and IGF-1 on the signalling. Western blotting showed that VO-Ohpic and IGF-1 significantly decreased PTEN expression and elevated the levels of P-PI3K and P-AKT (Figure 3E-H). After that, we explored that if the inhibition of adipogenesis was reversed after two activators treatment. The results showed that the level of the adipogenesis effect in the group with activators was significantly higher than that of aspirin group. The two activators reversed the aspirin-inhibited fatty droplets formation and related markers of PPARγ (Figure 3I-L).
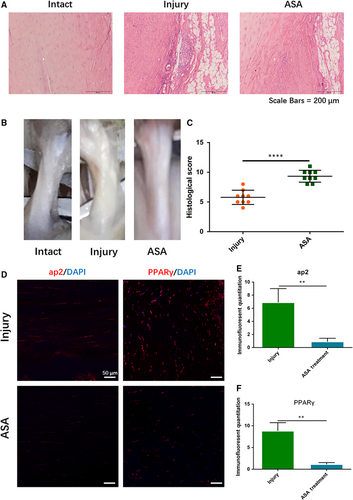
3.4 Aspirin inhibits lipids formation in injury tendon
Four weeks after ASA treatment, tendon samples were harvested for tendon healing analysis and adipogenic observation. H&E staining showed that fatty lipids in aspirin treatment group significantly decreased compared with injury group (Figure 4A). General observation showed that less fatty infiltration occurred in injury tendon after aspirin treatment (Figure 4B), and histological score in aspirin-treated group was significantly higher than that in injury group (Figure 4B,C). Immunostaining results showed that the levels of ap2 and PPARγ were significantly down-regulated after aspirin treatment (Figure 4D-F).
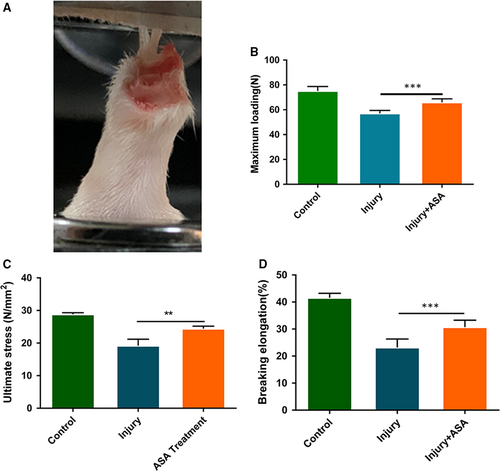
3.5 Aspirin increased the biomechanical properties of the injury tendon
Four weeks after aspirin treatment, tendon samples were harvested for biomechanical test (Figure 5A). Biomechanical test showed that maximum loading after aspirin treatment was significantly elevated compared with injury tendons (Figure 5B). Ultimate stress and breaking elongation in aspirin treatment group were also significantly increased compared to the injury group (Figure 5C,D). The results showed that aspirin treatment decreased rupture risk of injury tendon.
4 DISCUSSION
Fatty infiltration and metaplasia of adipose tissue is very common after tendon injury. Some factors induce TSCs differentiate into adipocytes and generate fatty lipids. How to inhibit and reverse the fatty infiltration is an important issue for injury tendon healing process. Aspirin and its ramification, a representative of NSAIDs, have been widely used in clinical for their pain relief and anti-inflammation effect through inhibiting the function of cyclooxygenase enzymes and prostaglandins,20 and TSCs play an irreplaceable role in repair process of tendinopathy, but the effect of aspirin on adipogenic differentiation of TSCs and fatty infiltration is still unclear. For the first time, the present study demonstrated that aspirin inhibited adipogenesis of TSCs through inhibition of PTEN/PI3K/AKT signalling pathway, decreased fatty infiltration and increased mechanical properties of injury tendon.
To make clear the effect of aspirin on adipogenic differentiation of TSCs, we firstly explored oil red staining and expression of fat formation related markers ap2, PPARγ and C/EBPα. The results revealed that aspirin inhibited the fat droplets and related markers formation. Some reports have demonstrated the effect of aspirin on cells differentiation. Yuan et al21 have proved that aspirin promoted the osteogenic potential and bone regeneration of human periodontal ligament stem cells (hPDLSCs) in vitro and in vivo. Also, Abd Rahman et al22 showed that low-dose aspirin promoted cell growth and osteogenic differentiation of PDLSCs through up-regulating the expression genes related to cell proliferation, tissue regeneration and differentiation. Zeng et al23also reported that aspirin inhibits osteoclastogenesis through inhibiting the activation of NF-κB and MAPKs in RAW264.7 cells induced by RANKL. Up to now, there are still no related reports on effect of aspirin on adipogenic differentiation and fatty acids formation.
To make clear the underlying mechanism, we utilized the RNA sequencing to explore the molecular difference of gene expression between the two groups. The results showed that PTEN, which was upstream of PI3K/AKT signalling, was up-regulated by aspirin. Based on this, we filtrated the PTEN/PI3K/AKT signalling pathway as our target. We found that the signalling pathway was inhibited in aspirin group compared with induction only group. Next, we explored if aspirin inhibited the adipogenesis of TSCs through inhibition of PTEN/PI3K/AKT signalling pathway. We added signalling activators IGF-1 and VO-Ohpic into the aspirin-treated TSCs. The results suggested that the two activators reversed adipogenesis inhibition of TSCs induced by aspirin. Many scholars have reported that PI3K/AKT signalling has taken part in adipogenic differentiation process of stem cells. Wang et al24 suggested that IGFBP2 promoted adipogenic differentiation of BMSCs through activating JNK and Akt signalling significantly. Song et al25 showed that PI3K/Akt/GSK-3β/β-catenin signalling pathway took part in the mechanical stress-induced changes of osteogenic and adipogenic differentiation in BMSCs. Our findings were in accord with the results above, which told us the important role of PTEN/PI3K/AKT in adipogenesis. This could be new target for reversing adipogenesis and fatty acids formation.
The present study also explored the effect of aspirin on injury tendon healing process. Many reports have stated that NSAIDs promoted healing process through moderating inflammation response of soft tissues such as tendon. COX-2 selective NSAIDs diminish endogenous resolution responses in systemic inflammation murine models.26, 27 Lipoxin A4 (LXA4), which promotes the resolution of inflammation, is released by low-dose aspirin.28, 29 Also, 15-epi LXA4, a kind of lipid mediator for lipid metabolism, has proven efficacious in many chronic inflammatory diseases like pulmonary inflammation30 and human infantile eczema.31 Dakin et al32 showed that 15-epi LXA4 increased the expression of CD206, ALOX15, and CCL22 mRNA and reduced the expression of IL12B mRNA of LPS-treated diseased tendon-derived stromal cells compared to no 15-epi LXA4. It is possible that low-dose aspirin and its metabolites have anti-inflammation and pro-resolution effect.29, 32 Cao et al33 showed that BMSC-promoted calvarial bone defects repair after treated with aspirin in a mini swine model. The results showed that aspirin played a vital role in BMSC-mediated calvarial bone regeneration. Our previous study revealed that aspirin inhibited inflammation of injury tendon and accelerated healing processes.18 The present study showed that aspirin reversed the adipogenic differentiation process, which may be independent of the inflammation regulation and pain relief, and this kind of effect provided a new viewpoint for us to understand that aspirin promoted healing process.
In the current study, the biomechanical properties of injury tendon were increased by aspirin treatment, suggesting that aspirin treatment decreased the risk of injury tendon rupture. Our previous study has proved that aspirin improved the biomechanical properties of injury tendon through regulating inflammation and extracellular matrix formation.18 The present study showed that aspirin reversed the fate of TSCs adipogenic differentiation and inhibited lipids accumulation and infiltration in injury tendon; hence, the biomechanical properties were improved. We can imagine that risk of rupture decreased.
There are still several limitations in this study. Firstly, we did not expand the study on patients in clinical, and TSCs were not derived from human beings. Secondly, we did not verify the effect of VO-Ohpic and IGF-1 on fatty formation in vivo. Lastly, some long-term following up should be made in clinical to verify effect of NSAIDs on fatty formation and healing quality of the injury tendon.
In summary, we found that aspirin inhibited adipogenic differentiation of TSCs and fatty infiltration, increased biomechanical properties of injury tendon and promoted healing process of injury tendon. We have demonstrated that PTEN/PI3K/AKT signalling pathway took part in the process above. We suggested that aspirin and its ramifications may contribute to treating tendon injury for their inhibition of fatty infiltration and promotion of biomechanical properties. All these provided new therapeutic potential and medicine evidence of aspirin in treating tendon injury and tendinopathy.
ACKNOWLEDGEMENTS
This study was supported by the National Natural Science Foundation of China (NSFC, Nos. 81230040 and 81572133), the National Key Research and Development of China (No. 2016YFC1100500) and Chongqing Science and Technology Commission (cstc2015shmszx0471).
CONFLICT OF INTEREST
The authors declare that there are no any conflicts of competing financial interests with the contents of this article.
AUTHOR CONTRIBUTION
YJW participated in the animal experiment, histological experiment, experimental design, acquisition of data, data analysis and interpretation, and manuscript writing. GH acquired the experimental data of the immunostaining, Western blot and qRT-PCR. FW, CKZ and XLZ joined the experimental design and manuscript revision. HHD and ZLG contributed to the experimental design. CSY, XT and BHZ modified grammar and polished the manuscript. JQZ took part in the conception and design. KLT conducted the conception, design and manuscript writing. All authors read and approved the final manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data supporting the findings of this study are available within the article and its supplementary information files.




